Abstract
Background
Pediatric generalized anxiety disorder (GAD) is debilitating and increasingly prevalent, yet its etiology remains unclear. Some believe the disorder to be propagated by chronic dysregulation of the limbic-hypothalamic-pituitary-adrenal (L-HPA) axis, but morphometric studies of implicated subcortical areas have been largely inconclusive. Recognizing that certain subcortical subdivisions are more directly involved in L-HPA axis functioning, this study aims to detect specific abnormalities in these critical areas.
Methods
Thirty-eight MRI scans of preschool children with (n = 15) and without (n = 23) GAD underwent segmentation and between-group volumetric comparisons of the basolateral amygdala (BLA), ventral hippocampal subiculum (vSC), and mediodorsal medial magnocellular (MDm) area of the thalamus.
Results
Children with GAD displayed significantly larger vSC compared to healthy peers, F(1, 31) = 6.50, pFDR = .048. On average, children with GAD presented with larger BLA and MDm, Fs(1, 31) ≥ 4.86, psFDR ≤ .054. Exploratory analyses revealed right-hemispheric lateralization of all measures, most notably the MDm, F(1, 31) = 8.13, pFDR = .024, the size of which scaled with symptom severity, r = .83, pFDR = .033.
Conclusion
The BLA, vSC, and MDm are believed to be involved in the regulation of anxiety and stress, both individually and collectively through the excitation and inhibition of the L-HPA axis. All were found to be enlarged in children with GAD, perhaps reflecting hypertrophy related to hyperexcitability, or early neuronal overgrowth. Longitudinal studies should investigate the relationship between these early morphological differences and the long-term subcortical atrophy previously observed.
Introduction
The prevalence of pediatric anxiety disorders is increasing, with the occurrence of clinically elevated anxiety symptoms in youth estimated between 11 and 25% [Citation1,Citation2]. Indeed, anxiety disorders are the single most common class of psychiatric diagnoses found in youths and adolescents, with the median age of onset estimated to be as early as 6 years [Citation3,Citation4]. These disorders tend to recur in adulthood [Citation5], and incur tremendous personal and societal costs through their association with suicide risk [Citation6], lower quality of life [Citation7], functional impairment [Citation8,Citation9], lower workforce capacity [Citation10], school dropout [Citation11], and poorer academic performance [Citation12]. Generalized anxiety disorder (GAD) is the most prevalent anxiety diagnosis in children [Citation3], and thus represents a prime target for neuropsychiatric research. However, improving diagnostic and interventive options for GAD presents a daunting challenge, not in the least because its etiology is poorly understood [Citation13]. Since fright, hypervigilance, and uneasiness are principal features of GAD [Citation2], the neural circuits subserving fear, arousal, and stress have long been research targets of interest, particularly in volumetric studies employing magnetic resonance imaging (MRI) [Citation14].
The amygdala, with its wide connections to subcortical and cortical areas, is believed to be a critical hub of fear and anxiety [Citation15–18], and has consistently been associated with the pathophysiology of GAD [Citation13]. Similarly, rodent, primate, and human models have delineated how the limbic-hypothalamic-pituitary-adrenal (L-HPA) axis, a neuroendocrine system responsible for the homeostatic regulation of arousal and stress [Citation19], is implicated in states of anxiety [Citation17]. While the L-HPA axis exerts its influence in numerous ways, such as through the innervation of brainstem noradrenergic circuits [Citation20], a component of its function particularly implicated in neuropsychiatric disorders is its release of glucocorticoids.
Through the release of corticotropin-releasing factor (CRF) by the paraventricular nucleus (PVN) of the hypothalamus, the L-HPA axis triggers the pituitary gland to secrete adrenocorticotropic hormone, resulting in elevated glucocorticoid release by the adrenal cortex, ultimately increasing arousal and redirecting energy resources to deal with a given stressor [Citation19]. Indeed, in vivo intraventricular administration of CRF in rodent models induced numerous signs analogous with human anxiety [Citation21], highlighting the direct role of the PVN in the modulation of these states. In healthy individuals, glucocorticoid levels are effectively monitored by the brain through negative feedback loops to allow for the adaptive regulation of their secretion [Citation19,Citation22]. This function is believed diminished in individuals with GAD [Citation14,Citation23], resulting in chronic states of elevated glucocorticoids. While CRF release is ultimately controlled by the hypothalamus, afferent inputs from adjacent areas, like the amygdala, hippocampus, and thalamus, project unto it, regulating its activity [Citation22,Citation24–28], and are thus frequent targets of interest in MRI research.
While some volumetric analyses of GAD have found abnormalities in these areas [Citation29–32], a recent mega-analysis showed no such tendency, except for a gender- and hemisphere-specific enlargement of the right ventral diencephalon in males [Citation33], which interestingly contains the hypothalamus, and, subsequently, the PVN. While often treated as coherent entities, subcortical structures are profoundly complex, both histologically and functionally. For example, the amygdala is known to consist of numerous distinct subnuclei.
