ABSTRACT
Background
The choroidal vasculature supplies the outer retina and is altered in many retinal diseases, including myopic traction maculopathy (MTM). Choroid health is typically assessed by measuring the choroidal thickness; however, this method has substantial limitations. The choroidal vascularity index (CVI) was recently introduced to provide quantitative information on the vascular flow in the choroid. This index has been evaluated in a wide range of diseases but has not been extensively used to characterize MTM.
Aim
This study aimed to investigate the CVI across different stages of MTM and the influence of macular surgery on choroidal perfusion markers in different surgically resolved MTM stages.
Methods
Eighteen healthy myopic eyes in the control group and forty-six MTM eyes in the surgical group were evaluated using enhanced optical coherence tomography (OCT) imaging. Binarized OCT images were processed to obtain the luminal choroidal area (LCA) and stromal choroidal area (SCA), which were used to calculate CVI in the form of a percentage ratio. CVI data were collected at baseline, one and four months postoperatively, and at the final clinical visit. MTM eyes were divided into four stages based on disease severity. The choriocapillaris flow area (CFA) and central subfield thickness (CSFT) were measured along side the CVI.
Results
No significant differences were observed between the two groups at baseline, except for visual acuity (p < 0.0001). Surgery significantly improved vision at all postoperative time points (p < 0.0001). At baseline, there were no significant differences in CVI, CFA, or CSFT scores between the control and surgical groups. However, all three measurements were lower at the final visit in the surgical group (p ≤0.0001). No significant differences were found in any of the parameters among the four stages of MTM (p > 0.05). Ultimately, correlation and multivariate linear regression analyses did not reveal any significant association between CVI and visual acuity.
Conclusions
This study did not find significant preoperative differences in CVI between healthy myopic eyes and eyes with MTM. However, the postoperative CVI and CFA values were significantly lower than those of the control eyes. Thus, CVI may not be a good biomarker for surgical outcomes, as the correlation between CVI and visual acuity was not statistically significant.The CVI and CFA decreased after surgery, providing evidence of choroidal changes after surgical management.
BACKGROUND
The choroid is a vascular tissue that supplies oxygen and nutrients to the retinal pigment epithelium (RPE) and the outer retina.Citation1 This tissue plays a crucial role in maintaining retinal homeostasis, and abnormalities in the structure of the choroid may indicate the presence of an underlying disease. Choroidal thickness measurements are widely used in clinical research to meaningfully evaluate the state of the choroid in healthy and diseased retinas.Citation2 However, choroidal thickness alone has important limitations, such as the inability to characterize the vascular flow between the stromal and luminal vascular areas.
In recent years, the choroidal vascularity index (CVI) has emerged as a popular measure to address this need because of its ease of use and the noninvasiveness of its calculation.Citation3–5 CVI can be calculated from optical coherence tomography (OCT) images via a series of digital binarization and quantification steps.Citation6,Citation7 As CVI captures both vascular and stromal changes within the choroid, it provides a more informative characterization of the state of the choroidal structure than choroidal thickness.
Since the initial introduction of CVI, a series of studies have linked changes in this index to the pathogenesis and progression of various retinal diseases.Citation5,Citation8,Citation9 The CVI has been shown to capture certain microcirculatory changes in the retinaCitation10–12 and has been correlated with visual function after vitrectomy.Citation13
As retinal diseases typically involve multiple processes that affect the choroid (e.g., inflammation, edema, and leakage), CVI has been suggested as a potentially useful biomarker for assessing the integrity of the vascular network within the choroid.Citation8,Citation14 Furthermore, earlier studies found that CVI is not sensitive to various confounding factors, such as axial length, blood pressure, or intraocular pressure.Citation15
Myopic traction maculopathy (MTM) is a vision-threatening condition associated with pathologic myopia (PM), the presence of posterior staphyloma (PS), evidence of inner and/or outer retinal layer-like thickening with or without epiretinal membrane (ERM) proliferation, or tractional elevation of Henle’s layer with or without evidence of a champagne flute-shaped schisis appearance, stretched retinal vessels, and an abnormally rigid inner limiting membrane (ILM), which may evolve into forms with more severe retinal complications.Citation16 Frequently, eyes with myopic foveoschisis (FS) and foveoretinal detachment (FRD) progress, leading to the formation of macular holes (MHs).Citation17,Citation18 Furthermore, highly myopic eyes have been shown to develop macular FS in nearly 34% of patients.Citation19–21 Due to the progressive nature of MTM, a system of four stages has been adopted to categorize the diseaseCitation21,Citation22: myopic FS at stage 1, FRD at stage 2, myopic MHs at stage 3, and MH retinal detachment (RD) at stage 4. In this context, monitoring disease and treatment outcomes through noninvasive methods such as CVI could substantially improve the management of MTM.Citation23
In a previous case series involving four eyes with MTM, we found that more patients with an advanced disease stage tended to have lower CVIs.Citation24 In this study, we obtained the following results: a more comprehensive series of healthy myopic control eyes and MTM eyes that underwent macular surgery and evaluated their CVIs at four different time points. This study aimed to investigate CVI values across different stages of MTM and influence of macular surgery on choroidal perfusion markers in successfully resolved surgical MTM stages.
We further assessed the differences between the preoperative and postoperative CVI, choriocapillaris flow area (CFA), central subfield thickness (CSFT) and the correlation between CVI and other variables using multivariate linear regression analysis.
