Abstract
Antiglomerular basement membrane (GBM) disease is characteristically described with linear deposition of IgG along GBM. However, the concurrent glomerular immune complex deposition was not rare and might be contributed to the development of anti-GBM disease. In the current series, glomerular immune complexes were identified in 10 of 47 patients who presented with renal-biopsy-proven anti-GBM disease. Six of the 10 patients complicated with a well-documented glomerulonephritis, including two patients with membranous nephropathy, one patient with IgA nephropathy, one patient with membranoproliferative glomerulonephritis, one patient with Schonlein–Henoch nephritis, and one patient with hepatitis B virus associated membranous nephritis. The other four patients had immune complexes with IgG or IgM predominance deposited in glomerular mesangium without a well-documented glomerulonephritis. Clinical and pathological data of patients with immune complex deposition (n = 10) were compared with those of patients with anti-GBM disease alone (n = 37). There was no significant difference in age, gender, clinical and pathological manifestations, and renal outcome between the two groups. In general, the association of glomerular immune complexes did not lead to a benign prognosis. Plasma exchange and extensive immunosuppressive therapy should be carried out as soon as possible. The immune complexes deposited in glomeruli might participate in the initiation of anti-GBM disease.
INTRODUCTION
An autoimmune response to the noncollagenous 1 (NC1) domain of α3 chains of type IV collagen in glomerular basement membrane (GBM) is the pathogenetic basis for the development of anti-GBM disease.Citation[1],Citation[2] Frequently, it manifests as Goodpasture syndrome with progressive glomerulonephritis and pulmonary hemorrhage, but it can also be confined to either the kidney or the lung.Citation[3],Citation[4] Most patients presented with a severe crescentic or necrotizing glomerulonephritis with transiently elevated titers of circulating anti-GBM antibodies. The pathology of anti-GBM glomerulonephritis is characterized by linear deposition of IgG in GBM.Citation[4] However, some patients exhibited granular or nonlinear IgG deposits, indicating the involvement of immune complex-mediated disease. Several studies described the occurrence of superimposed glomerular immune complexes in this condition. The coexistence of anti-GBM disease and immune complex-mediated disease such as membranous nephropathy, IgA nephropathy, Schonlein–Henoch nephritis, and membranoproliferative glomerulonephritis were reported.Citation[5–11] Whether these immune complexes are coincidental or primary to anti-GBM disease and whether they also have a major pathogenetic role in tissue injury are not elucidated.
In the current study, we investigated the clinical, histological, and laboratory data from a number of patients with anti-GBM nephritis, in order to determine the incidence of superimposed glomerular complexes in this disease, to reveal any distinguishing features of these cases, and to consider putative etiological factors in the development of anti-GBM disease.
PATIENTS AND METHODS
Patients
Clinical and pathological data from 47 patients who presented to the Renal Division and Institute of Nephrology at Peking University First Hospital with anti-GBM disease between 1994 and 2002 were studied retrospectively.
The diagnosis of anti-GBM disease was made by demonstration of linear immunoglobulin deposits on the GBM in renal biopsy specimens by direct immunofluorescence or detection of circulating anti-GBM antibodies by enzyme-linked immunosorbent assay (ELISA).Citation[12] Sera with low levels (<40%) of anti-GBM antibodies were further identified by Western-blot analysis using purified human α(IV)NC1 as solid-phase ligands.Citation[13]
Renal Biopsies
Renal biopsies were performed at presentation for all patients. The mean number of glomeruli in all 47 specimens was 20.1 ± 10.3, including five specimens from five to nine glomeruli and others more than 10 glomeruli.
Tissue for light microscopy was fixed in mercuric formalin, embedded in paraffin, and sectioned at 2 μm intervals. They were then stained with hematoxylin-eosin (HE), periodic acid-Schiff (PAS), Masson's trichrome, and silver methenamine-Masson's trichrome.
