ABSTRACT
Purpose
To investigate the efficacy and safety of brolucizumab in diabetic macular edema (DME) and diabetic retinopathy (DR).
Methods
In this systematic review and meta-analysis, an electronic search was done to acquire all articles describing brolucizumab use in patients with DME and DR. The review was prospectively registered on PROSPERO (CRD42022382625). Collected articles were filtered through two stages by independent reviewers. Data were extracted from the included articles and then analyzed accordingly.
Results
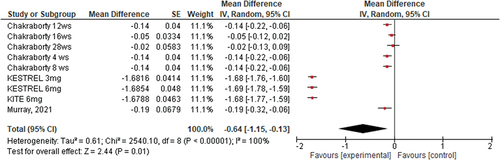
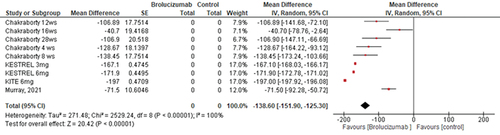
Brolucizumab induced significant improvement in best-corrected visual acuity and was either better or non-inferior to other types of anti-VEGF (MD −0.64 mu, 95% CI [−1.15, −0.13], P = .01); the same observation was noted with regards to central subfield macular thickness (CSMT) (MD −138.6 mu, 95% CI [−151.9, −125.3], P = .00001). Brolucizumab was reported to be relatively safe for use in diabetic patients, with few adverse events observed, with a higher frequency of adverse events in relation to the 3 mg dose compared to the 6 mg dose.
Conclusion
Brolucizumab is a new drug that has potential advantages in efficacy over other anti-VEGF agents in the treatment of DME and DR. It showed significant improvement in BCVA and CSMT with the possibility of a lower dosing schedule compared to other agents. Although observed in low frequency, sight-threatening adverse effects appear to occur more frequently compared to other anti-VEGF agents. The main observed adverse event was retinal vasculitis which was seen more commonly with the 3 mg dose versus the 6 mg dose.
INTRODUCTION
Diabetes mellitus (DM) is becoming more prevalent worldwide, with an estimated 440 million people suffering from the disease by 2030.Citation1 According to previous reports, DM is the primary cause of blindness in the U.S. between the ages of 20 and 74.Citation2 Diabetic retinopathy (DR) affects about 29% of diabetic individuals, while diabetic macular edema (DME) affects about 3%.Citation3 With the increasing prevalence of DM, a corresponding concern for DR complications is also growing. The global incidence of DR is predicted to increase from 126.6 million cases in 2010 to 191 million cases annually by 2030.Citation4,Citation5 DME is a major cause of vision loss among work-age individuals.Citation6 It can develop at any stage or severity of DR and can significantly impact vision, making it a serious complication of DM. The primary objective of treating DME is to enhance patients’ visual function and quality of life.Citation7 The gold standard for treating retinal vascular disorders, such as DME, is intravitreally injected anti-vascular endothelial growth factor (VEGF) agents.Citation8,Citation9 They are recommended as the first-line treatment by various clinical guidelines.Citation10 The US Food and Drug Administration (FDA) has approved multiple anti-VEGF molecules for intraocular use, with differing efficacy in the treatment of DME.Citation11,Citation12 A significant proportion of patients with DME, about 15–20% of cases, however, do not adequately respond to anti-VEGF medication which creates the need for further drug and treatment protocol development.Citation13
As evidenced by persistent macular edema despite 24 months of anti-VEGF medication, up to 56.7% of DME eyes treated with bevacizumab and 40% of those treated with ranibizumab are reported as non-responders.Citation14 Switching to a different medication, such as corticosteroids or an alternative anti-VEGF drug, is a viable option in cases of poor response,Citation15,Citation16 and several studies have shown benefit when switching from one anti-VEGF agent to another.Citation17,Citation18 Furthermore, in the protocol of the Diabetic Retinopathy Clinical Research Network (DRCR.net), aflibercept was superior to both bevacizumab but not ranibizumab in patients with vision worse than 20/50. It is essential to also emphasize the importance of treatment of persistent DME, as the MYRROR study has demonstrated that a delay in treatment can have an impact on visual outcomes possibly due to structural changes in the retina from chronic edema.Citation19