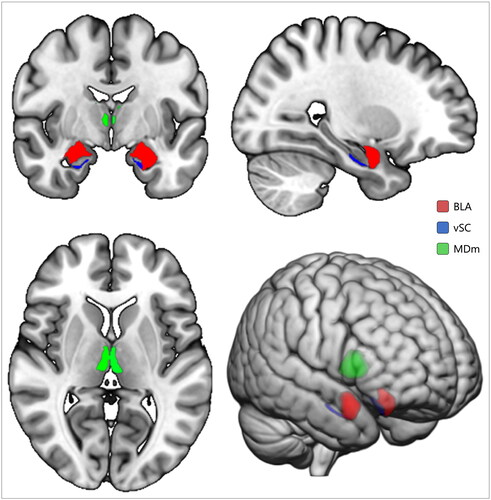
One amygdalar complex of nuclei, the basolateral amygdala (BLA), is uniquely implicated in fear and the regulation of arousal [Citation34,Citation35], with lesions in this area shown to block the encoding and activation of fear conditioning [Citation36]. Indeed, a recent study employing MRI and amygdalar sub-segmentation found distinct relationships between amygdalar subnuclei and psychiatric outcomes, associating the BLA specifically with symptoms of anxiety, depression, and substance abuse [Citation37]. Similarly, a mega-analysis revealed hippocampal subfields to be differentially implicated in psychiatric and neurological outcomes [Citation38]. For example, the ventral hippocampal subiculum (vSC) has been explicitly linked to stress response [Citation39], and lesions of this subdivision have been shown to result in elevated levels of glucocorticoids [Citation40]. Likewise, specific medial thalamic structures are implicated in the modulation of anxiety and stress through projections to the amygdala, hippocampus, and hypothalamus [Citation26,Citation41–44]. One of these areas is the mediodorsal medial magnocellular (MDm) area, linked to anxiety through a loop between it, the amygdala, and prefrontal cortex (PFC) [Citation42]. In addition to being implicated in fear learning and extinction through direct projections to the PFC and amygdala [Citation42], the MDm is specifically implicated in anxiety through one of its constituent nuclei, the paraventricular nucleus of the thalamus (PVT), which is understood to engage in an intricate network of projections between the BLA, vSC, and PFC [Citation26]. In addition to their separate roles, the BLA, vSC, and medial thalamus are all critically implicated in the regulation of the L-HPA axis by their projections unto the hypothalamus [Citation24,Citation27,Citation28,Citation45], and should thus be treated as research regions of interest when investigating disorders in which L-HPA axis dysfunction is considered a likely pathophysiological mechanism.
Aims
This study aimed to investigate whether volumetric abnormalities in select L-HPA-axis related subcortical regions are implicated in pediatric GAD. Specifically, morphological values were held against clinical phenotype to test the following hypotheses: (i) GAD is associated with volumetric abnormalities in BLA, vSC, and MDm and (ii) these differences are directly associated with symptom severity.
Methods and materials
Data and participants
This study employed an openly available dataset (https://openneuro.org/datasets/ds000144) [Citation46], pooling data from 22 ‘anxious’ and 23 ‘non-anxious’ preschool children aged 6–8 (M = 7.17, SD = 1.01).
To qualify for inclusion, anxious children had to (i) complete a laboratory assessment, (ii) be at least five and a half years old at recruitment, (iii) have completed a mock scanner scenario, and (iv) meet the criteria for an impairing anxiety disorder according to DSM-IV. Control participants were of similar age but were only included given they did not meet criteria for an anxiety disorder. Of 22 ‘anxious’ participants, only the 15 who met the criteria for GAD were included in the analysis.
MRI acquisition protocol
Fifteen participants (eight GAD, seven controls) were scanned using a 3T GE Signa EXCITE HD system, 23 (seven GAD, 16 controls) on a 3T GE MR750 unit (General Electric, Milwaukee, WI). Scanners were tested to ensure identical performance [Citation47]. T1-weighted image acquisition protocols were identical across-sites, TR = 8.096 ms; TE = 3.18 ms; inversion time = 450 ms; voxel size = 1 mm isotropic; interleaved-odd. The original study was approved by the local review board, and informed assent and consent was provided from children and parents, respectively [Citation47].