Frozen sections were examined for direct immunofluorescence (IF) after staining with fluorescein-conjugated (FITC) antisera specific for human IgG, IgM, IgA, C3, C1q, fibrin-relative antigen (FRA), and albumin (Dako, Denmark). They were then washed in phosphate-buffered saline, mounted in buffered glycerol, and examined in a fluorescent microscope (Nikon, Japan). Fluorescence was graded as absent (−) or of faint, mild, moderate, bright, or blazing intensity (± to 4+), and the staining pattern was described as linear, granular, or lumpish deposition along glomerular capillary wall or in mesangium.
Twenty-five specimens were additionally examined by electron microscopy. Biopsy material was fixed in glutaraldehyde, postfixed in osmium tetroxide, and embedded in Epon 812. Sections were stained with uranyl acetate and lead citrate and examined in a transmission electron microscope JEM-100CXII (JEOL, Japan).
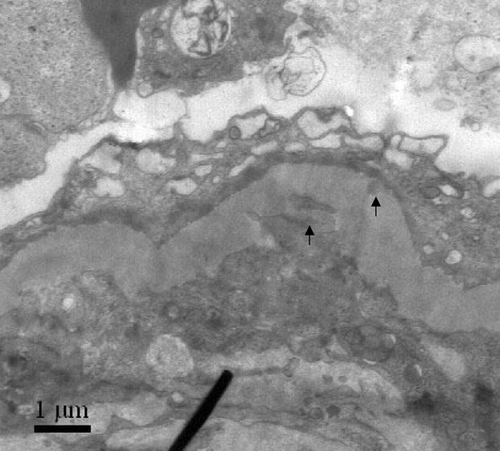
Patients with glomerular immune complexes deposits were characterized on light microscopic examination by mesangial, subepithelial, or subendothelial fuchsinophilic deposits seen on Masson-stained sections and were described as granular or lumpish deposits of IgG, IgA, or IgM, and complement (C3) with grade ≥2+ seen by direct immunofluorescence. The presence of glomerular immune complexes was confirmed by electron microscopic demonstration of immune complex-type electron-dense deposits in mesangial, subepithelial, or subendothelial area.
Detection of Anti-GBM Antibodies and Antineutrophil Cytoplasmic Antibodies (ANCA)
Anti-GBM antibodies were detected in all 47 sera by ELISA using purified bovine α(IV)NC1 as solid-phase ligands. ANCA were screened by indirect immunofluorescence (EUROIMMUN) and ELISA for myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA as described.Citation[12–14]
Statistical Analysis
All statistical analyses were carried out using the SPSS statistics package (SPSS Inc., Chicago, IL, USA), version 10.0.
RESULTS
Out of 47 patients, 10 patients (21.3%) presented with superimposed immune complexes in anti-GBM disease. These 10 patients could be divided into two groups. (1) Six patients had anti-GBM disease complicating with a well-documented glomerulonephritis, including two patients with membranous nephropathy, one patient with IgA nephropathy, one patient with membranoproliferative glomerulonephritis, one patient with Schonlein–Henoch nephritis, and one patient with hepatitis B virus associated membranous nephritis. There were five males and one female, with an average age of 31.7 ± 11.5 (18–45) years. Circulating anti-GBM antibodies were detected in all six patients, without any other autoantibodies, such as ANCA, antinucleus antibody, or rheumatoid factor. (2) Four patients had immune complexes (IgG or IgM) deposited in mesangium without a well-documented glomerulonephritis. There were three females and one male, with an average age of 51.8 ± 20.3 (22–67) years. Two of them were ANCA positive as well. Clinical and serological examinations about HIV, HBV, and SLE were all negative.
Clinical and pathological data of patients with immune complex deposition in kidney (n = 10) were compared with those of patients with anti-GBM glomerulonephritis alone (n = 37). There was no significant difference in age, gender, clinical and pathological manifestations, and renal outcome between these two groups ().