METHODS
Study Design
We conducted a nonrandomized retrospective analysis of the medical charts of successfully operated patients with different stages of MTM. All patients were treated between August 2016 and June 2022 operated on by the same surgeon (MAQR). This retrospective analysis was conducted in the Retina Department of Oftalmologia Integral ABC (Mexico City). The institutional review board approved the study design, and written informed consent was obtained from all patients. This study adhered to the guidelines outlined in the Declaration of Helsinki. No reference number was provided by the institution, owing to the retrospective nature of the study.
The inclusion criteria were as follows: patients over 18 years of age, with an equivalent spherical equivalent refractive error of > −6,0 diopters or axial length >26.5 mm, presence of any detected structural MTM stage due to PM, who had undergone vitrectomy with successful and uncomplicated macular surgery using different ILM peeling techniques for symptomatic MTM, at least six months of follow-up; and perfusional evaluation during follow-up, serial CVI, choriocapillaris flow area (CFA), and automated CSFT measurements of the macula according to the study protocol.
The exclusion criteria included patients with evidence of diffuse macular chorioretinal atrophy, patchy foveal-affected chorioretinal atrophy, or evidence of involuted or active myopic choroidal neovascularization based on the atrophy/traction/neovascularization (ATN) classification.Citation25 Additionally, eyes treated with macular laser photocoagulation or intravitreal injections during the study period, previous complicated vitreomacular surgeries, postoperative complications such as glaucoma and endophthalmitis, the presence of intraocular silicone oil, or those who did not fulfill the minimum postoperative functional, structural, and perfusional evaluation criteria and follow-up study protocols were also excluded.
Eighteen healthy myopic eyes were included in the control group. Eyes were matched for sex and age. The study groups and their corresponding inclusion criteria are listed in .
Table 1. Summary of study groups and inclusion criteria.
Surgical Procedure and Study Protocol Examinations
The surgical procedures were performed by a single highly experienced retina surgeon. (MAQR). The surgical techniques used in this study have been previously described in detail by the authors.Citation26 According to the study protocol, preoperative evaluation of the MTM stage was performed using a high-resolution spectral domain (SD) optical coherence a tomography (OCT) Spectralis HRA OCT system (Heidelberg Engineering, Heidelberg, Germany), with 25-line horizontal volume scans covering the area centered on the fovea. The CSFT measurements were obtained using standardized algorithms contained in the software of the instrument and were automatically generated. Perfusional choroidal evaluations were completed following a previously published protocol by the study authors using an OCT angiography (OCT-A) device (RTVue XR Avanti, OptoVue, Inc., Fremont, CA, USA).Citation26,Citation27 CFA was performed by segmenting the choriocapillaris subfoveal plexus (CSP) slabs using RTVue XR OCT Avanti with AngioVue Software (OptoVue Inc., Fremont, CA, USA) and automatically calculated from a 3.142 mm2 evaluation area.
Brief Description of the CVI Quantification Method by Image Binarization
Briefly, CVI values were derived from the images obtained using SD-OCT of the macula. High-resolution 9-mm horizontal OCT-B images were selected and uploaded to the ImageJ analysis software (version 1.53, http://imagej. nih. gov/ij/). The images were first converted into an 8-bit format and adjusted using the Niblack automatic local threshold. Next, an area of the subfoveal choroid was manually selected using the adaptable geometric polygon tool to manually select the total choroidal area (TCA) from 750 µm nasal to 750 µm temporal in the direction of the horizontal plane from the foveal center and from the RPE-Bruch membrane to the scleral border in the direction of the vertical plane. Subsequently, the stromal vascular tissue area was determined by the number of white pixels, and the luminal area (LA) at the enhanced choroid was determined by applying the threshold tool by quantifying the number of dark pixels. Finally, the dark-to-light pixel ratio was expressed as a percentage and defined as CVI, as previously described by Agrawal et alCitation4,Citation8 The protocol study method for binarization is illustrated in .
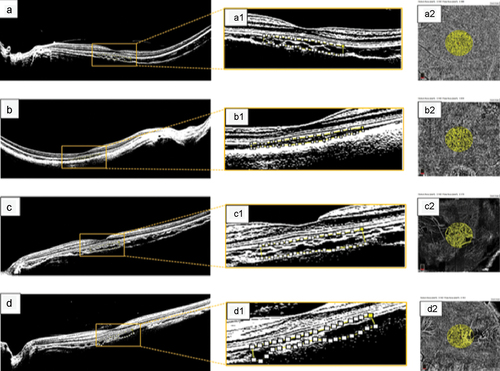
Figure 1. Protocol depicting the method used for quantifying the CVI in healthy myopic eyes. (a) binarized image designed to depict the intraretinal structure and choroidal layers in greater detail in a healthy, moderately highly myopic eye with an axial length of 27.8 mm. (a1) magnified image within the yellow square showing binarized processing of the subfoveal choroidal stroma and luminal vascular visualization of the subfoveal choroidal vessels to obtain a choroidal vascularity index (CVI) of 62.8% in a healthy, moderately myopic eye. The selected subfoveal area of choroidal flow is clearly delineated by the white dotted line. (a2) quantified choriocapillaris flow area (CFA) of 2.308 mm2 in the protocol-selected area of 3.142 mm2 in this healthy myopic eye. (b) binarized processing of choroidal flow in a healthy highly myopic eye with an axial length of 30.8 mm and enhanced choroidal vessel visualization, yielding a CVI of 59.4%. (b1) the magnified image within the white‒yellow dotted line clearly delineates a CVI of 1.972 mm2. (c) binarized image corresponding to a healthy, highly myopic eye in the control group; the CVI was 63.4% inside the selected choroidal flow area. (c1) magnified image depicting the selected area for the CVI measurements. The white and yellow dotted lines depict the binarized choroidal flow area selected to calculate the CVI. (c2) the CFA was 2.173 mm2 for the selected choriocapillaris. (d) binarized image in a healthy highly myopic eye with an axial length of 29.6 mm and a CVI of 58.2%. (d1) magnified image of the selected central subfoveal area clearly depicting the CVI area defined by the white dotted line. (d2) the CFA area was 1.737 mm2.