Structural data processing and quality assessment
Using the FreeSurfer analysis suite v7.3.2 (http://surfer.nmr.mgh.harvard.edu/), each T1-weighted image underwent a full subject-level processing workflow. This pipeline included skull-stripping, automated transformation to Talairach space, segmentation of the subcortical white matter and gray matter structures, intensity normalization, tessellation of grey/white matter boundaries, automated topology correction, and surface deformation [Citation48–55]. Three additional segmentation modules were employed: (1) an algorithm capable of the subdivision of 13 hippocampal subfields [Citation56], (2) an algorithm allowing the delineation of nine amygdalar structures [Citation57], and (3) an algorithm that parcellates the thalamus into 25 different nuclei [Citation58]. Post-processing results were visually inspected for any indication that the pipeline had failed or had compromised accuracy. In addition, a standardized quality assessment protocol was employed for outlier analyses and inspections of all hippocampal and amygdalar segmentations [Citation59]. Because no standardized quality analysis pipeline exists for thalamic nuclei, quality checks were appended with stem-and-leaf outlier analysis for these structures. Outliers were defined according to an interquartile range of 1.5 (Tukey’s method).
Preparation of regions of interest
Segmentations of lateral, basal, and accessory basal nuclei of the amygdala were combined to represent BLA. Similarly, heads of the hippocampal subiculum were combined to make up the vSC. Mediodorsal medial magnocellular areas of the thalamus were combined to represent the MDm. Once prepared, volumes (in mm3) of regions of interest were imported into the Statistical Packages for the Social Sciences (SPSS) v28 (IBM, Armonk, NY) for group-level analyses.
Clinical and demographic data
Variables contained in the dataset included baseline age, gender, ethnicity, poverty status, handedness, and IQ (). In addition, baseline and follow-up measures of functional impairment (World Health Organization’s International Classification of Functioning), diagnoses and symptomatology (GAD, social phobia (SOD), and/or separation anxiety disorder (SAD)), and total depressive and anxious symptomatology, were included. All psychiatric assessments were conducted using the Preschool Age Psychiatric Assessment (PAPA) tool.
Table 1. Demographics, descriptive statistics, and analyses between GAD and controls.
Group differences in volumetric measures
First, independent t-tests and Pearson’s Chi-square (χ2) were conducted on normally distributed (age, IQ) and non-parametric (sex, scanner site, handedness, ethnicity, and poverty status) demographic measures, respectively, to assess whether further covarying was needed. Then, data normality distribution in measures of interest was probed using a Shapiro–Wilk test [Citation60], along with Q–Q plot and histogram inspections. Finally, a general linear model was fit to assess group differences. The univariate model employed for all three a priori regions of interest () used group as a fixed effect (GAD vs. controls), as well as age, gender, IQ, scanner site, and intracranial volume as covariates. Of the three diagnoses in the dataset, GAD has been shown to have the highest level of comorbidity [Citation61], and, due to GAD, SOD, and SAD symptomatology in the sample all being significantly correlated (rs ≥ .39, ps ≤ .016), no covarying for comorbid symptoms or diagnosis status were done out of concerns that this would regress out important variance. The false discovery rate (FDR) method was used to correct for multiple comparisons [Citation62], set at q = 0.05 (5%).
Exploratory analyses of lateralization and post hoc correlations
There is evidence to suggest a right-hemispheric lateralization in the functional processing of anxiety [Citation63], as well as unilateral asymmetry of brain abnormalities observed in GAD [Citation64]. Therefore, analyses of volumetric differences in regions of interest were followed up by exploratory analyses utilizing identical fixed effects and covariates but employing unilateral measures instead. Highly significant findings were tested for associations between volume and clinical factors of interest (GAD symptomatology, total depressive and anxiety symptoms) using partial correlations adjusted for age, gender, IQ, scanner unit, and intracranial volume.
Results
Quality assessment of structural measures
Across 20 amygdalar, 44 hippocampal, and 52 thalamic submeasures, a total of 6, 9, and 18 outliers were discovered, respectively. Across the 10 unilateral measures that made up the three variables of interest, a single outlier was discovered for the right ventral subiculum. In total, 23 unique subjects had outlier values in at least one measure and were inspected accordingly. No failed segmentations were discovered and thus no exclusions warranted.
Demographic and clinical characteristics
Between-group comparisons in clinical measures are all reported in . In addition, t-tests probing demographic or clinical differences between participants across scanner sites revealed no such tendency, ts (42) ≤ 1.70, ps ≥ .10.
Main analysis
Means and standard deviations for all analyses are presented in . Children with GAD displayed significantly larger volumes of vSC (F(1, 31) = 6.50, pFDR = .048, η2 = .17) when compared to controls. In addition, trends toward larger BLA, F(1, 31) = 4.85, pFDR = .052, η2 = .14, and MDm, F(1, 31) = 4.01, pFDR = .054, η2 = .12, in GAD were demarcated.
