ABSTRACT
Purpose
To summarise the qualitative and quantitative parameters of bacterial orbital cellulitis (OC) on magnetic resonance imaging (MRI) and explore their clinical correlations.
Methods
Multi-centre retrospective study with inclusion of patients of all ages with OC who underwent MRI. Patients with isolated pre-septal cellulitis, bilateral disease and poor-quality scans were excluded. An enlargement ratio for extraocular muscles (EOMs) was calculated by dividing maximal EOM measurements from the affected side by the contralateral side.
Results
Twenty MRI scans from twenty patients (Mean age: 40.8 ± 24.3 years old, M: F = 15:5) between 2011 and 2022 were analysed. Three (15.0%) cases were paediatric patients (<18 years old). All cases had both pre-septal and orbital fat involvement. The EOM were affected in nineteen cases, with the superior muscle complex (18/19, 94.7%) most commonly affected. Mean enlargement ratio (1.30, Range: 1.04–1.82) was greatest for the medial rectus on axial views on T1 and fat-suppressed contrast-enhanced T1 (FS CE T1). Optic peri-neuritis was present in eleven (55.0%) patients, whilst two (9.5%) cases had optic neuritis. A greater degree of proptosis was observed in patients with optic neuropathy and those who underwent surgical intervention compared to those without (p = .002 and p = .002, respectively).
Conclusion
MRI remains an important imaging modality for evaluating complicated OC. However, qualitative features may lack accuracy and is not a reproducible means of analysis. Simple quantitative parameters, such as proptosis and EOM measurements, correlate with high-risk clinical features and may have utility in predicting clinical course.
INTRODUCTION
Bacterial orbital cellulitis (OC) is a sight and life-threatening condition. Expedient diagnosis and management help to ameliorate the risk of developing visual and intracranial complications.Citation1 Prior studies have investigated the utility of magnetic resonance imaging (MRI) in assessing OC. Jyani et al. described the spectrum of various simple qualitative features of OC and its complications on MRI, including adjacent paranasal sinus disease, orbital abscesses, optic neuritis/peri-neuritis and cerebral venous thrombosis.Citation2 The utility of quantitative measures in MRI, such as diffusion weighted imaging (DWI) and apparent diffusion coefficients (ADC), has also been investigated, with preliminary findings suggesting that DWI may improve diagnostic confidence in OC complicated by orbital abscesses when used alongside contrast-enhanced MRI.Citation3 Additionally, Kapur et al. have described the utility of DWI in differentiating OC from other inflammatory and lymphoid lesions.Citation4 The aim of the present study is to describe, in addition to the qualitative features, several simple quantitative parameters of bacterial OC on MRI that can be utilised. This study also explores the correlation of these radiological features with clinical presentation and outcomes.
METHODS
Subjects
This was a multi-centre retrospective case-series of patients diagnosed with unilateral bacterial OC who underwent MRI at initial presentation. Inclusion criteria were patients of any age with a clinico-radiological diagnosis of OC with MRI conducted during assessment. A diagnosis of OC was supported by clinical features, laboratory investigations (e.g., elevated white cell count and C-reactive protein), microbiological analysis, radiological features, and response to antimicrobial therapy and/or surgical intervention. Patients with isolated pre-septal cellulitis (i.e.: no post-septal involvement as demonstrated by clinico-radiological features), bilateral disease and poor-quality MRI scans were excluded.
Patients were identified from the Oculoplastic Unit at the Royal Adelaide Hospital (Adelaide, Australia), Women’s and Children’s Hospital (Adelaide, Australia), Flinders Medical Centre (Adelaide, Australia) and The Sussex Eye Hospital (Brighton, England). Data collected included patient demographics, clinical presentation at time of MRI, laboratory investigations (white-cell count, C-reactive protein and relevant microbiological analysis), MRI characteristics (qualitative and quantitative measures), course of management and clinical outcomes. All research was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board.