Outcome Measures
The primary outcome of this study was the pre- and postoperative quantitative evaluation and comparison of macular CVI, CFA, and CSFT values and their correlation with visual changes. The secondary outcomes included investigating the potential effects of vitrectomy and macular surgery on choroidal perfusion markers by comparing preoperative and postoperative values and correlating them with those obtained in the control group.
Statistical Analysis
All statistical tests were performed using GraphPad Prism software (version 9.2.0), with the significance threshold for all tests set at p < .05. Where appropriate, nonparametric tests were used because tests for the normality of distribution showed that the data were not normally distributed. Fisher’s exact test was used to test for differences in sex, eye laterality, and lens status between groups. The Mann‒Whitney U test was used to test for differences in age, axial length, and baseline and postoperative best-corrected visual acuity (BCVA) between the groups. The paired Wilcoxon signed-rank test was used to assess the changes in BCVA after surgery. The Kruskal–Wallis test was used to detect significant differences in the CVI, CFA, and CSFT. Pearson’s correlation analysis and multivariate linear regression were performed using the R programming environment (version 4.1.1).
RESULTS
General Characteristics of the Study Groups
The present study included 64 eyes, with 18 eyes in the healthy myopic control group and 46 eyes in the surgical group who underwent successful MTM surgery. No statistically significant differences were found between the two groups in terms of sex, eye laterality, lens status, age, or axial length. In the surgical group, the mean preoperative MTM was 11.35 months. Forty-one (89.0%) eyes showed an improvement in vision with CVI values lower than those in the control group. Twenty-eight (60.8%) eyes showed BCVA worse than 20/60 (0.48 logMAR units), and all of them showed CVI values below the mean of those in the control group; the reduction in the CVI was significant but did not correlate with the final BCVA (for further detail in the data analysis, consult the supplementary file). The eyes were further divided into four stages according to the severity of MTM (), with 11, 18, 9, and 8 eyes in stages 1, 2, 3, and 4, respectively. Demographic data are summarized in .
Table 2. Patient demographic data and clinical characteristics.
Surgical and Visual Outcomes
The baseline BCVA was significantly worse in the surgical group than in the healthy myopic group (p < .0001) (). After surgery, the BCVA improved from 1.179 to 0.753 logMAR at one month (p < .0001), 0.63 logMAR at four months (p < .0001), and 0.592 logMAR at the final visit (p < .0001). However, the postoperative BCVA in the surgical group was lower than that in the healthy myopic group (p < .0001).
Table 3. BCVA and surgery characteristics.
After surgery, MTM resolved in an average of 5.50 weeks, and the patients were followed up for a mean period of 25.41 months (). Half of the eyes were treated using fovea-sparing (FS)-ILM peeling, followed by an inverted flap in 17 eyes and classical ILM peeling in six eyes. Most eyes (63%) underwent gas tamponade, while the remaining eyes underwent silicone oil tamponade. More than half (52.2%) of the eyes did not require additional surgery; the majority of the second surgeries were performed to extract the silicone oil tamponade (37%). Ten (22%) surgical eyes developed postoperative complications, with MHs being the most common (10.9%), followed by residual extrafoveal FS (6.5%), rhegmatogenous RD (RRD) (2.2%), and diffuse chorioretinal atrophy (DCRA) (2.2%).
Retinal and Choroidal Characterization
SD-OCT and OCT-A were used to measure the CVI, CFA, and CSFT in both groups at baseline, one month after surgery, four months after surgery, and at the final visit in the surgical group. The analysis showed that in the first postoperative month, the choroidal perfusion markers did not differ from the preoperative values with an increase in CSFT. However, at four months and in the last quantification corresponding to the last visit, a significant tendency toward a reduction in the three markers was observed (). Compared with the healthy myopic group, the surgical group had a significantly lower CVI (p = .0001), smaller CFA (p < .0001), and smaller CSFT (p < .0001) at the final visit; however, there were no significant differences at baseline (, c, and e).
Figure 2. OCT measurements across study groups. OCT imaging was used to obtain the choroidal vascularity index (CVI, a-b), choriocapillaris flow area (CFA, c-d), and central subfield thickness (CSFT, e-f). (a, c, e) measurements across the healthy myopia group and surgical group at baseline and final visits. For all three measurements, no significant differences were detected at baseline between the study groups, but values were lower in the surgical group at the final visit. (b, d, f) measurements across time series in the surgical group. Significant changes were detected across time in all three measurements. p values are indicated by * (ns = not significant, *** = p ≤ .001, **** = p ≤ .0001). CFA, choriocapillaris flow area; CSFT, central subfoveal thickness; CVI, choroidal vascularity index.
