Abstract
Urinary guanidinoacetic acid (GAA) is a sensitive marker for gentamicin nephrotoxicity in rats. This study assesses the usefulness of GAA concentrations in the diagnosis of renal tubular injury in diabetic nephropathy. Serum, urine, and renal cortex samples were obtained from rats 1, 2, and 3 weeks after streptozotocin injection (65 mg/kg body weight). Guanidinoacetic acid levels were measured by high-performance liquid chromatography. N-acetyl-β-D-glucosaminidase (NAG) activity in urine was determined by an enzymatic method. GAA levels in serum, urine, and renal cortex were significantly decreased in diabetic rats compared with those in control rats. In contrast, urinary NAG activity was significantly increased in diabetic rats. Decreases in serum, urine, and renal cortical GAA levels were attenuated by insulin treatment. These results indicate that a high serum glucose level may affect GAA synthesis in the renal cortex and that urinary GAA may be a clinically useful indicator of renal tubular injury in diabetic nephropathy.
INTRODUCTION
Although the glomerulus, particularly the mesangium, has been the focus of intensive investigation in relation to diabetic nephropathy, it is now widely accepted that the rate of deterioration in renal function correlates best with the degree of tubulointerstitial injury.[Citation[1],Citation[2]] The measurement of renal tubular enzymes such as N-acetyl-β-D-glucosaminidase (NAG) in patients with diabetes mellitus is useful to detect diabetic nephropathy.[Citation[3],Citation[4]]
Guanidinoacetic acid (GAA) is synthesized from arginine and glycine by glycine amidinotransferase in the renal tubules.[Citation[5],Citation[6]] A fraction of GAA synthesized in the kidney is excreted in the urine,[Citation[7]] and urinary GAA excretion is decreased in patients with chronic renal failure.[Citation[8],Citation[9]] We previously showed that urinary excretion of GAA is decreased in rats with gentamicin nephrotoxicity.[Citation[10]] One dose of gentamicin at 5 mg/kg body weight was sufficient to reduce urinary GAA levels. Results suggested that urinary GAA levels reflect the metabolic function of the proximal tubule and that, ultimately, measurement of urinary GAA may be useful for the early diagnosis of proximal tubular injury in humans. Thus, this study assesses whether GAA is a sensitive marker as NAG in the tubular injury in rats with streptozotocin-induced diabetes with or without insulin treatment.
MATERIALS AND METHODS
Eighty male Sprague-Dawley rats (Charles River Japan, Kanagawa, Japan), weighing 150 g, were used in this study. Diabetes was induced in 60 rats by a single tail-vein injection of streptozotocin (65 mg/kg body weight). Streptozotocin was obtained from Sigma Chemical Co. (St. Louis, MO, USA). After 24 h, diabetes was confirmed in streptozotocin-treated rats by measurement of tail-vein plasma glucose levels by the o-toluidine method.[Citation[11]] Rats with a plasma glucose level <250 mg/dL were excluded. Twenty age-matched control rats were injected with saline solution. Diabetic and control rats were killed at 0, 1, 2, or 3 weeks after the streptozotocin or saline injection. Six rats from each group were used for determination of GAA in serum, urine, and renal cortex at each time point. Seven days after the injection of streptozotocin, 20 rats received NPH insulin (NovoNordisk, Copenhagen, Denmark). The appropriate dose of insulin was adjusted between 8 and 14 units daily to maintain the plasma glucose level at approximately 130 to 160 mg/dL. Insulin-treated rats and non-insulin-treated rats were studied after 1 week of insulin or vehicle injection. Twenty-four-hour urine specimens and blood samples were collected for the determination of glucose, creatinine (Cr), and GAA. The kidneys were removed, and renal cortex was isolated for the GAA assay.
GAA was determined by high-performance liquid chromatography (Guanidinopack II, Japan Spectroscopic Co., Tokyo, Japan) and a fluorescence detector (Model FP 110-C, Japan Spectroscopic Co.), as reported previously.[Citation[9]] NAG activity was measured in the remaining urine specimens by an enzymatic test (NAG test Shionogi, Shionogi Pharmaceutical Co., Osaka, Japan) with m-cresolsulfophthalyl-N-acetyl-β-D-glucosaminide as the substrate.
Results are expressed as mean ± SE. Statistical significance was determined by the Mann-Whitney U test and ANOVA, with p < 0.05 considered statistically significant.
RESULTS
Serum glucose and Cr levels in diabetic rats are shown in . Although serum glucose levels increased markedly in diabetic rats, there was no change in serum Cr in diabetic rats compared with the level in control rats.