Lateralization analyses and post hoc correlations
Exploratory analyses revealed right-hemispheric lateralization in all findings; BLA, F(1, 31) = 5.13, pFDR = .031, η2 = .14, vSC, F(1, 31) = 5.33, pFDR = .031, η2 = .15, and MDm, F(1, 31) = 8.13, pFDR = .024, η2 = .21. Volumes of right MDm were subsequently correlated with GAD symptomatology, revealing a significant positive association between volume and symptom severity in GAD (r = .38, pFDR = .033) and total depressive and anxiety symptoms (r = .37, pFDR = .033). Leaving the single vSC outlier out of the analysis did not change the significance of the results.
Discussion
Employing an open-source dataset, this MRI study investigated structural abnormalities in 15 preschool children with GAD compared with 23 demographically matched diagnosis naïve peers. Regions of interest were selected for analysis based on their associations with the emotional and cognitive processing believed to be adversely affected in GAD. Initially, children with GAD presented with significantly enlarged BLA and vSC, the former turning nonsignificant following FDR-correction. The BLA and MDm trended on significance, both at p = .05. When looking at unilateral measures, GAD had significantly larger volumes across all right-hemispheric measures than control participants. Brief considerations on these findings will be offered below.
The BLA is believed to play a critical role in the L-HPA axis; integrating multimodal stimuli and projecting them onto adjacent amygdalar subdivisions responsible for elevating stress responses through disinhibition of the L-HPA axis [Citation22]. Studies have suggested how prolonged states of stress and chronic glucocorticoid exposure stimulate dendritic hypertrophy and synaptic connectivity in the BLA [Citation65], ultimately promoting responsivity to stress and anxiety [Citation22]. Indeed, BLA neuroadaptation related to chronic stress has been hypothesized to diminish the ability of the PFC to inhibit stress responses [Citation66], tipping the balance between executive cognitive circuits and limbic emotional pathways in favor of the latter. Previously, amygdalar hyperactivity and PFC hypoactivity have been demarcated in fMRI studies employing emotion regulation tasks in pediatric GAD [Citation13]. Thus, the present finding of enlarged BLA in GAD might represent hypertrophy related to the hyperexcitability of pathways responsible for the innervation and elevation of fear and stress responses and might be directly implicated in GAD pathophysiology through its potentiation of stress responsiveness and the resulting negative effects of this on the emotion regulation capacity of the brain.
The hippocampus has been widely implicated in disorders involving L-HPA-axis dysregulation, such as depression and GAD, but structural findings are usually inverse, with patient volumes being lower [Citation13,Citation64,Citation67]. These reductions in volume are assumed to be atrophic in nature and have been speculated to be caused by toxic chronic glucocorticoid exposure [Citation68]. The subiculum specifically has not been widely investigated in GAD, and even within the field of depression research, results have been ambiguous, with a study reporting it as the only hippocampal subfield unaffected by diagnosis [Citation69]. However, studies of post-traumatic stress syndrome (PTSD) and GAD have found specific left-lateralized reductions in subiculum volume related to childhood maltreatment [Citation70], while subfield investigations in obsessive compulsive disorder (OCD), a separate but phenotypically related disorder through overlaps in anxious symptomatology, have found smaller volumes isolated to the right hemisphere [Citation71]. Curiously, a recent study by Vattimo et al. [Citation72] found increased hippocampal subfield volumes in pediatric OCD, including the subiculum, which the authors interpreted to reflect early neuronal overgrowth. Within a neurodevelopmental frame of reference, these seemingly contradictory findings of reduced and enlarged hippocampal volumes in anxiety disorders could be interpreted to reflect windows into two different stages of anxiety-disorder pathophysiology. If early overgrowth of neurons instigates abnormal hippocampal processing, this could lead to aberrant inhibition of the intricate projections that manage the L-HPA-axis, driving the long-term glucocorticoid-exposure related reductions in volume mentioned above. While the cross-sectional design of this study does not allow for such inferences, such a relationship between early hypertrophy and long-term atrophy may explain the present findings and should be investigated further.
The MDm is efferently and afferently connected with the BLA and PFC in circuits related to the innervation of fear responses and the allocation of affective significance to stimuli and the environment [42, Citation44]. Accordingly, rat models have shown evidence of increased anxiety-like behavior when the mediodorsal thalamus is lesioned [Citation43]. The PVT, included in the MDm mask employed here, is itself reciprocally connected to the vSC and BLA in pathways involved in the tuning of stress responsiveness [Citation22], and these connections have been hypothesized to be involved in the habituation of the L-HPA axis to chronic states of stress [Citation73]. Indeed, the PVT has been uniquely associated with anxiety specifically through its strong input from the vSC and hypothalamus [Citation26], with some suggesting how increased volume of the thalamus might be associated with the abnormal cognitive and emotional processing found in GAD [Citation74]. Curiously, enlarged thalami that normalize with treatment have been found in pediatric OCD populations [Citation75,Citation76], believed to reflect abnormal neurodevelopmental mechanisms [Citation76]. Considering this and the vSC finding above, the enlarged MDm and vSC found here could reflect a shared early biomarker in pediatric anxiety disorders more generally and may not reflect changes unique to GAD. To probe this hypothesis, comparisons with overlapping phenotypical measures across anxiety diagnoses, including OCD and GAD, as well as longitudinal data, would be required.