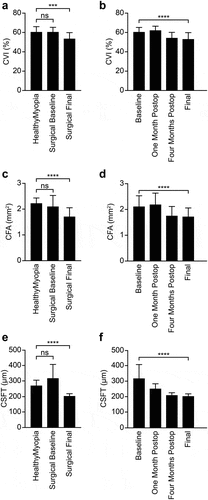
Table 4. Choroidal perfusion markers and OCT measurements for both study groups.
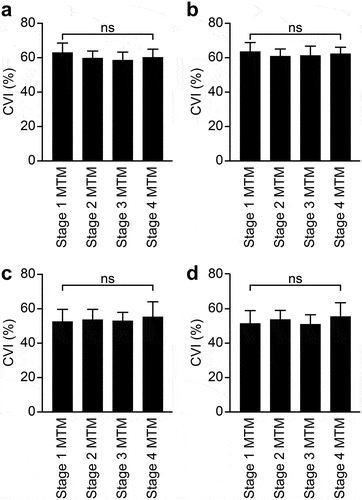
In the surgical group, SD-OCT and OCT-A measurements showed significant differences across the time points (p < .0001) (, d, and f). Specifically, all three values decreased postoperatively. When the surgical group was separated according to the MTM stage (), no significant differences in CVI were identified at any of the time points among the stages (p = .164, 0.375, 0.820, and 0.432 at baseline, one month after surgery, four months after surgery, and at the final visit, respectively).
Figure 3. CVI measurements across MTM stages. The surgical group was separated based on the MTM stage. CVI at baseline (a), one month postsurgery (b), four months postsurgery (c), and the final visit (d) are plotted. p values are indicated by * (ns = not significant). CVI, choroidal vascularity index; MTM, myopic traction maculopathy.
Correlation and Linear Regression Analyses
Analysis of the correlation between the three OCT measurements and BCVA did not reveal any significant correlation with the final surgical outcome. In the surgical group at the final visit, the Pearson’s correlation coefficient between CVI and BCVA was 0.096 (p = .527), that between CFA and BCVA was 0.016 (p = .915), and that between CSFT and BCVA was 0.183 (p = .224).
Multivariate linear regression analysis was performed to identify any relationship between the selected patient factors and the final postoperative BCVA in surgically treated eyes. The model contained six variables: age, preoperative MTM duration, postoperative time to MTM resolution, final CVI, final CFA, and final CSFT. None of the patient factors showed a significant relationship with final postoperative BCVA (p > .05) (). Preoperative MTM duration tended to have the best relationship with BCVA (p = .051), and the corresponding coefficient was positive (i.e., a longer preoperative MTM duration was associated with a higher logMAR value and therefore a worse BCVA).
Table 5. Multivariate linear model relating final postoperative BCVA with patient factors.
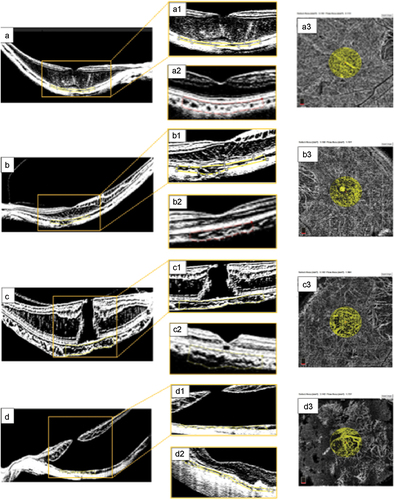
Representative images from the surgical participants who underwent enhanced OCT imaging evaluation and CVI calculations are shown in .
Figure 4. Surgical cases. (a) preoperative OCT findings consistent with myopic foveoschisis (FS) due to macular thickening and schisis-like thickening of the inner and outer retina layers. (a1) magnified image from the yellow inset depicting a preoperative choroidal vascularity index (CVI) of 49.8% calculated within the area clearly delineated with the yellow dotted line. (a2) long-term postoperative binarized image, showing a CVI of 47.3% within the area delineated with the red dotted line. (a3) the choriocapillaris flow area (CFA) was 2.308 mm2 at the selected subfoveal choriocapillaris area of 3.142 mm2. (b) preoperative image of a symptomatic female patient with FS and an axial length of 29.7 mm. (b1) corresponding binarized image of this complex FS eye with outer and inner retinal layer-like thickening, tractional elongation of Henle’s layer, and a thin superficial foveal layer with a preoperative CVI of 40.4%. (b2) long-term postoperative horizontal B-scan image following binarization showing an almost normal foveal profile, diffuse thinning of the retinal layers, and no evidence of outer or inner retinal layer thickening. The postoperative CVI was 49.6% in the subfoveal choroidal flow area, as delineated by the red dotted line. (b3) the image shows a CFA of 2.138 mm2 at the selected subfoveal choriocapillaris area of 3.142 mm2. (c) highly myopic eye with an axial length of 31.2 mm and a moderate posterior staphyloma. Evidence of a full-thickness myopic MH with tractional elongation of Henle’s layer and thickening of the macula without evidence of macular detachment. (c1) magnified preoperative binarized image with a CVI of 56.6%. (c2) postoperative structural evaluation showed a flat macula with a recovered foveal profile, an external limiting membrane (ELM) line lucency defect, and a well-preserved retinal pigment epithelium (RPE) layer. The calculated postoperative CVI was 54.4%. (c3) CFA of 1.808 mm2 at the selected subfoveal choriocapillaris area of 3.142 mm2. (d) preoperative image of an eye with extensive macular hole retinal detachment (MHRD). (d1) the preoperative CVI was 44.7%. (d2) postoperative appearance shows extrafoveal chorioretinal atrophic areas, an irregular foveal profile, a thin foveal roof, a closed macular hole, macular reattachment without evidence of residual subretinal fluid (SRF), attenuated internal and external retinal layers, and a subfoveal ellipsoid zone (EZ). The quantified choroidal perfusion indices were lower than those obtained in the control myopic group, with a CVI of 42.7%. (d3) the CFA value was 1.704 mm2 at the selected subfoveal choriocapillaris area of 3.142 mm2.