Table 1 Comparison of clinical and laboratory data of patients with anti-GBM disease, with and without glomerular immune complexes
Case History of Patients with Well-Documented Glomerulonephritis
Patient 1 with Membranous Nephropathy
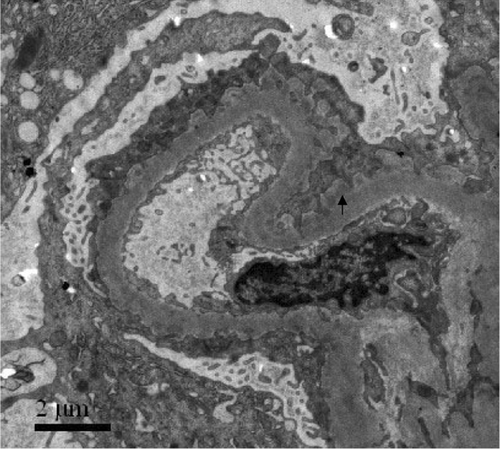
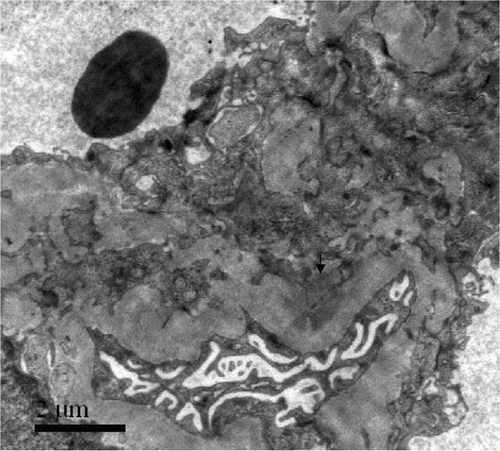
A 20-year-old man with a history of cigarette smoking and hemoptysis 50 days before admission presented with macroscopic hematuria and generalized edema. His serum creatinine at presentation was 832 μmol/L, his urinary sediment contained more than 100 RBC/HP, and his maximum 24 h urinary protein excretion was 13.9 g. Circulating anti-GBM antibodies were detected with 85%. Tests for C-reactive protein, rheumatoid factor, antistreptolysin O, anti-DNA antibody, and anti-nucleus antibody were negative. Viral serology was unmarkable throughout, including analysis of hepatitis B antigens and antibodies. Renal biopsy showed extensive rupture of capillary basement membranes and 100% large crescent formation in 19 glomeruli, including 14 cellular and five cellulofibrous crescents. Linear staining of IgG +++ and C3 +++ along GBM were demonstrated by direct immunofluorescence. The presence of glomerular immune complexes was confirmed by the subepithelial electron-dense deposits seen by electron microscope (). The patient was hemodialysis dependent.
Patient 2 with Membranous Nephropathy
A 40-year-old male smoker presented with macroscopic hematuria. His creatinine on admission was 728 μmol/L and his maximum 24 h urinary protein excretion was 2.4 g. Circulating anti-GBM antibodies were 67%. Renal biopsy showed 37 large crescents, including 19 cellular and 18 cellulofibrous crescents, and two small cellular crescents in all 39 glomeruli. By direct immunofluorescence, it could be seen that there was linear staining of IgG +++ and C3 ++ along GBM. The glomerular subepithelial immune complex deposits were demonstrated when using electron microscopy (). Again, no other circulating antibody or any other cause for membranous lesion could be found. Although plasma exchange was instituted 11 times combined with methylprednisolone pulse and cyclophosphamide therapy, the patient was dialysis dependent.
Patient 3 with IgA Nephropathy
A 27-year-old male smoker presented with a recent upper respiratory tract infection, macroscopic hematuria, and oligo-anuria. His creatinine at presentation was 1347 μmol/L. Circulating anti-GBM IgG antibodies were 134%; anti-GBM IgA antibodies were not detectable. In IgG subclasses study, besides IgG1 and IgG4, IgG2 could be detected.Citation[15] Renal biopsy showed extensive rupture of capillary basement membranes and 100% large cellular crescents formation in 17 glomeruli. Direct immunofluorescence showed granular deposits of IgA +++ and IgG + along glomerular capillary walls and in mesangium. Electron microscopy was not performed for the patient. He was hemodialysis dependent.