Image Analysis
MRI sequences conducted included a combination of T1- and T2-weighted imaging (T1 and T2), fat-suppressed contrast-enhanced T1 (FS CE T1), and DWI with ADC mapping. MRI scans were reviewed by an experienced consultant neuroradiologist (SP), noting qualitative radiological features, such as local involvement of various orbital structures (e.g., pattern of extraocular muscle, lacrimal gland, orbital apex, optic nerve and sheath involvement), and regional structures (e.g., paranasal sinuses, intracranial extension). Analysis and interpretation of radiological data were conducted by experienced ophthalmologists (JT and VJ). Quantitative assessment and orbital measurements were conducted on Phillips Vue picture archiving and communication systems (PACS) Version 12 (Phillip Medical Systems). Proptosis was measured as the perpendicular distance between the interzygomatic line perpendicular to the posterior globe on axial T1.Citation5 Maximal extraocular muscle (EOM) diameters were measured in both T1 and FS CE T1 sequences.Citation6,Citation7 Medial rectus and lateral rectus muscle thickness was measured on axial scans perpendicular to the muscle belly. The superior rectus and levator palpebrae were measured together as the superior muscle complex, as they could not be reliably distinguished. The medial rectus, inferior rectus, superior muscle complex, superior oblique and inferior oblique were measured on coronal views. Due to its oblique course within the orbit, the lateral rectus was not measured on coronal scans.Citation6,Citation7 An enlargement ratio for EOMs was calculated by dividing maximal EOM measurements from the affected side by the contralateral side.
Statistical Analysis
Statistical analysis was performed with SPSS (IBM corporation, New York). Where applicable, results are expressed as means ± standard deviation (σ) and presented in relevant tables. Differences in means were analysed by the Independent Sample’s t-test with p < .05 deemed statistically significant. Fisher’s exact test was used to analyse associations between categorical variables.
RESULTS
Patient Demographics & Clinical Features
This study included twenty MRI orbital scans from twenty patients (M: F = 15:5) presenting between 2011 and 2022. Mean age for all patients was 40.8 ± 24.3 years old (Range: 2 to 85 years old). Three (15.0%) cases were paediatric patients (<18 years old), and all cases were unilateral.
The most common clinical features were periorbital oedema and erythema (19/20, 95.0%), and orbital pain (18/20, 90.0%). Other common features included diplopia secondary to restricted EOM (17/20, 85.0%), conjunctival injection (15/20, 75.0%), clinical proptosis (14/20, 70.0%) and optic neuropathy (6/20, 30%). Mean duration of symptoms prior to presentation (where recorded) was 3.4 ± 4.1 days (Range: 0 to 14 days). Sixteen (80%) patients, of which three patients were paediatric, proceeded to surgical intervention, including functional endoscopic sinus surgery (FESS), and/or orbital abscess drainage. Complete resolution of visual function and extraocular motility was achieved in 18 (90%) patients and there were no mortalities. In the remaining two patients, one was lost to follow up, and one had persistent optic neuropathy.
Qualitative Analysis
Indications for an MRI scan included clinical concern for intracranial extension and further delineation of orbital involvement. summarises all qualitative radiological findings from the following twenty cases. DWI sequences and ADC mapping were conducted in sixteen (80%) patients and helped to confirm the presence of abscesses/collections via the presence of diffusion restriction.
Table 1. Summary of qualitative radiological features encountered in this series.
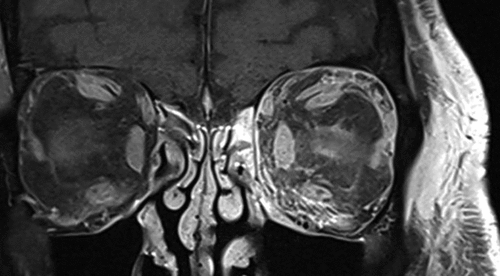
Pre-septal involvement was observed in all cases, which was demonstrated as oedema and hyperintensity on FS T2. Orbital fat involvement was observed as focal or diffuse regions of heterogenous contrast-enhancement on FS CE T1 (). Both extra- and intra-conal involvement was observed in thirteen patients, and isolated extraconal involvement in seven patients. Inflammation of the lacrimal gland, observed as contrast-enhancement and/or loss of distinct margins, was present in eighteen (90%) cases.