Brolucizumab, a monoclonal antibody that binds to VEGF-A, was approved in 2019 by the FDA and has a unique single-chain antibody fragment and low molecular weight, providing durability and potential advantages over other anti-VEGF agents.Citation20,Citation21 Brolucizumab is administered as an intraocular injection, with a recommended dosage of 6 mg every 4 weeks for the first 3 months, followed by every 8–12 weeks. Brolucizumab was reported non-inferior to aflibercept in phase 3 clinical trials of HAWK and HARRIER, with superior anatomical outcomes obtained with quarterly (q12-week) doses in the treatment of nAMD.Citation22 In 200 sites in 36 countries, two more 3 phase clinical trials, KESTREL and KITE, are now being conducted to determine whether brolucizumab is non-inferior to Aflibercept in terms of functional and morphological improvement for the treatment of DME over a 52-week period.Citation23 The preliminary findings from these trials have revealed encouraging visual acuity and anatomical results following one year.Citation23 Brolucizumab has, however, been linked to instances of retinal vasculitis and retinal vascular occlusion, usually in the presence of other indications of intraocular inflammation (IOI) as suggested by several recent case series and case reports.Citation24 This has created some concern regarding its intraocular use.
Several reviews have been performed to evaluate the use of brolucizumab for the treatment of AMD. This is the first systematic review and meta-analysis to evaluate the up-to-date data on the safety and efficacy of brolucizumab in the treatment of DME and DR.
MATERIALS AND METHODS
Study Protocol and Database Search
This research was carried out in accordance with the Preferred Reporting for Systematic Review and Meta-Analysis (PRISMA) recommendations.Citation25,Citation26 The study adhered to the tenets of the Declaration of Helsinki and the necessity for institutional review board (IRB) approval was not required since it did not involve human subjects. In December 2022, our protocol was registered on PROSPERO [registration number: CRD42022382625]. Meanwhile, on December 25–26, 2022, we searched six electronic databases [PubMed, Scopus, EMBASE, Web of Science, CENTRAL, and Google Scholar] to retrieve all studies that reported the use of brolucizumab in diabetic macular edema and/or diabetic retinopathy using the following keywords: ((Brolucizumab or BOVU) AND (macular edema OR diabetic retinopathy)). Medical Subject Headings (MeSH) terms were also added whenever applicable to retrieve all relevant studies based on their indexed terms in included databases. Of note, only the first 200 records from Google Scholar were retrieved and screened as per the recent recommendations.Citation25 Noteworthy, an updated database search was carried out on March 15th, 2023 to include any newly published studies before the official synthesis of collected data.
Additionally, on February 1st, 2023, after finishing the screening process, we conducted a manual search of references to identify any relevant studies that we could not identify through the original database search. This search was conducted through: (1) searching similar articles of the finally included articles in our review through the “similar articles” option on PubMed, (2) searching the reference list of finally included articles in our review, and (3) searching through Google with the keywords used in the original database search.
Eligibility Criteria
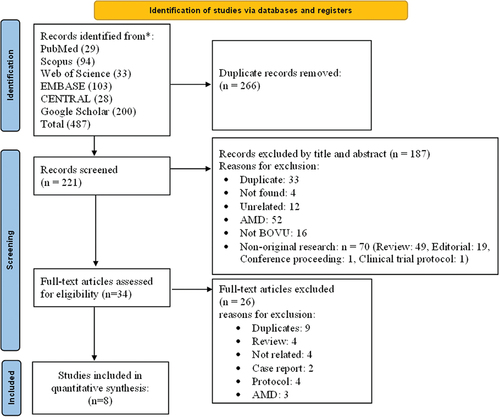
We included original research papers that discussed the use of brolucizumab in DME and/or DR. We included all of the following study designs: randomized controlled clinical trials (RCT), retrospective studies, case series and case reports. Studies were included regardless of the language of publication. Meanwhile, studies were excluded if they were (1) non-original research (i.e., reviews, commentaries, guidelines, editorials, correspondence, letters to editors, etc.), (2) unavailable full-texts, (3) duplicated records or records with overlapping datasets, (4) studies reporting using brolucizumab for indications other than DME or DR, where outcomes for DME or DR were not reported separately (5) studies not involving brolucizumab.