Patient 4 with Schonlein–Henoch nephritis
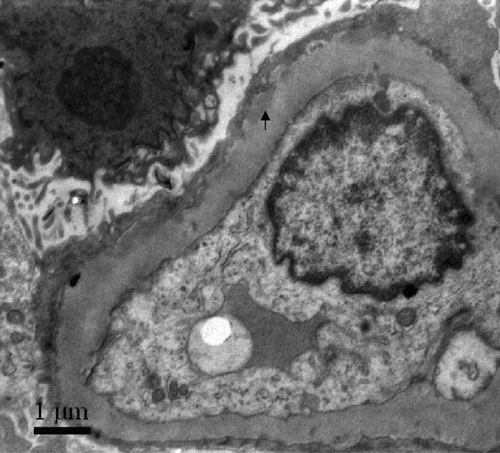
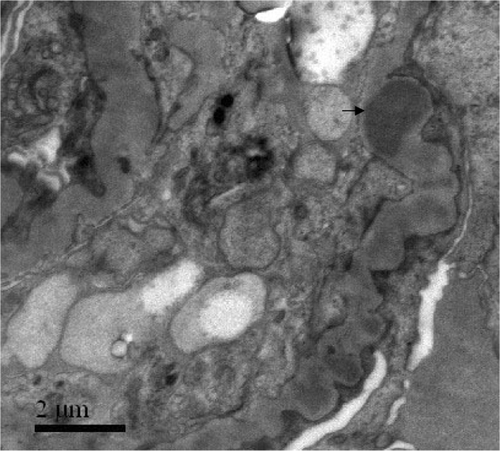
An 18-year-old man presented with an upper respiratory tract infection, intermittent abdominal pain, arthralgia, skin rash, and hematuria lasting for 1 year and oligo-anuria on admission. His creatinine was 1093 μmol/L. Circulating anti-GBM antibodies were 82%. Renal biopsy showed diffuse proliferation of mesangial and endothelial cells and extensive rupture of capillary basement membranes. There were six large cellulofibrous crescents and two small cellular crescents in all the eight glomeruli. Direct immunofluorescence showed granular deposits of IgG ++, IgA ++, C3 ++ and IgM + along glomerular capillary walls and in mesangium. Electron-dense deposits in mesangium were identified by electron microscopy (). He was hemodialysis dependent.
Patient 5 with Membranoproliferative Glomerulonephritis
A 45-year-old male smoker presented with macroscopic hematuria and generalized edema. His serum creatinine at presentation was 515 μmol/L, and his maximum 24 h urinary protein excretion was 13.1 g. Circulating anti-GBM antibodies were only 24%, the anti-GBM specificity was further confirmed by Western-blot analysis using human α(IV)NC1 as solid-phase ligands.Citation[12] Renal biopsy showed diffuse GBM thickening and mesangial hypercellularity. A double-contour of GBM with interposition of mesangial cells and matrix were seen. There was extensive rupture of capillary basement membrane and 100% large cellular crescent formation in 28 glomeruli. Direct immunofluorescence showed petaline deposits of IgG++++, IgM++, C3+, and FRA++ along glomerular capillary walls. Subendothelial and subepithelial electron-dense deposits were demonstrated by electron microscopy (). The patient was hemodialysis dependent and subsequently received a renal transplant.
Patient 6 with Hepatitis B Virus Associated Membranous Nephritis
A 40-year-old female presented with an upper respiratory tract infection and hematuria. Her serum creatinine at presentation was 490 μmol/L, and her maximum 24 h urinary protein excretion was 0.81 g. Circulating anti-GBM antibodies were 164%. Serological examinations about hepatitis B were negative, including HBs-Ag, HBs-Ab, HBe-Ag, HBe-Ab, and HBc-Ab. Renal biopsy showed 15 large crescents, including two cellular, seven cellulofibrous, and six fibrous crescents, and two glomeruli with global sclerosis in all the 21 glomeruli. In the other four glomeruli, there were mild diffuse mesangioproliferation, diffuse thickening of GBM and subepithelial immune complex deposits on Masson-stained sections. Direct immunofluorescence showed linear deposits of IgG+++ and granular deposits of IgM++, IgA++, C3++, C1q++, FRA++, HBsAg+++, and HBcAg+++ along glomerular capillary walls. Electron microscopy was not performed. She was treated with pulse methylprednisolone, followed by prednisolone and cyclophosphamide therapy. Six months later, her serum creatinine was 161 μmol/L.