Numerous studies of GAD have found right-hemispheric lateralization in discovered subcortical functional and structural abnormalities [Citation33,Citation77], with some speculating this right unilateral involvement to be uniquely tied to GAD pathophysiology [Citation78]. Structurally, increased right-hemispheric amygdaloid volume has previously been associated with pediatric GAD [Citation29], and functionally, fMRI findings have elucidated a rightward lateralization specific to trait anxiety [Citation63], that is, anxiety characterized by continuity and chronicity. Trait anxiety is considered a clinical [Citation79] and genetic [Citation80] risk factor for GAD, and right amygdalar hyperactivity has been specifically associated with this trait and this disorder in resting-state fMRI [Citation79]. Ultimately, the finding of amygdalar abnormalities in anxious children has now been multimodally delineated in this very sample, with these present structural findings converging on an earlier fMRI study discovering right-lateralized functional amygdaloid irregularities during an emotional cognitive task [Citation81], and the original study in which these data appeared finding amygdaloid-PFC connectivity aberrancies [46]. This strengthens the assumption that the present morphometric findings have functional, and therefore clinical, implications.
Strengths and limitations
To the benefit of this study, numerous demographic variables known to influence brain structure were provided with the data, allowing for elaborate covarying across all analyses. For example, IQ, age, and gender are known to have widespread effects on brain structural measures [Citation82,Citation83]. Scanner unit was also covaried for, despite evidence that it does not affect structural analyses in any remarkable fashion [Citation84], even in analyses applying sub-segmentations [Citation85]. Importantly, the use of subcortical structure subdivisions likely increases the sensitivity of this present analysis as specific subareas of the three implicated subcortical structures play very different roles in cognitive and emotional functions. Choosing specific subdivisions based on a priori hypotheses grounded in multiple research modalities, such as neuroendocrinology and neurophysiology, increases triangulation potential and advocates an agenda in which neural findings should make sense on several levels of analysis.
Limitations include the small sample size, which increases the likelihood that effects are driven by sample heterogeneity. Unfortunately, efforts aimed at securing additional data were not successful, likely owed in part to difficulties in recruiting participants for such studies. In addition, the requirement of GAD as a diagnosis in the patient sample disqualified seven participants, which is a noticeable decrease in statistical power. Currently, one of the strongest morphometric findings in GAD is an enlargement in the ventral diencephalon as found in an ENIGMA mega-analysis [Citation33]. This finding was only present for males when compared with their healthy peers and only became apparent when more participants were included and no control for intracranial volume was employed [Citation33]. The present dataset included three males with GAD, deemed too little of a sample to allow for an attempted replication of this finding.
Another limitation pertains to the phenotypical variables included in the dataset. First, total depressive and anxious symptoms were given as a single score, with no means to divide the two domains for further exploratory analyses. Correlations between depressive symptomatology and brain measures specifically could help delineate the relationship between the high comorbidity of GAD with depression [Citation61]. Similarly, more elaborate measures of domain-wise symptomatology in GAD could have sensitized this analysis further, as some symptoms of GAD, like uneasiness and fatigue, could be speculated to be more related to L-HPA-axis dysregulation than others. Moreover, no data were given on illness history or treatment, all variables likely to influence brain structure [Citation74,Citation77,Citation86]. Ultimately, the correlation between MDm volume and psychiatric symptoms is difficult to interpret, as such a relationship is to be expected in a cohort where the clinical group was found to differ significantly in volume. However, as control participants also presented with a range of non-clinical anxiety symptoms, they were included in the correlation analysis to allow for an investigation of a wider continuum of phenotypes.
The cross-sectional design of this study also limits extrapolation, as several interpretations, particularly regarding the BLA and vSC, are based on previous longitudinal findings of neuroplastic changes in these sites, with the lack of follow-up data limiting any inferences on progressivity. Another issue is the way the groupings were set up: Several GAD children had a comorbid anxiety disorder (N = 8), and two presented with numerous. No covarying for these was done out of risk that the small sample and genetic and phenotypical overlap of GAD, SAD, and SOD, would regress out important variance. Indeed, consistent evidence suggests that GAD and phobias share a genetic basis [Citation87], with some authors suggesting even more ambiguity in younger populations [Citation88]. Covarying for these variables, such as the ENIGMA mega-analysis mentioned earlier did [Citation33], might have shown different results, and, indeed, because we did not, the results may not be specific to GAD, but may instead represent abnormalities apparent in children with the most severe anxious phenotypes, presenting with numerous diagnoses. Thus, while there is a possibility that results in this study are isolated to this small and heterogeneous sample, the conclusion that they triangulate with assumptions on the neuroendocrinology and neurophysiology of anxiety increases their potential. Notably, this low-powered study managed to find significant and/or trending differences across every measure of interest, controlled for likely confounding covariates, which bears potential for future investigations of these structures in similar phenotypes.