Screening and Study Selection
Retrieved records from the database search were exported into EndNote software for duplicate removal before the beginning of the screening phase. Records were then imported into an Excel (Microsoft, USA) Sheet for screening. The screening was divided into two steps: title and abstract screening and full-text screening. The full texts of eligible articles were then retrieved for screening before being finally included in the review. Both steps were carried out by three reviewers [SI, LAS, QAS]. Any differences between reviewers were solved through group discussions, and the senior authors [HAS, AGE] were consulted if reviewers could not reach an agreement.
DATA EXTRACTION AND ASSESSMENT OF METHODOLOGICAL QUALITY AND RISK OF BIAS
The data extraction was performed by three reviewers [SI, LAS, QAS] through a data extraction sheet that was formatted through Excel (Microsoft, USA). This sheet consisted of six parts. The first part included the baseline characteristics of included studies [title, authors’ names, year of publication, country, and study design] and patients as well [sample size, age, and gender]. The second part included data on brolucizumab (number of injections, laterality, optical coherence tomography (OCT) findings post-injection, baseline and post-treatment best corrected visual acuity (BCVA), baseline and post-treatment Diabetic Retinopathy Severity Scale (DRSS) score, the indication for treatment, and whether the patient was treatment-naive or not). The third part summarized the medical history of reported cases (i.e., systemic diseases, cardiovascular diseases, cerebrovascular diseases, immunological diseases, history of eye trauma, previous eye diseases, and previous ocular surgeries). The fourth part included a thorough assessment of adverse events following the injections including intraocular inflammation, systemic adverse events, the rate of progression to PDR, and the rate of persistent DME. The fifth part included the assessment of fluorescein angiography and OCT angiography findings if available. The sixth part included the quality assessment of included studies. Methodological quality and risk of bias were assessed using the NIH Quality Assessment Tool for the specific study type.Citation27 (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
Data Synthesis
Acquired data was tabulated and reorganized, then qualitative and quantitative analysis were performed. Qualitative analysis was done through table columns comparison using the Statistical Package for Social Sciences (SPSS) version 27 (IBM SPSS Corp, SPSS Statistics ver. 26, USA). Descriptive analysis was used to display categorical variables as percentages and frequencies while presenting numerical variables as a mean and standard deviation to evaluate the data quantitatively. The significance of the data was determined using a categorical Chi-square test. All statistical tests were conducted with a 95% confidence interval and a 5% error margin. A p-value of less than 0.05 was considered statistically significant. Quantitative analysis was performed on categorical basis through meta-analysis executed using Cochrane’s RevMan software. In RCTs, visual acuity (VA) was frequently quantified and reported as an Early Treatment Diabetic Retinopathy Study (ETDRS) letter score. When the logarithm of the minimum angle of resolution (logMAR) or Snellen chart scores were used to measure VA, the score was converted to approximate ETDRS letter scores using the method proposed by Gregori et al.,Citation28 which was used in quantitative analysis.
logMAR = −1 × log (Snellen fraction)
Approximate ETDRS letter scores = 85 + 50 × log (Snellen fraction)
RESULTS
Study Selection
Our search yielded 487 articles. 453 were excluded at the title and abstract level, leaving 34 articles for full-text review. Eight articles with a total of 926 patients satisfied the inclusion criteria and were included in the qualitative and quantitative analyses. shows PRISMA chart for the selection of included articles.