DISCUSSION
MTM is a progressive and debilitating condition that can lead to severe loss of visual function without proper treatment.Citation16,Citation28 CVI has recently been introduced as a promising biomarker for choroidal health and has potential applications in disease diagnosis and management.Citation8 Since then, numerous studies have evaluated CVI in various retinal diseases; however, its detailed application in MTM is lacking. In this study, we report CVI-quantified findings across both treatment times and MTM stages to evaluate their potential as biomarkers for the disease and the possible deleterious effects of surgery. We believe that the findings of this study can provide insights into the suitability of preoperative and postoperative CVI and CFA as choroidal perfusion markers and how they behave after noncomplicated macular surgery in different MTM surgical stages. The relationships among CVI, CFA, CSFT, visual function, and other postoperative outcomes of MTM were also explored. Collectively, these results provide novel insights into the choroidal state of eyes with MTM before and after surgery and may help guide the potential use of CVI as a biomarker for the clinical management of the disease.
The study consisted of two cohorts: healthy myopic eyes and MTM eyes, the latter of which was further divided into four stages based on disease severity. Except for worse visual acuity in the eyes with MTM, there were no significant differences between the two groups in terms of sex composition, eye laterality, lens status, age, or axial length. In eyes with MTM, the surgical procedure quickly improved visual function one month postsurgery, with the average vision improving progressively through the last clinical visit. However, the visual acuity at the last visit was worse than that in the healthy myopic control group, indicating that some visual loss was irreversible, which is consistent with previously reported surgical outcomes.Citation29
The CVI, CFA, and CSFT were used to assess choroidal and retinal health across the study groups. Intriguingly, none of the three parameters was significantly different between the myopic control and preoperative values in the surgical group. Furthermore, all three parameters showed a notable trend of reduction in values, which were significantly reduced at the final visit, with statistically significant differences from those in the healthy control myopic group. Comparisons across the time series confirmed that these three parameters decreased after surgery. This finding is in contrast to an earlier study that found that CVI increased at both one and three months after macular buckling surgery, accompanied by thickening of the choroid in the initial postoperative period,Citation30 without mention of the axial length of the eyes, and having in the scientific thinking that the eyes in our study belong to a highly myopic eye spectrum given the presence of PS and complex vitreoretinal interfaces and the lack of any kind of either equatorial or macular buckling elements. Some of these changes may be attributed to the macular surgical procedures performed, which can have different effects on the long-term postoperative perfusion markers of the choroid due to transurgical vitrectomy-related perfusion changes at the level of the microcirculation in these highly myopic eyes. Additionally, a reduction in CSFT after surgery has been previously reported in MTM by Yi et al,Citation31 whereas changes in CVI and CFA associated with surgery in MTM have not yet been described. Therefore, we hypothesized that both thickness and poor final postoperative vision are the result of damage due to retinal-choroid perfusion, represented by lower CVI and CFA values, as a consequence of the degenerative and tractional processes inherent in elevated myopia or the changes and alterations in retinochoroidal perfusion due to macular surgery. However, prospective, randomized, and multicenter studies are required to define these concepts in the absence of meta-analyses and systematic studies.
In a previous preliminary study, we reported a progressively smaller CVI at more advanced stages of MTM.Citation24 However, when the same assessment was performed for the cohorts in this study, no significant differences were found among the four stages of MTM at any time point evaluated, although CVI tended to decrease over time. Given the significantly larger sample size in the present study, the previously observed variations in CVI may have been due to sampling noise rather than biological variations. Collectively, these results suggest that the CVI was not significantly different between the MTM stages but was significantly reduced after surgery. A possible implication is that the structural worsening in these highly myopic eyes depends more on the long-term stage and chronic tractional component of the MTM in combination with the lower values in the choroidal perfusional stage of the choriocapillaris and choroid according to our postoperative quantitative evaluation of these biomarkers or is due to the impact of the surgery on the perfusion of the posterior pole. However, further long-term prospective studies are required to confirm this hypothesis.