Conclusion
To the knowledge of the author, this is the first study to find volumetric enlargement in the MDm in GAD, as well as the first to find coherent structural anomalies across numerous limbic and thalamic subdivisions that are each interconnected within the L-HPA axis, likely involved in the pathophysiology of GAD. Abnormalities in structures multimodally associated with fear, arousal, and stress response, converge on the clinical presentation of GAD, a disorder characterized by chronic vigilance and uneasiness, and are likely to be involved in its pathophysiology. Future studies employing larger samples and longitudinal data should attempt to replicate these results, particularly those of the thalamus, an often-overlooked structure in neuroimaging analyses of psychiatric diagnoses that often focus on the limbic system.
Acknowledgements
The present research was self-funded. Special thanks to colleagues Maya Tranter, Rubina Fray Gogulu, and Zacharias Kalle Obel for contributing with feedback during the original drafting of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). Alexander Tobias Ysbæk-Nielsen works, teaches, and studies at the Department of Psychology, University of Copenhagen.
Data availability statement
The employed dataset, titled “Preschool Anxiety Disorders”, is hosted by OpenNeuro (OpenNeuro.org) and has the accession number: ds000144 (https://openneuro.org/datasets/ds000144/versions/00002). All data are freely available under the Creative Commons CC0 Universal license. For additional details, refer to the original paper in which these data appeared [Citation47].
References
- Racine N, McArthur BA, Cooke JE, et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175(11):1142–1150. doi: 10.1001/jamapediatrics.2021.2482.
- Tiirikainen K, Haravuori H, Ranta K, et al. Psychometric properties of the 7-item GEneralized Anxiety Disorder Scale (GAD-7) in a large representative sample of Finnish adolescents. Psychiatry Res. 2019;272:30–35. doi: 10.1016/j.psychres.2018.12.004.
- Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593.
- Merikangas KR, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication – Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017.
- Pine DS, Cohen P, Gurley D, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56.
- Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the great smoky mountains study. Arch Gen Psychiatry. 2006;63(9):1017–1024. doi: 10.1001/archpsyc.63.9.1017.
- Ramsawh HJ, Chavira DA. Association of childhood anxiety disorders and quality of life in a primary care sample. J Dev Behav Pediatr. 2016;37(4):269–276. doi: 10.1097/DBP.0000000000000296.
- Sherbourne CD, Sullivan G, Craske MG, et al. Functioning and disability levels in primary care out-patients with one or more anxiety disorders. Psychol Med. 2010;40(12):2059–2068. doi: 10.1017/S0033291710000176.
- Stein MB, Roy-Byrne PP, Craske MG, et al. Functional impact and health utility of anxiety disorders in primary care outpatients. Med Care. 2005;43(12):1164–1170. doi: 10.1097/01.mlr.0000185750.18119.fd.
- Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60(7):427–435. doi: 10.4088/JCP.v60n0702.
- Egger HL, Costello JE, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry. 2003;42(7):797–807. doi: 10.1097/01.CHI.0000046865.56865.79.
- Mazzone L, Ducci F, Scoto MC, et al. The role of anxiety symptoms in school performance in a community sample of children and adolescents. BMC Public Health. 2007;7(1):347. doi: 10.1186/1471-2458-7-347.
- Maron E, Nutt D. Biological markers of generalized anxiety disorder. Dialogues Clin Neurosci. 2017;19(2):147–158. doi: 10.31887/DCNS.2017.19.2/dnutt.
- Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022.
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33(4):653–663. doi: 10.1016/s0896-6273(02)00588-3.
- Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(3):290–299.e2. doi: 10.1016/j.jaac.2012.12.010.
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83.
- Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318.
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith.
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic‐pituitary‐adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11(5):361–369. doi: 10.1046/j.1365-2826.1999.00337.x.
- Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. In: Meerlo P, Benca RM, Abel T, editors. Sleep, neuronal plasticity and brain function. Vol. 25. Berlin Heidelberg: Springer; 2014. p. 379–410.
- Herman JP, Nawreen N, Smail MA, et al. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress. 2020;23(6):617–632. doi: 10.1080/10253890.2020.1859475.
- Faravelli C, Sauro CL, Godini L, et al. Childhood stressful events, HPA axis and anxiety disorders. World J Psychiatry. 2012;2(1):13–25. doi: 10.5498/wjp.v2.i1.13.
- Cole AB, Montgomery K, Bale TL, et al. What the hippocampus tells the HPA axis: hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF + PVN neurons. Neurobiol Stress. 2022;20:100473. doi: 10.1016/j.ynstr.2022.100473.