Study Characteristics
Included studies were 4 interventional studies and 4 observational studies. Duration of study ranged from 2 to 52 weeks. Patients were mainly in the older age group and males were of higher frequency than females (81.425%). Included studies were good to high in quality. A brief summary of the characteristics of included studies is available in . Two studies utilized brolucizumab 3 mg, three trials used brolucizumab 6 mg, and one research compared both doses, the remaining studies did not mention a specific dose. All studies looked at how brolucizumab affected BCVA and central subfield macular thickness (CSMT) except for one study that solely looked into the existence of antibodies to brolucizumab in both the serum and vitreous fluid, with no consideration for dosages, CSMT, or BCVA. The effect of brolucizumab was evaluated in one study on the contralateral eye, and the results were significant in both the injected and contralateral eye,Citation32 as shown in . The main study was the KESTREL and KITE study,Citation36 which reported two major clinical trials that used brolucizumab in the treatment of DME []. The statistical meta-analysis was limited to BCVA and CSMT measurements because they’re the only variables reported in all included studies.Citation23
Table 1. Summary of studies included in this review.
Table 2. Overview of BCVA changes after Brolucizumab across included studies.
Table 3. Summary of CST results in included studies.
BCVA
All studies showed significant improvement in the BCVA from the baseline to different endpoint measurements. In addition, brolucizumab was reported to be non-inferior to other anti-VEGF drugs (aflibercept).Citation23
The overall mean difference of the effect of brolucizumab on BCVA from the baseline to different endpoints showed the following results (Mean Difference −0.64 on LogMAR, 95% CI [−1.15, −0.13], P = .01); and the pooled studies were heterogonous (Heterogeneity: Chi2 = 2540.1, (p = .00001)) ().
Central Subfield Macular Thickness (CSMT)
The overall mean difference of the effect of brolucizumab on CSMT from the baseline to different endpoints yielded the following: (Mean Difference −138.6 micrometer, 95% CI [−151.9, −125.3], P = .00001); and the pooled studies were heterogenous (Heterogeneity: Chi2 = 2529.24, (p < .01)) ().
Other Significant Effects
Subretinal Fluid And/Or Intraretinal Fluid
According to Brown et al., at week 52, a lower proportion of subjects in the brolucizumab arms had intraretinal (IRF) and/or subretinal (SRF) compared with aflibercept in both KESTREL and KITE with a treatment difference of − 14.1% (95% CI: −23.3, −4.6), and − 13.2% (95% CI: −23.2, −3.8) between brolucizumab 3 mg and 6 mg, respectively, and aflibercept.Citation23
Diabetic Retinopathy Status
Brown et al. reported on the improvement in the DRSS score following treatment. They found that the proportion of subjects with an improvement greater than 2-steps was higher in the brolucizumab 3 mg arm (28.6%) and 6 mg arm (29.6%) compared with the aflibercept arm (21.7%) in KESTREL. On the other hand, the proportions were comparable in the KITE study (brolucizumab 6 mg, 29.0%; aflibercept, 27.7%).Citation23
Safety
Brolucizumab was linked to 6 incidences of retinal vasculitis in DME patients. However, with the exception of one patient who had severe visual loss (the author indicates that this patient has been diagnosed with past head trauma, which could explain the poor prognosisCitation23), the majority of patients have had a favorable prognosis and complete resolution. Ocular adverse effects were more likely in patients who received brolucizumab 3 mg rather than 6 mg. In these patients, many more eye problems have been described. According to Busch et al.,Citation29 in a study on 192 blood samples and 59 vitreous bodies to investigate the role of immunity in the incidence of ocular problems after brolucizumab in comparison to other Anti-VEGF alternatives, the following results were obtained: (1) Higher antibody concentrations were associated with female gender and diabetic retinopathy; (2) Anti-drug antibodies (ADAs) can develop in both patients with or without prior brolucizumab exposure. (3) Furthermore, ADAs were less common in vitreous samples compared to serum samples (13% vs. 20.4%) expect for DR patients ().
Table 4. Safety of brolucizumab as observed by included studies.
DISCUSSION
By merging the data from 8 studies that included a total of 926 diabetic patients, this systematic review and meta-analysis attempted to provide an up-to-date investigation of the safety and efficacy of brolucizumab in the treatment of DME and DR. Our findings demonstrated that the use of brolucizumab had considerable positive effects on BCVA, CSMT, IRF, SRF, and the status of DR. Furthermore, four included studies mentioned ocular side effects that emerged after using brolucizumab.