The CVI has been proposed as a novel OCT-based parameter for quantifying structural vascular changes in the choroid with potential applications in a variety of retinal diseases, such as retinitis pigmentosa (RP), Stargardt disease, diabetic choroidopathy, geographic atrophy, and retinal vein occlusion.Citation9,Citation32–34 In RP, choriocapillaris loss has been reported in extracted human eyes,Citation35 and Tan et alCitation36 reported significantly lower CVIs in eyes with RP. Similarly, Wei et alCitation32 found lower CVI in a cohort of eyes with retinal dystrophies. In another study, Ratra et alCitation37 found that CVI was a more robust biomarker than CSFT for capturing choroidal alterations in eyes with Stargardt disease. Furthermore, the study found no significant correlation between visual acuity and CVI.
In the present study, there were no significant differences in the CVI between the control and MTM eyes before surgery, suggesting that it may not be useful for the diagnosis of MTM. In contrast, Wang et alCitation38 reported a greater subfoveal choroidal capillary vessel density in eyes with MTM and retinoschisis. However, choroidal atrophy is a known part of the pathogenesis of MTM, along with RPE atrophy and a reduction in adhesion between the RPE and retina.Citation39 It is unclear why there were no significant differences in CVI between the control eyes and eyes with different stages of MTM before surgery in this study. A study with a larger sample size, especially in more severe cases of MTM, is needed to identify these differences. However, it is important to note significant differences in the postoperative perfusion evaluations, where lower CVI and CFA-quantified values were observed when compared with the estimated values of the highly myopic eyes conforming to the control group. Consideration of the impact of complex surgery on the microcirculation of the posterior pole in these eyes may be warranted, as it could lead to the development of more sophisticated low-pressure control perfusion vitrectomy techniques with detailed intraoperative control of perfusion to preserve the choroid and choriocapillaris intact and potentially obtain better functional results. However, this hypothesis needs to be proven in future randomized controlled trials.
Another important question is whether any relevant patient characteristics and choroidal/retinal parameters are correlated with visual acuity. In this study, in a series of analyses, we found that none of the relevant variables, including CVI, were predictive of final visual acuity. Notably, the duration of MTM before treatment and, consequently, a more advanced stage of MTM characterization tended toward significance (p = .051); that is, a longer preoperative duration was associated with worse visual outcomes. Currently, the timing of MTM treatment remains controversial because of its progressive pathogenesis.Citation40 Although no statistically significant difference was found when we analyzed the values among the different MTM stages in the long term, these observations highlight the need for early diagnosis and treatment to preserve vision.
The present study has several limitations that are worth addressing. First, these findings should be confirmed in a larger cohort of healthy eyes with myopia or MTM. Second, additional assessments of the retina-choroid complex, such as multifocal electroretinography, autofluorescence imaging, and microperimetry, may further enhance the current understanding of CVI as a potential biomarker for MTM. Third, more detailed studies are required to assess the cause of postoperative CVI reduction. Despite these limitations, the present findings are consistent with the various comparisons and parameters among the study groups. Although we did not find convincing evidence for CVI as a biomarker for MTM, it may be used in combination with other anatomical features to provide a more comprehensive assessment of choroidal health.
CONCLUSION
In conclusion, MTM is a progressive degenerative disease characterized by alterations in the choroidal vasculature. The CVI was significantly reduced after surgery, but there was no statistically significant difference between MTM stages and between control and MTM eyes presurgery. However, significant differences were found between the long-term postoperative CVI and CVI in the healthy myopic control group. Further research is required to better understand these complex conditions. These observations suggest that long-term CVI measurement alone may be a reliable biomarker for the presence of MTM and can capture changes in the choroidal vasculature after surgical management. Additional studies are needed to confirm these findings and to further evaluate the utility of CVI as a biomarker for MTM.
ABBREVIATIONS
| ATN | = | atrophic (A), tractional (T), and neovascular (N) |
| BCVA | = | best-corrected visual acuity |
| CFA | = | choriocapillaris foveal area |
| CFT | = | central foveal thickness |
| CSP | = | choriocapillaris subfoveal plexus |
| CSFT | = | central subfield thickness |
| CVI | = | choroidal vascularity index |
| ERM | = | epiretinal membrane |
| FS-ILM | = | foveal sparing ILM peeling technique |
| FRD | = | foveoretinal detachment |
| ILM | = | internal limiting membrane |
| LCA | = | luminal choroidal area |
| LogMAR | = | logarithm of the minimum angle of resolution |
| FS | = | foveoschisis |
| MH | = | macular hole |
| MHRD | = | macular hole retinal detachment |
| MTM | = | myopic traction maculopathy |
| OCT | = | optical coherence tomography |
| OCT | = | A optical coherence tomography angiography |
| PPV | = | pars plana vitrectomy |
| PM | = | pathologic myopia |
| PS | = | posterior staphyloma |
| PVR | = | proliferative vitreoretinopathy |
| RPE | = | retinal pigment epithelium |
| RRD | = | rhegmatogenous retinal detachment |
| RD | = | retinal detachment |
| SD-OCT | = | spectral-domain OCT |
| SCA | = | stromal choroidal area |
| SRF | = | subretinal fluid |
| TCA | = | total choroidal area |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study adhered to the tenets of the Declaration of Helsinki and received full approval from the appropriate research ethics committee, institutional review committee, and institutional teaching department (the institutions did not provide reference numbers for the retrospective studies).
AUTHOR CONTRIBUTIONS
MAQR, study conception, writing the manuscript, dataset interpretation, statistical analysis interpretation, final revision, conclusions; EAQG, figure artwork, tables, material compilation; MAQG, figure and table construction; VLG, statistical analysis and final revision. All the authors have approved the manuscript for submission.