Case History of Patients Without Well-Documented Glomerulonephritis
Patient 1
A 22-year-old female presented with an upper respiratory tract infection, hematuria, and edema. Her serum creatinine at presentation was 700 μmol/L, and her maximum 24 h urinary protein excretion was 4.13 g. Circulating anti-GBM antibodies were 76%. Renal biopsy showed extensive rupture of capillary basement membranes and 100% large crescent formation in 26 glomeruli, including 16 cellular and 10 cellulofibrous crescents. Direct immunofluorescence showed linear deposits of IgG+++ and C3++ along glomerular capillary walls. Electron-dense deposits in mesangium were demonstrated by electron microscopy (). She was hemodialysis dependent.
Patient 2
A 67-year-old female presented with hematuria and hemoptysis for 14 days. On admission, she had oligo-anuia, and her serum creatinine was 645 μmol/L. Circulating anti-GBM antibodies were 204%. In all the 14 glomeruli, there were 13 large crescents, including four cellular, seven cellulofibrous, and two fibrous crescents, and one small cellular crescent with segmental fibrinoid necrosis and neutrophils infiltration. Direct immunofluorescence showed granular deposits of IgG++, IgM+−++, IgA+−++, and C3++−+++ along glomerular capillary walls. Electron-dense deposits in mesangium were demonstrated by electron microscopy. She was hemodialysis dependent.
Patient 3
A 56-year-old female with a history of exposure to hydrocarbons presented with hematuria, proteinuria, fever, malaise, fatigue, weight loss, and anesthesia of extremities. Her serum creatinine at presentation was 660 μmol/L. Circulating anti-GBM antibodies were 51%, and p-ANCA was positive with anti-MPO antibodies 107%. Renal biopsy showed 14 large cellular crescents in 21 glomeruli. In other glomeruli, mild diffuse mesangial proliferation, segmental fibrinoid necrosis, and infiltration of neutrophils could be found. Direct immunofluorescence showed lumpish deposits of IgG+−++, IgM++, IgA+, and C3+++ along glomerular capillary walls and in mesangium. Electron microscopy was not performed. She was hemodialysis dependent.
Patient 4
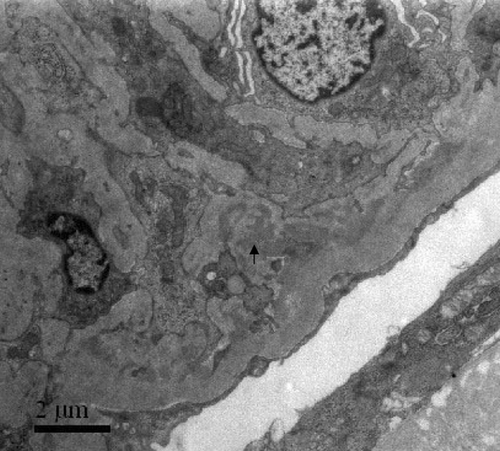
A 62-year-old male smoker presented with hematuria, proteinuria, oligo-anuria, general edema, and hemoptysis for 1 week. His serum creatinine at presentation was 991 μmol/L. Circulating anti-GBM antibodies were 176%, and p-ANCA was positive with anti-MPO antibodies 32%. Renal biopsy showed extensive rupture of the capillary basement membranes with segmental fibrinoid necrosis. Large cellular crescents were seen in all 18 glomeruli. Direct immunofluorescence showed granular deposits of IgG+++, C3+++, and IgM+ along glomerular capillary walls and in mesangium. Electron-dense deposits in GBM and mesangium were demonstrated by electron microscopy (). He died of hemoptysis and heart failure.