The main outcome of this study was that brolucizumab has a beneficial role in reducing macular thickness in DME patients. The mean reduction in CSFT was 138.6 micron, CI [−151.9, −125.3], from the CSFT baseline. The best improvement was associated with using brolucizumab at a dose of 6 mg in KITE study (−197 micron reduction in CSFT) at week 52 of follow up, after receiving a median of 7 intravitreal injections. These results were similar to the findings of the Dugel et al. study. In the Dugel et al. study, significant CSFT reductions from baseline were observed at Week 48 with brolucizumab 6 mg. The reduction was reported as (−172.8 micron) in HAWK and (−193.8 micron) in HARRIER studies (i.e., Dugel et al. study).Citation22 On the contrary, results of the Hänsli et al. study were less encouraging, as they reported a reduction of only −74 microns (baseline 394.2, 12 months follow-up 320.0).Citation36 This can be attributed to the difference in population; in our cohort, both naïve and previously treated eyes were investigated, while the Hänsli et al. paper only included patients who switched to brolucizumab after either complaining of persistent disease activity with intra- and/or subretinal fluid and/or receiving a high treatment demand with other anti-VEGF agents.
In terms of BCVA, the total improvement from baseline through various measurement points is 53.07 in the ETDRS score. The best reduction was associated with a similar dose and follow-up of the CSMT (KITE study, dose of 6 mg, week 52 of follow-up, median of 7 IVT), which is much higher than the results of the previous systematic review conducted by Hänsli C et al.,Citation36 which showed a change of only 69.41 to 75.48 ETDRS. An observation that could be explained by the same reasons for differences in CST outcome.Citation38 However, the results of the main studies on the effect of brolucizumab on nAMD were similar in the least squares [LS] mean: +6.6 [6 mg] and + 6.1 [3 mg] letters with brolucizumab in HAWK; +6.9 brolucizumab 6 mg in HARRIER in comparison to + 7.3 [6 mg] and + 9.2 [3 mg] letters with Brolucizumab in KESTREL; and + 10.6 brolucizumab. These numbers indicate that brolucizumab offers similar improvement indices as compared to other intravitreal injections for similar cases of DME. In a meta-analysis on the effects of ranibizumab in BCVA improvement, the mean effect was + 7.01 (2.56–11.39) which is comparable to our findings.Citation39 Intravitreal aflibercept, however, was reported to elicit better improvement in terms of BCVA, with a mean change of + 13.30 as reported by Xie et al.Citation40 In addition, Zhang et al.Citation39 meta-analysis showed that ranibizumab resulted in less CSMT reduction with a reported mean reduction around −14.67, which is significantly less than the reduction observed with brolucizumab in the present study. Similarly, CSMT mean reduction with aflibercept injection was around −33.76 in Xie et al. meta-analysis.Citation40 Thus, brolucizumab, as seen in the present analysis resulted in a significantly higher mean reduction of CSMT, suggesting higher efficacy in this regard. Nevertheless, these numbers are subject to patients group characteristics and baseline conditions; therefore, future prospective head-to-head randomized clinical trials are recommended to investigate these differences.
Regarding the safety of brolucizumab use, six patients had developed retinal vasculitis. Nevertheless, the majority of them had a favorable prognosis and complete remission. These findings are consistent with the findings of HAWK and HARRIER studies, which found that brolucizumab had an overall well-tolerated safety profile. In this article, the overall assessment demonstrated that the combined IOI (iritis and uveitis) was higher in the brolucizumab 6 mg group, which contradicts our findings. Four patients who had received a 3 mg dose of brolucizumab reported adverse ocular events, while only 2 patients with the 6 mg dose complained of such issue. This could be explained by the fact that one of the included studies in the analysis investigated the 3 mg dose only without comparison with 6 mg dose, which results in a larger sample size for 3 mg ocular side effects.Citation34 In addition, in KESTLER study,Citation23 the follow-up period was longer in patients received 3 mg brolucizumab compared to 6 mg brolucizumab (mean of 12.5 and 9.4 months respectively) which could lead to detection of more cases with ocular side effects.