INSTITUTIONAL REVIEW BOARD STATEMENT
This study was conducted at the Retina Department of the Oftalmologia Integral ABC Institution in Mexico City. The institutional review board approved the study according to the institutional guidelines. No reference number was provided for the retrospective studies at this institution.
ACKNOWLEDGMENTS
We express our deep appreciation to the technical staff of the Retina Department of Oftalmologia Integral ABC (a Medical and Surgical Nonprofit Organization), Mexico City, Mexico, which is affiliated with The Postgraduate Division Studies at the National Autonomous University of Mexico.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
DATA AVAILABILITY STATEMENT
The datasets used in this study have been included in the main text. Photographs and figures from this study may be released via a written application to the Photographic Laboratory and Clinical Archives Retina Department at the Oftalmologia Integral ABC, Medical and Surgical Assistance Institution (Nonprofit Organization), Av. Paseo de las Palmas 735 suite 303, Lomas de Chapultepec, Mexico City 11,000, Mexico and the corresponding author upon request. The data analysis can be found in the supplementary file.
Additional information
Funding
REFERENCES
- Xie R, Qiu B, Chhablani J, Zhang X. Evaluation of choroidal thickness using optical coherent tomography: a review. Front Med. 2021;8:783519. doi:10.3389/fmed.2021.783519.
- Tan KA, Gupta P, Agarwal A, Chhablani J, Cheng CY, Keane PA, et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol. 2016;61(5):566–581. doi:10.1016/j.survophthal.2016.02.007.
- Agarwal A, Agrawal R, Khandelwal N, Invernizzi A, Aggarwal K, Sharma A, et al. Choroidal structural changes in tubercular multifocal serpiginoid choroiditis. Ocul Immunol Inflamm. 2018;26(6):838–844. doi:10.1080/09273948.2017.1370650.
- Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016;6(1):21090. doi:10.1038/srep21090.
- Agrawal R, Li LK, Nakhate V, Khandelwal N, Mahendradas P. Choroidal vascularity index in Vogt-Koyanagi-Harada disease: an EDI-OCT derived tool for monitoring disease progression. Trans Vis Sci Tech. 2016;5(4):7. doi:10.1167/tvst.5.4.7.
- Yazdani N, Ehsaei A, Hoseini-Yazdi H, Shoeibi N, Alonso-Caneiro D, Collins MJ. Wide-field choroidal thickness and vascularity index in myopes and emmetropes. Ophthalmic Physiol Opt. 2021;41(6):1308–1319. doi:10.1111/opo.12875.
- Iovino C, Au A, Hilely A, Violanti S, Peiretti E, Gorin MB, et al. Evaluation of the choroid in eyes with retinitis pigmentosa and cystoid macular edema. Invest Ophthalmol Vis Sci. 2019;60(15):5000–5006. doi:10.1167/iovs.19-27300.
- Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, et al. Choroidal vascularity index (CVI)–a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS One. 2016;11(1):e0146344. doi:10.1371/journal.pone.0146344.
- Aribas YK, Hondur AM, Tezel TH. Choroidal vascularity index and choriocapillary changes in retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2389–2397. doi:10.1007/s00417-020-04886-3.
- Sakata K, Funatsu H, Harino S, Noma H, Hori S. Relationship between macular microcirculation and progression of diabetic macular edema. Ophthalmology. 2006;113(8):1385–1391. doi:10.1016/j.ophtha.2006.04.023.
- EK C, DY K, Hunter AA III, Pilli S, Wilson M, RJ Z, et al. Staging of macular telangiectasia: power-Doppler optical coherence tomography and macular pigment optical density. Invest Ophthalmol Vis Sci. 2013;54:4459–4470. doi:10.1167/iovs.12-11116.
- Veverka KK, AbouChehade JE, R I Jr, Pulido JS. Noninvasive grading of radiation retinopathy: the use of optical coherence tomography angiography. Retina. 2015;35(11):2400–2410. doi:10.1097/IAE.0000000000000844.
- Marques JH, Marta A, Castro C, Baptista PM, José D, Almeida D, et al. Choroidal changes and associations with visual acuity in diabetic patients. Int J Retina Vitreous. 2022;8(1):6. doi:10.1186/s40942-021-00355-z.
- Kim M, Kim RY, Park YH. Choroidal vascularity index and choroidal thickness in human leukocyte antigen-B27-associated uveitis. Ocul Immunol Inflamm. 2019;27(8):1280–1287. doi:10.1080/09273948.2018.1530364.
- Singh SR, Invernizzi A, Rasheed MA, Cagini C, Goud A, Vupparaboina KK, et al. Wide-field choroidal vascularity in healthy eyes. Am J Ophthalmol. 2018;193:100–105. doi:10.1016/j.ajo.2018.06.016.
- Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CMG, Saw SM, Verhoeven VJ, et al. International photographic classification and grading system for myopic maculopathy. American Journal Of Ophthalmology. 2015;159(5):877–883.e7. doi:10.1016/j.ajo.2015.01.022.
- Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156(5):948–957.e1. doi:10.1016/j.ajo.2013.06.031.
- Shimada N, Ohno-Matsui K, Baba T, Futagami S, Tokoro T, Mochizuki M. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142(3):497–500. doi:10.1016/j.ajo.2006.03.048.