- Dedovic K, Duchesne A, Andrews J, et al. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074.
- Kirouac GJ. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front Behav Neurosci. 2021;15:627633. doi: 10.3389/fnbeh.2021.627633.
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis: serotonergic systems and the HPA axis. J Neuroendocrinol. 2002;14(11):911–923. doi: 10.1046/j.1365-2826.2002.00861.x.
- O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207(3):271–282. doi: 10.1111/j.1469-7580.2005.00446.x.
- De Bellis MD, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48(1):51–57. doi: 10.1016/S0006-3223(00)00835-0.
- Gold AL, Steuber ER, White LK, et al. Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology. 2017;42(12):2423–2433. doi: 10.1038/npp.2017.83.
- Moon C-M, Jeong G-W. Abnormalities in gray and white matter volumes associated with explicit memory dysfunction in patients with generalized anxiety disorder. Acta Radiol. 2017;58(3):353–361. doi: 10.1177/0284185116649796.
- Moon C-M, Kim G-W, Jeong G-W. Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. NeuroReport. 2014;25(3):184–189. doi: 10.1097/WNR.0000000000000100.
- Harrewijn A, Cardinale EM, Groenewold NA, et al. Cortical and subcortical brain structure in generalized anxiety disorder: findings from 28 research sites in the ENIGMA-Anxiety Working Group. Transl Psychiatry. 2021;11(1):502. doi: 10.1038/s41398-021-01622-1.
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812.
- Lingawi NW, Laurent V, Westbrook RF, et al. Acquisition and extinction of second-order context conditioned fear: role of the amygdala. Neurobiol Learn Mem. 2021;183:107485. doi: 10.1016/j.nlm.2021.107485.
- Sah P, Faber ESL, Lopez De Armentia M, et al. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003.
- Oshri A, Gray JC, Owens MM, et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24(4):400–410. doi: 10.1177/1077559519839491.
- Van Der Meer D, Rokicki J, Kaufmann T, et al. Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatry. 2020;25(11):3053–3065. doi: 10.1038/s41380-018-0262-7.
- Ghasemi M, Navidhamidi M, Rezaei F, et al. Anxiety and hippocampal neuronal activity: relationship and potential mechanisms. Cogn Affect Behav Neurosci. 2022;22(3):431–449. doi: 10.3758/s13415-021-00973-y.
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118.
- Kark SM, Birnie MT, Baram TZ, et al. Functional connectivity of the human paraventricular thalamic nucleus: insights from high field functional MRI. Front Integr Neurosci. 2021;15:662293. doi: 10.3389/fnint.2021.662293.
- Lee S, Shin H-S. The role of mediodorsal thalamic nucleus in fear extinction. J Anal Sci Technol. 2016;7(1):13. doi: 10.1186/s40543-016-0093-6.
- Ouhaz Z, Ba-M’hamed S, Mitchell AS, et al. Behavioral and cognitive changes after early postnatal lesions of the rat mediodorsal thalamus. Behav Brain Res. 2015;292:219–232. doi: 10.1016/j.bbr.2015.06.017.
- Timbie C, García-Cabezas MÁ, Zikopoulos B, et al. Organization of primate amygdalar–thalamic pathways for emotions. PLoS Biol. 2020;18(2):e3000639. doi: 10.1371/journal.pbio.3000639.
- McKlveen JM, Myers B, Flak JN, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024.
- Carpenter KLH, Angold A, Chen N-K, et al. Preschool anxiety disorders (ds000144) [dataset]; 2018.
- Carpenter KLH, Angold A, Chen N-K, et al. Preschool anxiety disorders predict different patterns of amygdala–prefrontal connectivity at school-age. PLOS One. 2015;10(1):e0116854. doi: 10.1371/journal.pone.0116854.
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395.
- Dale AM, Sereno I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162.
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797.
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426.
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X.
- Fischl B, Salat DH, Van Der Kouwe AJW, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016.
- Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032.
- Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364.
- Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042.
- Saygin ZM, Kliemann D, Iglesias JE, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046.
- Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology (arXiv:1806.08634). arXiv; 2018. Available from: http://arxiv.org/abs/1806.08634
- Sämann PG, Iglesias JE, Gutman B, et al. FreeSurfer‐based segmentation of hippocampal subfields: a review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum Brain Mapp. 2022;43(1):207–233. doi: 10.1002/hbm.25326.
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3–4):591–611. doi: 10.1093/biomet/52.3-4.591.
- Franz L, Angold A, Copeland W, et al. Preschool anxiety disorders in pediatric primary care: prevalence and comorbidity. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1294–1303.e1. doi: 10.1016/j.jaac.2013.09.008.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodol). 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x.