Figure 1. Coronal CE FS T1 of left orbital cellulitis. There is predominantly inferomedial orbital fat involvement and diffuse contrast-enhancement of the EOMs.
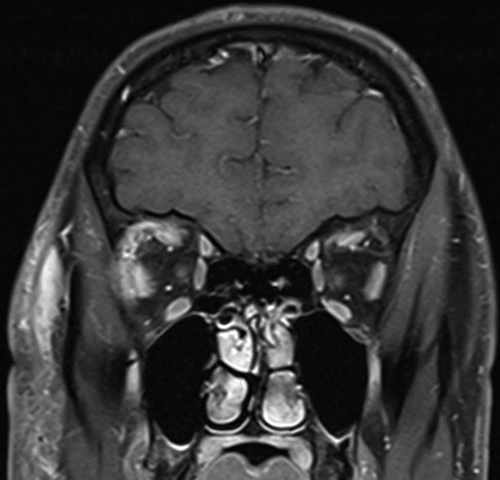
EOM involvement was observed as loss of EOM margins, hyperintensity on T2, contrast-enhancement and/or gross enlargement in areas contiguous with orbital fat inflammation ( and ). Nineteen patients had EOM involvement with the superior muscle complex (18/19, 94.7%) most commonly affected, followed by the superior oblique (14/19, 73.7%), lateral rectus (12/19, 63.2%), medial rectus (10/19, 52.6%), inferior rectus (7/19, 36.8%) and inferior oblique (5/19, 26.3%).
Figure 2. Coronal MRI orbits of right orbital cellulitis. A, demonstrates contrast-enhancement of the enlarged right lateral rectus and adjacent supero-lateral extraconal fat on CE FS T1. B, demonstrates the prior stated changes, along with the loss of distinct margins of the lateral rectus, and peri-neuritis on CE FS T1. C, demonstrates high T2 signal of the respective lateral rectus and orbital fat.
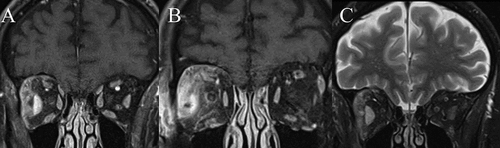
Figure 3. Coronal CE FS T1 of right orbital cellulitis. Extraocular muscle involvement in bacterial orbital cellulitis demonstrated by loss of distinct margins of the right lateral rectus and superior muscle group in areas contiguous with orbital fat inflammation.
Optic peri-neuritis was present in eleven (55.0%) patients, observed as contrast-enhancement and focal hyperintensity of the optic nerve sheath on FS T2. Meanwhile, two (9.5%) cases had optic neuritis observed as a hyperintensity on FS T2. Orbital apex involvement (i.e., orbital inflammation present in the posterior third of the orbit) was demonstrated in eleven patients (55.0%). Of the six patients with clinical optic neuropathy, one had peri-neuritis only (Case 3); one had peri-neuritis and orbital apex involvement (Case 1); two had peri-neuritis, optic neuritis and orbital apex involvement (Cases 5 and 6); and two had no abnormalities in the optic nerve, sheath or orbital apex (Cases 4 and 18).
Adjacent paranasal sinus disease was observed in fifteen (75.0%) patients. Intracranial extension was evidenced by dural enhancement, presence of empyema and/or dural collections. Intracranial extension of infection was observed in twelve (60.0%) patients. One patient had a superior sagittal sinus thrombosis; however, the remaining cases had no cerebral vascular complications.
Quantitative Radiological Analysis
Proptosis measurements were conducted in all twenty MRI orbital scans. A greater degree of proptosis was observed in patients with optic neuropathy compared to those without (7.33 ± 2.31 mm vs. 3.52 ± 2.11 mm, respectively) (p = .002), and in patients with surgical intervention compared to those who required medical management only (5.2 ± 2.9 mm vs. 2.5 ± 0.5 mm, respectively) (p = .002). There was no statistically significant difference in mean proptosis measurement between patients who had intracranial extension and those without (4.9 ± 2.8 mm vs 4.36 ± 2.9 mm, respectively) (p = .664).