- Ikuno Y, Tano Y. Early macular holes with retinoschisis in highly myopic eyes. Am J Ophthalmol. 2003;136(4):741–744. doi:10.1016/s0002-9394(03)00319-2.
- Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122(10):1455–1460. doi:10.1001/archopht.122.10.1455.
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi:10.1016/j.ajo.2008.12.008.
- Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi:10.1097/IAE.0b013e3181be0a83.
- Fan H, Chen HY, Ma HJ, Chang Z, Yin HQ, Ng DS, et al. Reduced macular vascular density in myopic eyes. Chin Med J. 2017;130(4):445–451. doi:10.4103/0366-6999.199844.
- Quiroz-Reyes MA, Quiroz-Gonzalez EA, Quiroz-Gonzalez MA, Lima-Gomez V. Association of the choroidal vascularity index with myopic traction maculopathy: a preliminary case-series report. Lat Am J Ophthalmol. 2023;6:2. doi:10.25259/LAJO_14_2022.
- Ruiz-Medrano J, Flores-Moreno I, Ohno-Matsui K, Cheung CMG, Silva R, Ruiz-Moreno JM. Validation of the recently developed ATN classification and grading system for myopic maculopathy. Retina. 2020;40(11):2113–2118. doi:10.1097/IAE.0000000000002725.
- Quiroz-Reyes M, Moreno-Andrade B, Quiroz-Gonzalez EA, Quiroz-Gonzalez MA, Kim HJ, Nieto-Jordan A, et al. Long-term postoperative structural and functional evaluation in myopic foveoretinal detachment. Int J Ophthalmol Clin Res. 2021;8:132. doi: 10.23937/2378-346X/1410132.
- Quiroz-Reyes MA, Quiroz-Gonzalez EA, Quiroz-Gonzalez MA, Alsaber A, Lima-Gomez V. Long-term post-operative perfusion outcomes in giant retinal tears treated with and without scleral buckling. Lat Am J Ophthalmol. 2022;5:2. doi:10.25259/LAJO_2_2022.
- Li S, Li T, Wang X, Cai X, Lu B, Chen Y, et al. Natural course of myopic traction maculopathy and factors influencing progression and visual acuity. BMC Ophthalmol. 2021;21(1):347. doi:10.1186/s12886-021-02087-y.
- Fang D, Liang J, Chen S, Huang C, Li K, Mao X, et al. Surgical outcomes of myopic foveoschisis according to the ATN classification system. Ophthalmol Ther. 2023;12(1):71–85. doi:10.1007/s40123-022-00582-z.
- Tang N, Zhao X, Chen J, Liu B, Lu L. Changes in the choroidal thickness after macular buckling in highly myopic eyes. Retina. 2021;41(9):1858–1866. doi:10.1097/IAE.0000000000003125.
- Yi HC, Kim H, Bae SH. Long-term outcomes of vitrectomy used to treat myopic traction maculopathy. J Korean Ophthalmol Soc. 2020;61(1):34–40. doi:10.3341/jkos.2020.61.1.34.
- Wei X, Mishra C, Kannan NB, Holder GE, Khandelwal N, Kim R, et al. Choroidal structural analysis and vascularity index in retinal dystrophies. Acta Ophthalmol. 2019;97(1):e116–e121. doi:10.1111/aos.13836.
- Nicolini N, Tombolini B, Barresi C, Pignatelli F, Lattanzio R, Bandello F, et al. Assessment of diabetic choroidopathy using ultra-widefield optical coherence tomography. Transl Vis Sci Technol. 2022;11(3):35. doi:10.1167/tvst.11.3.35.
- Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Moscardelli F, Iovino C, et al. Choroidal vascularity index quantification in geographic atrophy using binarization of enhanced-depth imaging optical coherence tomographic scans. Retina. 2020;40(5):960–965. doi:10.1097/IAE.0000000000002459.
- Henkind P, Gartner S. The relationship between retinal pigment epithelium and the choriocapillaris. Trans Ophthalmol Soc U K. 1983;103:444–447.
- Tan R, Agrawal R, Taduru S, Gupta A, Vupparaboina K, Chhablani J. Choroidal vascularity index in retinitis pigmentosa: an OCT study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(3):191–197. doi:10.3928/23258160-20180221-07.
- Ratra D, Tan R, Jaishankar D, Khandelwal N, Gupta A, Chhablani J, et al. Choroidal structural changes and vascularity index in stargardt disease on swept source optical coherence tomography. Retina. 2018;38(12):2395–2400. doi:10.1097/IAE.0000000000001879.
- Wang SW, Hung KC, Tsai CY, Chen MS, Ho TC. Myopic traction maculopathy biomarkers on optical coherence tomography angiography—an overlooked mechanism of visual acuity correction in myopic eyes. Eye. 2019;33(8):1305–1313. doi:10.1038/s41433-019-0424-0.
- Ouyang PB, Duan XC, Zhu XH. Diagnosis and treatment of myopic traction maculopathy. Int J Ophthalmol. 2012;5(6):754–758. doi:10.3980/j.issn.2222-3959.2012.06.19.
- Frisina R, Gius I, Palmieri M, Finzi A, Tozzi L, Parolini B. Myopic Traction Maculopathy: Diagnostic and Management Strategies. Clin Ophthalmol. 2020;14:3699–3708. doi:10.2147/OPTH.S237483.