- Bourne VJ, Vladeanu M. Lateralisation for processing facial emotion and anxiety: contrasting state, trait and social anxiety. Neuropsychologia. 2011;49(5):1343–1349. doi: 10.1016/j.neuropsychologia.2011.02.008.
- Kolesar TA, Bilevicius E, Wilson AD, et al. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 2019;24:102016. doi: 10.1016/j.nicl.2019.102016.
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171.
- McEwen BS, Eiland L, Hunter RG, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014.
- McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54.
- Fu CHY, Fan Y, Davatzikos C. Widespread morphometric abnormalities in major depression. Neuroimaging Clin N Am. 2020;30(1):85–95. doi: 10.1016/j.nic.2019.09.008.
- Huang Y, Coupland NJ, Lebel RM, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry. 2013;74(1):62–68. doi: 10.1016/j.biopsych.2013.01.005.
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109.
- Zhang L, Hu X, Lu L, et al. Abnormalities of hippocampal shape and subfield volumes in medication-free patients with obsessive–compulsive disorder. Hum Brain Mapp. 2019;40(14):4105–4113. doi: 10.1002/hbm.24688.
- Vattimo EFQ, Dos Santos AC, Hoexter MQ, et al. Higher volumes of hippocampal subfields in pediatric obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2021;307:111200. doi: 10.1016/j.pscychresns.2020.111200.
- Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress: integration of cortical influences on HPA axis. J Comp Neurol. 2015;523(18):2769–2787. doi: 10.1002/cne.23815.
- Chen Y, Cui Q, Fan Y-S, et al. Progressive brain structural alterations assessed via causal analysis in patients with generalized anxiety disorder. Neuropsychopharmacology. 2020;45(10):1689–1697. doi: 10.1038/s41386-020-0704-1.
- Rosenberg DR, Benazon NR, Gilbert A, et al. Thalamic volume in pediatric obsessive–compulsive disorder patients before and after cognitive behavioral therapy. Biol Psychiatry. 2000;48(4):294–300. doi: 10.1016/S0006-3223(00)00902-1.
- Weeland CJ, Kasprzak S, De Joode NT, et al. The thalamus and its subnuclei—a gateway to obsessive-compulsive disorder. Transl Psychiatry. 2022;12(1):70. doi: 10.1038/s41398-022-01823-2.
- Abdallah CG, Coplan JD, Jackowski A, et al. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol. 2013;23(4):276–284. doi: 10.1016/j.euroneuro.2012.05.009.
- Madonna D, Delvecchio G, Soares JC, et al. Structural and functional neuroimaging studies in generalized anxiety disorder: a systematic review. Braz J Psychiatry. 2019;41(4):336–362. doi: 10.1590/1516-4446-2018-0108.
- Du Y, Li H, Xiao H, et al. Illness severity moderated association between trait anxiety and amygdala-based functional connectivity in generalized anxiety disorder. Front Behav Neurosci. 2021;15:637426. doi: 10.3389/fnbeh.2021.637426.
- Gottschalk MG, Domschke K. Genetics of generalized anxiety disorder and related traits. Dialog Clin Neurosci. 2017;19(2):159–168. doi: 10.31887/DCNS.2017.19.2/kdomschke.
- Sawalha J, Yousefnezhad M, Selvitella AM, et al. Predicting pediatric anxiety from the temporal pole using neural responses to emotional faces. Sci Rep. 2021;11(1):16723. doi: 10.1038/s41598-021-95987-4.
- Basten U, Hilger K, Fiebach CJ. Where smart brains are different: a quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 2015;51:10–27. doi: 10.1016/j.intell.2015.04.009.
- Pangelinan MM, Zhang G, VanMeter JW, et al. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage. 2011;54(4):3093–3100. doi: 10.1016/j.neuroimage.2010.11.021.
- Hedges EP, Dimitrov M, Zahid U, et al. Reliability of structural MRI measurements: the effects of scan session, head tilt, inter-scan interval, acquisition sequence, FreeSurfer version and processing stream. Neuroimage. 2022;246:118751. doi: 10.1016/j.neuroimage.2021.118751.
- Brown EM, Pierce ME, Clark DC, et al. Test–retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage. 2020;210:116563. doi: 10.1016/j.neuroimage.2020.116563.
- Brown GG, Ostrowitzki S, Stein MB, et al. Temporal profile of brain response to alprazolam in patients with generalized anxiety disorder. Psychiatry Res. 2015;233(3):394–401. doi: 10.1016/j.pscychresns.2015.06.016.
- Ask H, Cheesman R, Jami ES, et al. Genetic contributions to anxiety disorders: where we are and where we are heading. Psychol Med. 2021;51(13):2231–2246. doi: 10.1017/S0033291720005486.
- Waszczuk MA, Zavos HMS, Gregory AM, et al. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry. 2014;71(8):905–916. doi: 10.1001/jamapsychiatry.2014.655.