EOM diameters were determined on fifteen T1 orbital scans for fifteen patients, and twelve FS CE T1 orbital scans for twelve patients. For both T1 and FS CE T1 orbital scans, there was no statistically significant difference in the total sum of the EOM for patients with optic neuropathy (p = .539 and p = .396, respectively). Similarly, no difference was observed for patients undergoing surgical intervention on both MRI sequences (p = .674 and p = .346, respectively). Within this study, an enlargement ratio represented a quantitative comparison between the affected and unaffected orbits. depicts the enlargement ratio for each involved EOM for both T1 and FS CE T1 sequences.
Table 2. Mean enlargement ratio for involved EOM.
DISCUSSION
Radiological assessment of bacterial OC with CT and/or MRI provides confirmation of post-septal involvement and characterisation of orbital or intracranial complications which may require surgical intervention.Citation1 CT orbital imaging is a rapid, practical, low-cost and easily accessible modality to acutely identify complications. CT features of OC have been well described such as proptosis; pre-septal swelling; intra- and/or extra-conal fat stranding; enlargement and contrast-enhancement of EOMs; bony deossification; and adjacent paranasal sinus disease.Citation1,Citation8–10 MRI scans are less frequently performed, however, in certain situations, supplement the initial imaging. Consequently, literature describing the spectrum and utility of MRI features in OC remains limited. ,Citation2–4 , Citation9 In addition to describing the qualitative features of OC, this present study aims to characterise several simple quantitative parameters of OC.
The radiological features of OC on MRI are summarised as follows. Pre-septal involvement was demonstrated as oedema and hyperintensity on FS T2. Orbital fat involvement was indicated by focal or diffuse regions of heterogenous contrast-enhancement on FS CE T1. EOM involvement was observed as loss of EOM margins, hyperintensity on FS T2, contrast-enhancement and/or gross enlargement which was contiguous with regions of orbital fat inflammation. Optic peri-neuritis was observed as contrast-enhancement and focal hyperintensity of the optic nerve sheath on FS T2. Upon analysis of the various quantitative MRI parameters, a greater degree of proptosis was observed in patients with optic neuropathy and those who underwent surgical intervention (p = .002 and p = .002, respectively). EOM measurements have also helped to define various quantitative parameters of EOM enlargement that may be present in OC.
Spectrum of MRI Features
The spectrum of MRI features encountered within this study are consistent with those previously described.Citation2–4,Citation9 Firstly, inflammatory changes in the intra- and/or extra-conal orbital fat were visualised as heterogenous focal or diffuse regions of hyperintensity on FS T2, and demonstrated contrast-enhancement on FS CE T1.Citation9 Subperiosteal abscesses (SPAs) may appear as a region of low to intermediate signal on T1 and hyperintensity on T2, with peripheral contrast-enhancement.Citation9 Diffusion weighted imaging would reveal restricted diffusion within an abscess.Citation11 SPAs are commonly located in the superomedial or inferomedial orbit due to the thin lamina papyracea.Citation12,Citation13 Depending on the size of the orbital collection, other radiological features encountered include globe displacement and/or enlargement and displacement of the adjacent EOM.Citation14 Furthermore, some patients may demonstrate a transitional stage between inflammatory oedema and SPA development, with minimal periosteal detachment and inflammation of adjacent extraconal fat.Citation9 Superior ophthalmic vein (SOV) thrombosis may be seen, although the internal signal will vary with time. In the acute stage it would be low signal in T1 and often low signal on T2 (due to deoxyhaemoglobin). In the subacute phase, the thrombus will generally be hyperintense on T1 and intermediate on T2.Citation15,Citation16 Cavernous sinus thrombosis (CST) results in engorgement of the cavernous sinus and ophthalmic veins and usually engorgement of EOM.Citation17,Citation18 On contrast-enhanced MRI, CST may reveal only peripheral enhancement around the thrombus or reduced enhancement compared to the normal side. Within our study, intracranial extension was readily demonstrated on MRI by dural and/or leptomeningeal enhancement, subdural collections and cerebral abscesses. However, there were no cases of SOVT or CST.