DISCUSSION
We confirmed previous reports by demonstrating that glomerular immune complexes were a common finding in patients with anti-GBM disease. The frequency was 21.3% in our 47 cases. If patients who had undergone electron microscopic examination were selected, the prevalence reached 28.0% (7/25). Anti-GBM disease might be combined with membranous nephropathy, IgA nephropathy, Schonlein–Henoch nephritis, membranoproliferative glomerulonephritis, and other immune complex-mediated glomerulonephritis, as reported previouslyCitation[5–11],Citation[16]; we reported hepatitis B virus associated membranous nephritis as well.
None of the ten patients with glomerular immune complexes deposit was previously diagnosed as having immune complex-mediated renal disease. Considering the rapid onset of hematuria and hemoptysis, the rapid decline of renal function, and the high percentage and synchrony of crescent formation without previous diagnosis of glomerulonephritis, it was reasonable to speculate that anti-GBM disease might occur secondary to or simultaneously with the immune-complex-mediated disease.
The glomerular immune complexes found in patients with anti-GBM disease might be helpful to elucidate the mechanism of anti-GBM antibody production. The exposure of GBM antigen produced by glomerular damage of immune-complex-mediated glomerulonephritis might lead to the production of anti-GBM antibodies. That was demonstrated in animal experimentsCitation[17], Citation[18] and supported by reports of human anti-GBM disease secondary to membranous nephropathy.Citation[7–9] On the other hand, autoimmunity was thought to cause most varieties of glomerulonephritis, including membranous nephropathy, minimal-change nephropathy, Goodpasture's disease, and possibly IgA nephropathy. So, the coexistence of anti-GBM disease with IgA nephropathy or membranous nephropathy could not be explained merely as occasional incident.
Anti-GBM disease might be preceded and actually facilitated by IgA-related immune complex deposition. The release of IL-1, TNF, IL-6, oxygen radicals, and eicosanoids by infiltrating glomerular and interstitial monocytes/macrophages and mesangial cells was well documented in experimental models and in in vivo studies of IgA nephropathy.Citation[19–23] The paracrine effects of some of these mediators might initiate immunologic and inflammatory events resulting in conformational changes of glomerular basement membrane and exposure of GBM antigens, thus facilitating anti-GBM antibody production.
Abnormalities of IgA molecules in IgA nephropathy might be another factor in the pathogenesis of anti-GBM disease with IgA nephropathy. Recent studies identified that there was a defect in galactosylation of IgA1, both in serum and in elution from nephrectomy specimens obtained from patients with IgA nephropathy.Citation[24] Serum autoantibodies against IgA1 with aberrant polysaccharide chain were IgG2 subclass predominant.Citation[25] Deposits of IgA1 with aberrant polysaccharide chain along GBM and anti-GBM IgG2 detected in patients with anti-GBM disease combined with IgA nephropathy led us to speculate that the deposition of aberrant IgA1 along GBM might induce novel antigen formation and consequently lead to the production of anti-GBM antibodies.
Many case reports revealed the coexistence of anti-GBM disease with membranous nephropathy.Citation[7–10] In most of these cases, an initially membranous nephropathy evolved into anti-GBM disease. It had been hypothesized that damage to GBM in the course of membranous nephropathy might result in the release of normal or altered basement membrane materials, leading to the formation of anti-GBM antibodies.Citation[7–10] However, Kielstein et al.Citation[10] reported a patient presented with Goodpasture's syndrome who subsequently developed membranous glomerulonephritis.