Four of our included trials, on the other hand, were associated with no ocular vascular events, which is consistent with the findings provided by Hänsli et al..Citation36 IOI is a potential adverse effect of all anti-VEGF medications that have been observed in numerous prior research,Citation38,Citation41,Citation42 and it had been highlighted in the context of brolucizumab recently, particularly in nAMD patients.Citation43 The use of brolucizumab is a risk-benefit balance in nAMD patients since it has shown treatment benefits, and experts have attempted to devise criteria for patient selection for the use of brolucizumab for nAMD, highlighting risk factors such as female gender and Japanese ethnicity in which higher incidence of IOI was reported.Citation41,Citation44,Citation45 Nevertheless, in patients with DME, brolucizumab showed safer results, as demonstrated by the KITE and KRESTEL trials.Citation46 This could partly be explained by demographic differences between patients with DME and nAMD, especially since anti-brolucizumab antibodies were observed in patients who had never received the drug, suggesting a preexisting antibody that was linked to specific human-leukocyte antigen (HLA) subtypes.Citation29,Citation47 Furthermore, Hirano, T. et al.Citation48 studied 23 eyes with DME who received brolucizumab in a Japanese population and reported that no patient had IOI. This variation in the safety profile of brolucizumab is yet under investigation. Recently, it has been suggested in the literature that DR and its progression are directly linked to an immunological dysregulation.Citation49 This goes in accordance with the findings of Busch et al., where DR patients had higher concentration of brolucizumab ADAs in DR patients, especially in vitreous samples compared to serum.Citation29 Despite this, IOI was reported more commonly with nAMD compared to DME following brolucizumab treatment. It is also possible that more patients with nAMD received the medication compared to patients with DME, leading to a larger number of reports of adverse events from patients with nAMD. This should be further evaluated in future studies.
In our study, the mean (range) time to the event from the last brolucizumab was 115.67 (30–203) days, which is longer than the previous systematic review, which showed that the mean (range) time to the event from the last brolucizumab is 19.4 (0–63) days,Citation50 and also longer than the results of the HAWK and HARRIER study, which reported that the majority of IOI occurred within the first 12 weeks of treatment.Citation22 The main limitation of this review is the paucity of studies using brolucizumab in the treatment of DME or DR, with most results being driven by the two large RCTs. Since brolucizumab is a new medication, our understanding of its efficacy and safety profiles is yet to be deepened and refined. Our review sheds some light on the difference between both doses of brolucizumab with regards to the risk of ocular vascular adverse events. Furthermore, for unexplainable reasons, the risk of IOI associated with brolucizumab may be less when treating DME compared to wet AMD. These findings should be confirmed in future studies.
CONCLUSION
Brolucizumab is a relatively new anti-VEGF agent that has been used in the treatment of DME and DR. It showed great potential in improving BCVA and CSMT of patients with DME with the possibility of less frequent injections compared to other anti-VEGF agents. Brolucizumab safety was also assessed, and adverse effects were relatively few, most concerning of which are retinal vasculitis and retinal vascular occlusion, with possible less adverse events with the 6 mg dose vs the 3 mg and in DME patients vs nAMD patients that requires further studies.
Authors’ Contributions
Conceptualization: HAS, AA; Data curation: MJT, MTA, AA; Formal analysis: SI, LAS; Funding acquisition: HAS; Investigation: QAS, NA; Methodology: HAS, AGE, AA; Project administration: BA, AGE; Resources: MJT, MTA; Software; HAS, SI; Supervision: AGE, HAS, AA; Validation: MJT, MTA, AA; Visualization: LAS, NA; Roles/Writing - original draft: HAS, MJT, AGE, AA; Writing - review & editing: QAS, NA, HAS. All authors read and approved the final manuscript.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi:10.1016/j.diabres.2009.10.007.
- Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720.
- Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of Diabetic Retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. doi:10.1001/jama.2010.1111.
- Bandello F. Diabetic Macular Edema. Dev Ophthalmol. 2017;58:102–138.
- Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi:10.4103/0301-4738.100542.
- Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720.
- Jain A, Varshney N, Smith C. The evolving treatment options for diabetic macular edema. Int J Inflam. 2013;2013:1–10. doi:10.1155/2013/689276.
- Lanzetta P, Loewenstein A. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–1273. doi:10.1007/s00417-017-3647-4.
- Yorston D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health. 2014;27:44–46.
- Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi:10.1159/000458539.
- Li E, Donati S, Lindsley KB, et al. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;5(5):Cd012208. doi:10.1002/14651858.CD012208.pub2.
- Mansour SE, Browning DJ, Wong K, et al. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;14:653–678. doi:10.2147/OPTH.S236637
- Bressler NM, Beaulieu WT, Maguire MG, et al. Early response to anti–vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. American Journal Of Ophthalmology. 2018;195:93–100. doi:10.1016/j.ajo.2018.07.030
- Sorour OA, Levine ES, Baumal CR, et al. Persistent diabetic macular edema: definition, incidence, biomarkers, and treatment methods. Surv Ophthalmol. 2023;68(2):147–174. doi:10.1016/j.survophthal.2022.11.008.
- Busch C, Zur D, Fraser-Bell S, et al. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55(8):789–796. doi:10.1007/s00592-018-1151-x.
- Sivaprasad S, Crosby-Nwaobi R, Heng LZ, et al. Injection frequency and response to bevacizumab monotherapy for diabetic macular oedema (BOLT report 5). Br J Ophthalmol. 2013;97(9):1177–1180. doi:10.1136/bjophthalmol-2013-303168.
- Laiginhas R, Silva MI, Rosas V, et al. Aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):83–89. doi:10.1007/s00417-017-3836-1.
- Bahrami B, Hong T, Schlub TE, et al. AFLIBERCEPT for PERSISTENT DIABETIC MACULAR EDEMA: forty-eight-week outcomes. Retina. 2019;39(1):61–68. doi:10.1097/IAE.0000000000002253.
- Bruè C, Pazzaglia A, Mariotti C, et al. Aflibercept as primary treatment for myopic choroidal neovascularisation: a retrospective study. Eye (Lond). 2016;30(1):139–145. doi:10.1038/eye.2015.199.
- Rahman EZ, Singer MA. Brolucizumab as treatment of wet age-related maculopathy. Drugs Today (Barc). 2020;56(11):699–704. doi:10.1358/dot.2020.56.11.3199812.
- Tadayoni R, Sararols L, Weissgerber G, et al. Brolucizumab: a newly developed anti-VEGF molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica. 2021;244(2):93–101. doi:10.1159/000513048.
- Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of Brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028.
- Brown DM, Emanuelli A, Bandello F, et al. KESTREL and KITE: 52-week results from two phase III pivotal trials of Brolucizumab for Diabetic Macular Edema. Am J Ophthalmol. 2022;238:157–172. doi:10.1016/j.ajo.2022.01.004
- Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following Intravitreal Brolucizumab. J Vitreoretin Dis. 2020;4(4):269–279. doi:10.1177/2474126420930863.
- Muka T, Glisic M, Milic J, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60. doi:10.1007/s10654-019-00576-5.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Study Quality Assessment Tools | NHLBI, NIH | https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi:10.1097/IAE.0b013e3181d87e04.
- Busch M, Pfeil JM, Dähmcke M, et al. Anti-drug antibodies to brolucizumab and ranibizumab in serum and vitreous of patients with ocular disease. Acta Ophthalmol. 2022;100(8):903–910. doi:10.1111/aos.15124.
- Chakraborty D, Mondal S, Parachuri N, Kumar N, Sharma A. Brolucizumab—early experience with early extended interval regime in chronic centre involved diabetic macular oedema. Eye (Lond). 2022;36(2):358–360. doi:10.1038/s41433-021-01816-3.