Quantitiative Parameters
This present study explored various simple quantitative MRI parameters including proptosis measurements and EOM diameters. The utility of quantitative radiological parameters has been explored in the literature for a range of orbital diseases, such as dysthyroid optic neuropathy.Citation19–22
Proptosis, along with poor pre-treatment visual acuity and large SPA volume, are important predictors of surgical intervention on CT scans.Citation23,Citation24 Analysis of proptosis measurements on MRI was consistent with these conclusions (p = .002). Additionally, a greater degree of proptosis was observed in patients with optic neuropathy (p = .002). Meanwhile, qualitative features such as peri-neuritis and orbital apex involvement were not associated with the presence of optic neuropathy, though comparison remains limited by the small sample sizes and relative power of this study. However, the development of optic neuropathy in OC remains multifactorial. Optic nerve compromise in OC may occur via several mechanisms including inflammatory causes from adjacent infection, ischemia from vascular complications or compression.Citation25
Calculation of an EOM enlargement ratio has previously been explored for use in thyroid ophthalmopathy, albeit via a different method. Nugent et al. previously described calculation of an enlargement ratio by comparison of mean muscle diameters of orbits with thyroid ophthalmopathy with normal orbits on CT.Citation26 Contrastingly, this study assessed affected orbits with the contralateral unaffected orbit. details the mean and range of the calculated enlargement ratio for each involved EOM. Mean enlargement ratio was greatest for the medial rectus on axial views, whilst less pronounced enlargement was observed for the inferior oblique, on both T1 and FS CE T1 sequences. Several factors are likely to affect the calculation and interpretation of an EOM enlargement ratio for involved EOMs. Firstly, inflammatory changes in EOM may be demonstrated by various MRI features beyond overt EOM enlargement, including contrast-enhancement, T2 hyperintensity, or loss of distinct EOM margins in regions contiguous with orbital fat inflammation. Additionally, although prior literature has reported no statistically significant difference in EOM diameters between left and right orbits of healthy subjects, marginal differences still exist, ranging from 0.9 mm to 1.2 mm.Citation27 These marginal differences may influence the accurate calculation and interpretation of an enlargement ratio, and hence the ratio should be interpreted alongside other radiological features. Finally, interpretation of such measures would be difficult in bilateral disease. Thus, it remains important to analyse the quantitative measurements with normative data.Citation6,Citation7 Additionally, the interpretation of quantitative orbital measurements should occur alongside the clinical presentation and other qualitative abnormalities identified, to guide management and prognostication.
Advantages and Disadvantages of MRI
Within our study, the main indications for conducting an MRI orbital scan were clinical suspicion of intracranial extension and/or CST. Additionally, DWI and ADC mapping helps to identify subdural empyemas and intraorbital/cerebral abscesses. MRI also provides superior visualisation of any EOM, optic nerve and optic nerve sheath changes. It may depict EOM involvement via loss of EOM margins, contrast-enhancement and hyperintensity on T2, even in the absence of gross EOM enlargement (), as was demonstrated by the range of EOM enlargement ratios observed ().
Specific MRI sequences, such as DWI, and their ability to aid in the identification of abscesses and differentiate inflammation from malignancy, have garnered interest.Citation3,Citation4 Abscesses exhibit diffusion restriction due to reduced water content within the purulent material, appearing as hyperintense on DWI and hypointense on ADC mapping within the central, non-enhancing region of the abscess cavity.Citation3 Sepahdari et al. reported that DWI helped to increase diagnostic confidence of the presence of an abscess in cases without contrast-enhanced imaging, and in certain situations, was also equivalent to contrast-enhanced MRI sequences. However, MRI is less readily available, has a longer scan time, greater operational costs, is susceptible to artefact and a general anaesthetic may be required for young children.Citation2 These factors are reflected in the sample included within this study, notably with inclusion of a smaller number of paediatric cases.