Although a pathogenetic relationship between these two diseases remained speculative, it was conceivable that a common autoimmune pathogenesis might account for the combination of membranous nephropathy and anti-GBM disease. Based on an animal model of membranous nephropathy, that is, passive Heymann nephritis, in which antibodies to podocyte antigens were injected, it was currently assumed that an (auto-) immune response to podocyte might contribute to the pathogenesis of human membranous nephropathy.Citation[26] Furthermore, IgG subclasses restriction of IgG1 and IgG4 were observed both in anti-GBM disease and in membranous nephropathy,Citation[27] which suggested that there might be some pathway similar in antibody production and in the way of renal damage.Citation[28]
Alternatively to a common autoimmune pathogenesis between anti-GBM disease and immune complex-mediated disease, anti-GBM antibodies might alter the permeability of glomerular basement membrane, allowing circulating immune complexes to access otherwise inaccessible parts of GBM. It was demonstrated that anti-GBM antibodies facilitated the access of anti-gp330 antibodies to antigens on glomerular epithelial cells.Citation[29] The presence of glomerular-bound anti-GBM antibodies might also predispose to the deposition of molecules (antigen, rheumatoid factors, and anti-idiotypic antibodies) with a particular affinity to these antibodies.Citation[30], Citation[31]
In the current study, a patient with hepatitis B virus associated membranous nephritis and anti-GBM disease was reported. Whether hepatitis B virus might play a role in the pathogenesis of anti-GBM disease needed further clarification.
There was no clinical or laboratory feature that distinguished patients with superimposed glomerular immune complexes from patients with anti-GBM disease alone. The association of glomerular immune complexes did not lead to a benign prognosis. Plasma exchange and extensive immunosuppressive therapy should be carried out as soon as possible.Citation[32]
Thus, glomerular immune complexes were found to be relatively common in anti-GBM disease. In some cases, immune complexes developed independently of anti-GBM disease, in other circumstances, their origins might be interrelated. Autoimmune response might participate in the association between anti-GBM disease and immune-complex-mediated disease.
ACKNOWLEDGMENTS
The technical assistance of Jie Ao and Xin Zheng is greatly appreciated. The study was supported by a grant of the Chinese Medical Foundation (ORF9802).
REFERENCES
- Turner N, Mason PJ, Brown R, et al. Molecular cloning of human Goodpasture antigen demonstrates it to be the α3 chain of type IV collagen. J Clin Invest 1992; 89: 592–601, [PUBMED], [INFOTRIEVE], [CSA]
- Hudson BG, Tryggvason K, Sundaramoorthy M, et al. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 2003; 348: 2543–2556, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003; 64: 1535–1550, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Falk RJ, Jennette C, Nachman PH. Primary glomerular disease. The Kidney6th, FC Rector, BM Brenner. Saunders, Philadelphia 2001; 2: 1263–1349
- Trpkov K, Abdulkareem F, Jim K, et al. Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol 1998; 49: 124–128, [PUBMED], [INFOTRIEVE], [CSA]
- Carreras L, Poveda R, Bas J, et al. Goodpasture syndrome during the course of a Schonlein-Henoch purpura. Am J Kidney Dis 2002; 39: E21, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Klassen J, Elwood C, Grossberg AL, et al. Evolution of membranous nephropathy into anti-glomerular basement membrane glomerulonephritis. N Engl J Med 1974; 290: 1340–1344, [PUBMED], [INFOTRIEVE], [CSA]
- Pettersson E, Tornroth T, Miettinen A. Simultaneous anti-glomerular basement membrane and membranous glomerulonephritis: case report and literature review. Clin Immunol Immunopathol 1984; 31: 171–180, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Thitiarchakul S, Lal SM, Luger A, et al. Goodpasture's syndrome superimposed on membranous nephropathy. A case report. Int J Artif Organs 1995; 18: 763–765, [PUBMED], [INFOTRIEVE], [CSA]