- Murray JE, Gold AS, Latiff A, et al. Brolucizumab: evaluation of compassionate use of a complex anti-VEGF therapy. Clin Ophthalmol. 2021;15:4731–4738. doi:10.2147/OPTH.S339393
- Chakraborty S, Sheth JU, Campa C. Contralateral effect following intravitreal brolucizumab injection in diabetic macular Edema. Case Rep Ophthalmol Med. 2022;2022:1–4. doi:10.1155/2022/3755249.
- Mahapatra SK, Das A. Initial experience in off-label use of brolucizumab in diabetic macular ededama and retinal vein occlusion. Current Indian Eye Res J Ophthalmic Res Group. 2021;54(49):51.
- Hirano T, Toriyama Y, Takahashi Y, et al. Retinal arterial occlusive vasculitis after multiple intravitreal brolucizumab injections for diabetic macular edema. Am J Ophthalmol Case Rep. 2023;29:101788. doi:10.1016/j.ajoc.2022.101788
- Chakraborty D, Sheth JU, Boral S, et al. Off-label intravitreal brolucizumab for recalcitrant diabetic macular edema: a real-world case series. Am J Ophthalmol Case Rep. 2021;24:101197. doi:10.1016/j.ajoc.2021.101197
- Hänsli C, Schild C, Pfister I, Garweg JG. Brolucizumab in pretreated neovascular age-related macular degeneration: case series, systematic review, and meta-Analysis. Life. 2023;13(3):814. doi:10.3390/life13030814.
- Table 25 , all ocular serious adverse events in the study eye (Safety Analysis Set). 2015.
- Hikichi T. Sub-Tenon’s capsule triamcinolone acetonide injection to prevent brolucizumab-associated intraocular inflammation. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2529–2535. doi:10.1007/s00417-022-05611-y.
- Zhang L, Wang W, Gao Y, et al. The efficacy and safety of Current treatments in diabetic macular Edema: a systematic review and network meta-analysis. PLoS One. 2016;11(7):e0159553. doi:10.1371/journal.pone.0159553.
- Xie X, Lian C, Zhang Z, et al. Aflibercept for long-term treatment of diabetic macular edema and proliferative diabetic retinopathy: a meta-analysis. Front Endocrinol (Lausanne). 2023;14:1144422. doi:10.3389/fendo.2023.1144422
- Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi:10.1016/j.oret.2020.09.020.
- Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after Intravitreal Injection of Brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi:10.1016/j.ophtha.2020.04.017.
- Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion–related events with Brolucizumab. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011.
- Holz FG, Iida T, Maruko I, Sadda SR. A CONSENSUS on RISK MITIGATION for BROLUCIZUMAB in NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: patient selection, evaluation, and treatment. Retina. 2022;42(9):1629–1637. doi:10.1097/IAE.0000000000003556.
- Ogura Y, Jaffe GJ, Cheung CMG, et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2022;106(7):994–999. doi:10.1136/bjophthalmol-2021-319090.
- Garweg JG. A randomized, double-masked, multicenter, phase III study assessing the efficacy and Safety of Brolucizumab versus aflibercept in patients with visual impairment due to Diabetic Macular Edema (KITE). Klin Monbl Augenheilkd. 2020;237(4):450–453. doi:10.1055/a-1101-9126.
- Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: preclinical assessment and mitigation. Clin Immunol. 2013;149(3):534–555. doi:10.1016/j.clim.2013.09.006.
- Hirano T, Kumazaki A, Tomihara R, Ito S, Hoshiyama K, Murata T. Evaluating initial responses to brolucizumab in patients undergoing conventional anti-VEGF therapy for diabetic macular edema: a retrospective, single-center, observational study. Sci Rep. 2023;13(1):10901. doi:10.1038/s41598-023-37726-5.
- Pan WW, Lin F, Fort PE. The innate immune system in diabetic retinopathy. Prog Retinal Eye Res. 2021;84:100940. doi:10.1016/j.preteyeres.2021.100940.
- Wykoff CC, Matsumoto H, Barakat MR, et al. Retinal vasculitis or vascular occlusion after brolucizumab for neovascular age-related macular degeneration: a systematic review of real-world evidence. Retina. 2023;43(7):1051–1063. doi:10.1097/IAE.0000000000003769.