There are several limitations to this study including the retrospective nature and small sample size, with limitations on statistical power. At our tertiary institutions, MRI scans were generally reserved for cases of diagnostic uncertainty and/or suspicion of severe complications, such as suspected intracranial extension. Evidence of selection bias may also be supported by the increased incidence of superior orbital collections and involvement of the superior muscle complex, supero-medial and supero-lateral orbital fat involvement. Thus, the inclusion criteria may depict cases of greater disease severity, and this pattern of superior orbital involvement, as evident within our cases, has been described in the literature.Citation28 Additionally, not all patients underwent DWI sequences as part of the MRI scans, and significant motion artefact limited visualisation on some sequences or planes, preventing a complete radiological assessment.
In conclusion, MRI scans remain an important diagnostic imaging modality for evaluating complicated OC. In addition to identification of intracranial extension, there is further delineation of orbital fat involvement, EOM and optic nerve changes which can provide prognostic characterisation of severe cases of OC. Further research into quantitative MRI parameters may provide a greater adjunct for clinical evaluation, and correlation with clinical presentation and predicting clinical course.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [TA], upon reasonable request.
Additional information
Funding
REFERENCES
- Tsirouki T, Dastiridou AI, Ibánez Flores N, Cerpa JC, Moschos MM, Brazitikos P et al., Orbital cellulitis. Surv Ophthalmol. Jul-Aug, 2018;63(4):534–553. doi:10.1016/j.survophthal.2017.12.001.
- Jyani R, Ranade D, Joshi P. Spectrum of orbital cellulitis on magnetic resonance imaging. Cureus. Aug 11 2020; 12(8):e9663. doi:10.7759/cureus.9663.
- Sepahdari AR, Aakalu VK, Kapur R, Michals EA, Saran N, French A et al., MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. Sep, 2009;193(3):W244–50. doi:10.2214/ajr.08.1838.
- Kapur R, Sepahdari AR, Mafee MF, Putterman AM, Aakalu V, Wendel LJ, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol. Jan, 2009 ;30(1):64–70. doi:10.3174/ajnr.A1315.
- Schmidt P, Kempin R, Langner S, et al. Association of anthropometric markers with globe position: A population-based MRI study. PLOS ONE. 2019;14(2):e0211817. doi:10.1371/journal.pone.0211817.
- Rana K, Juniat V, Rayan A, Patel S, Selva D. Normative measurements of the superior oblique and inferior oblique muscles by magnetic resonance imaging. Surg Radiol Anat. Apr, 2022;44(4):521–525. doi:10.1007/s00276-022-02915-w.
- Rana K, Juniat V, Rayan A, Patel S, Selva D. Normative measurements of orbital structures by magnetic resonance imaging. Int Ophthalmol. Dec, 2022;42(12):3869–3875. doi:10.1007/s10792-022-02407-1.
- Caruso PA, Watkins LM, Suwansaard P, Yamamoto M, Durand ML, Romo LV, Odontogenic orbital inflammation: clinical and CT findings–initial observations. Radiology. Apr, 2006;239(1):187–194. doi:10.1148/radiol.2391041243.
- Eustis HS, Mafee MF, Walton C, Mondonca J. MR imaging and CT of orbital infections and complications in acute rhinosinusitis. Radiol Clin North Am. Nov, 1998;36(6):1165–1183. xi. doi:10.1016/s0033-8389(05)70238-4.
- Pereira FJ, Velasco e, Cruz AA, Anselmo-Lima WT, Elias Júnior J. Computed tomographic patterns of orbital cellulitis due to sinusitis. Arq Bras Oftalmol. Jul-Aug 2006;69(4):513–518. doi:10.1590/s0004-27492006000400011.
- Ferreira TA, Saraiva P, Genders SW, Buchem MV, Luyten GPM, Beenakker JW. CT and MR imaging of orbital inflammation. Neuroradiology. Dec 2018;60(12):1253–1266. doi:10.1007/s00234-018-2103-4.
- Lee S, Yen MT. Management of preseptal and orbital cellulitis. Saudi J Ophthalmol. Jan, 2011;25(1):21–29. doi:10.1016/j.sjopt.2010.10.004.