- Kielstein JT, Helmchen U, Netzer KO, et al. Conversion of Goodpasture's syndrome into membranous glomerulonephritis. Nephrol Dial Transplant 2001; 16: 2082–2085, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Deodhar HA, Marshall RJ, Sivathondan Y, et al. Recurrence of Goodpasture's syndrome associated with mesangiocapillary glomerulonephritis. Nephrol Dial Transplant 1994; 9: 72–75, [PUBMED], [INFOTRIEVE], [CSA]
- Cui Z, Zhao MH. Avidity of anti-glomerular basement membrane autoantibodies was associated with disease severity. Clin Immunol 2005; 116: 77–82, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cui Z, Zhao MH, Xin G, et al. Characteristics and prognosis of Chinese patients with anti-glomerular basement membrane disease. Nephron Clin Pract 2005; 99: c49–55, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Liu N, Zhao MH, Zhang YK, et al. Anti-glomerular basement membrane autoantibodies and anti-neutrophil cytoplasmic autoantibodies in Chinese patients with rapidly progressive glomerulonephritis and their clinical implication. Clin Nephro 1999; 3: 1–5, [CSA], [CROSSREF]
- Yan Y, Cui Z, Zhao MH. The distribution and clinical significance of IgG subclasses of anti-glomerular basement membrane antibodies. J Nephrol, in press, [CSA]
- Savige JA, Dowling J, Kincaid-Smith P. Superimposed glomerular immune complexes in anti-glomerulonephritis. Am J Kidney Dis 1989; 14: 145–153, [PUBMED], [INFOTRIEVE], [CSA]
- Kalluri R, Gunwar S, Reeders ST, et al. Goodpasture syndrome: Localization of the epitope for the autoantibodies to the carboxyl-terminal region of the alpha 3 (IV) chain of basement membrane collagen. J Biol Chem 1991; 266: 24018–24024, [PUBMED], [INFOTRIEVE], [CSA]
- Kalluri R, Cantley LG, Kerjaschkii D, et al. Reactive oxygen species expose cryptic epitopes associated with autoimmune Goodpasture syndrome. J Biol Chem 2000; 275: 20027–20032, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yoshioka K, Takemura T, Murakami K, et al. In situ expression of cytokines in IgA nephritis. Kidney Int 1993; 44: 825–833, [PUBMED], [INFOTRIEVE], [CSA]
- Lan HY, Nikolic-Paterson DJ, Mu W, et al. Local macrophage proliferation in the progression of the glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int 1995; 48: 753–760, [PUBMED], [INFOTRIEVE], [CSA]
- Libetta C, Rampino T, Palumbo G, et al. Circulating serum lectins of patients with IgA nephropathy stimulate IL-6 release from mesangial cells. J Am Soc Nephrol 1997; 8: 208–213, [PUBMED], [INFOTRIEVE], [CSA]
- Ikezumi Y, Hurst LA, Masaki T, et al. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int 2003; 63: 83–95, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kamimura H, Honda K, Nitta K, et al. Glomerular expression of alpha2(IV) and alpha5(IV) chains of type IV collagen in patients with IgA nephropathy. Nephron 2002; 91: 43–50, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med 2002; 347: 738–748, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tomana M, Novak J, Julian B, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999; 104: 73–81, [PUBMED], [INFOTRIEVE], [CSA]
- Kerjaschki D, Neale TJ. Molecular mechanism of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol 1996; 7: 2518–2526, [PUBMED], [INFOTRIEVE], [CSA]
- Segelmark M, Butkowski R, Wieslander J. Antigen restriction and IgG subclasses among anti-GBM autoantibodies. Nephrol Dial Transplant 1990; 5: 991–996, [PUBMED], [INFOTRIEVE], [CSA]
- Noel LH, Aucouturier P, Monteiro RC, et al. Glomerular and serum immunoglobulin G subclasses in membranous nephropathy and anti-glomerular basement membrane nephritis. Clin Immunol Immunopathol 1988; 46: 186–194, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barabas AZ, Boyd N, Cornish J, et al. Progressive passive Heymann nephritis in the rat. Lab Invest 1982; 47: 400–405, [PUBMED], [INFOTRIEVE], [CSA]
- Unanue ER, Dixon FJ. Experimental glomerulonephritis. VI. The autologous phase of nephrotoxic serum nephritis. J Exp Med 1965; 121: 715–718, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dwyer DS, Bradley RJ, Urquhart CK, et al. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature 1983; 301: 611–614, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 2001; 134: 1033–1042, [PUBMED], [INFOTRIEVE], [CSA]