- Chaudhry IA, Shamsi FA, Elzaridi E, Al-Rashed W, Al-Amri A, Al-Anezi F, Outcome of treated orbital cellulitis in a tertiary eye care center in the middle East. Ophthalmology. 2007; Feb;114(2):345–354. doi:10.1016/j.ophtha.2006.07.059.
- Harris GJ. Subperiosteal inflammation of the orbit. A bacteriological analysis of 17 cases. Arch Ophthalmol. 1988;106(7):947–952. doi:10.1001/archopht.1988.01060140093032.Jul
- Lee AG, Johnson MC, Policeni BA, Smoker WR. Imaging for neuro-ophthalmic and orbital disease – a review. Clin Exp Ophthalmol. 2009;37(1):30–53. doi:10.1111/j.1442-9071.2008.01822.x.
- Sotoudeh H, Shafaat O, Aboueldahab N, Vaphiades M, Sotoudeh E, Bernstock J. Superior ophthalmic vein thrombosis: What radiologist and clinician must know? Eur J Radiol Open. 2019;6:258–264. doi:10.1016/j.ejro.2019.07.002.
- Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. Oct, 2006;26(1):S19–41. doi:10.1148/rg.26si055174. Suppldiscussion S42-3.
- Igarashi H, Igarashi S, Fujio N, Fukui K, Yoshida A. Magnetic resonance imaging in the early diagnosis of cavernous sinus thrombosis. Ophthalmologica. 1995;209(5):292–296. doi:10.1159/000310635.
- Berger M, Matlach J, Pitz S, Berres M, Axmacher F, Kahaly GJ, Imaging of the medial rectus muscle predicts the development of optic neuropathy in thyroid eye disease. Sci Rep. Apr 15 2022;12(1):6259. doi:10.1038/s41598-022-10043-z.
- Higashiyama T, Iwasa M, Ohji M. Quantitative analysis of inflammation in orbital fat of thyroid-associated ophthalmopathy using MRI signal intensity. Sci Rep. 2017;7(1):16874. doi:10.1038/s41598-017-17257-6. 2017/12/04.
- Xu L, Li L, Xie C, Guan M, Xue Y. Thickness of extraocular muscle and orbital fat in mri predicts response to glucocorticoid therapy in graves’ ophthalmopathy. Int J Endocrinol. 2017;2017:3196059. doi:10.1155/2017/3196059.
- Zou M, Wu D, Zhu H, Huang X, Zhao X, Zhao J, Multiparametric quantitative MRI for the evaluation of dysthyroid optic neuropathy. Eur Radiol. 2022;32(3):1931–1938. 2022/03/01. doi:10.1007/s00330-021-08300-2.
- Aryasit O, Aunruan S, Sanghan N. Predictors of surgical intervention and visual outcome in bacterial orbital cellulitis. Medicine. Jun 25 2021 100(25):e26166. doi:10.1097/md.0000000000026166.
- Rahbar R, Robson CD, Petersen RA, DiCanzio J, Rosbe KW, McGill TJ, Management of orbital subperiosteal abscess in children. Arch Otolaryngol Head Neck Surg. 2001; Mar;127(3):281–286. doi:10.1001/archotol.127.3.281.
- Chaudhry IA, Al-Rashed W, Arat YO. The hot orbit: orbital cellulitis. Middle East Afr J Ophthalmol. Jan 2012;19(1):34–42. doi:10.4103/0974-9233.92114.
- Nugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, et al. Graves orbitopathy: correlation of CT and clinical findings. Radiology. 1990; Dec;177(3):675–682. doi:10.1148/radiology.177.3.2243967.
- Ozgen A, Aydingöz U. Normative measurements of orbital structures using MRI. J Comput Assist Tomogr. 2000;24(3):493–496. May-Jun. doi:10.1097/00004728-200005000-00025.
- Chaturvedi R, O’Rourke M, Rostron E, Cook A, Dharmasena A. Complete manuscript title: Neurological complications from sinugenic orbital cellulitis. Eur J Ophthalmol. 2022;32(4):2469–2474. Jul. doi:10.1177/11206721211044637.