Table 1 Serum glucose and creatinine levels in diabetic nephropathy and control rats
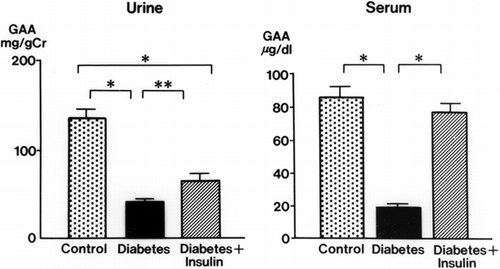
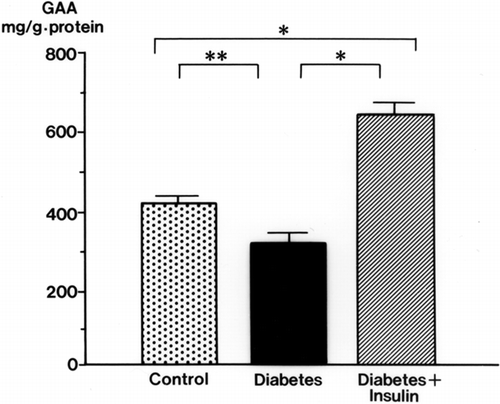
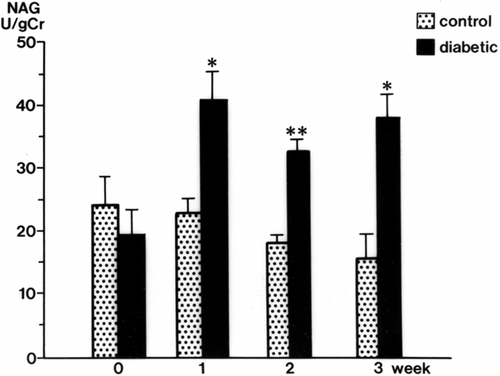
Urinary GAA concentrations in diabetic rats were decreased significantly at 2 weeks (34.3 ± 3.9 mg/g Cr, p < 0.01) and 3 weeks (32.6 ± 4.3 mg/g Cr, p < 0.01) after streptozotocin injection compared with levels in control rats (105.3 ± 17.9 mg/g Cr, both weeks) (). The serum GAA concentration was also decreased significantly 1 week after streptozotocin injection (p < 0.001) and thereafter decreased further with the progression of diabetic nephropathy (p < 0.001) (). There was a time lag between the reduction of serum GAA and the reduction of urine GAA. The renal cortical GAA concentration was significantly decreased at 1 week in diabetic rats (p < 0.05) and decreased progressively until 3 weeks (p < 0.01) (). Urinary NAG activity was increased 1 week after diabetes was induced (p < 0.01) and was still significantly increased in the diabetic rats at 3 weeks (p < 0.001) ().
Figure 1. Urinary and serum guanidinoacetic acid (GAA) concentrations in the control and diabetic rats. mean ± SE, *p < 0.001, **p < 0.01.
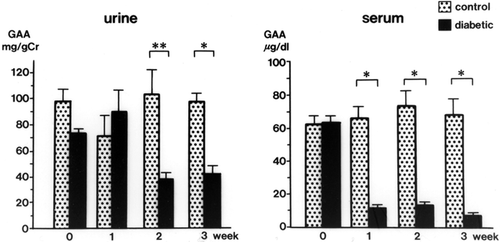
Figure 2. Renal cortical guanidinoacetic acid (GAA) concentrations in streptozotocin-induced diabetic rats. mean ± SE, *p < 0.01, **p < 0.05.
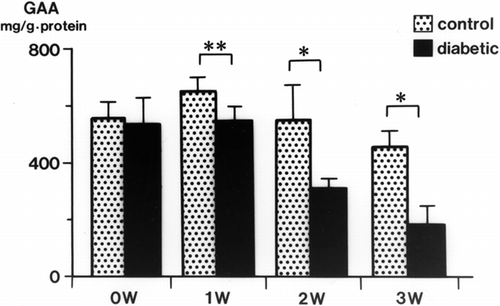
Figure 3. Urinary N-acetyl-β-D-glucosaminidase (NAG) levels in the control and diabetic rats. mean ± SE, *p < 0.001, **p < 0.01.
Insulin treatment blunted the decrease in urinary and serum GAA in diabetic rats (). The effects of insulin on GAA levels in the renal cortex of diabetic rats are shown in . With insulin, the decrease in GAA in the renal cortex was attenuated. The renal cortical GAA concentration was higher in insulin-treated rats than in control rats.
DISCUSSION
Tubulointerstitial injury is a major feature of diabetic nephropathy and an important predictor of renal dysfunction. The measurement of a variety of tubular markers in urine, including lysosomal and brush-border enzymes and tubular proteins, has become useful in the early detection of tubulointerstitial injury. Urinary NAG, a lysosomal enzyme in the proximal tubular cells, is used widely as a marker for renal tubular function in patients with diabetic nephropathy.[Citation[12–14]]
GAA is a precursor of creatine, which is essential for energy metabolism in skeletal muscle, and is synthesized from glycine and arginine by glycine amidinotransferase in the kidney, the pancreas, and the liver in humans.[Citation[15]] McGuire et al.[Citation[16]] reported that glycine amidinotransferase immunoreactivity was found by immunohistochemistry within specific rat tissues, including cells in the proximal tubules of the kidney, hepatocytes, and alpha cells of the pancreatic islets. A large part of GAA synthesized in the kidney is transported to the liver, where it is methylated to creatine by guanidinoacetate methyltransferase[Citation[17]] and is taken up by skeletal muscle. The remainder of GAA is excreted in the urine.[Citation[7]] We have previously shown in isolated renal tubules that GAA is synthesized from arginine or canavanine and glycine, but not from canaline, hydroxyurea, citrulline, and argininosuccinic acid,[Citation[6]] suggesting that the guanidine cycle proposed by Natelson and Sherwin[Citation[18]] may not be fully functional in the renal tubules. We also reported previously that, by microdissecting individual nephron segment from the rat kidney, GAA synthesis was limited to the first portion (S1) and the second portion (S2) of the proximal tubule with arginine and glycine as substrates, and that the S1 portion showed significantly higher activity for GAA synthesis than the S2 portion.[Citation[19]] These results suggest that urinary GAA levels may indicate the metabolic function of the proximal renal tubule.
In this study, decreased excretion of GAA in diabetic rats may have been due to proximal tubule impairment and a decrease in serum GAA. It is likely that the decrease in serum GAA levels was due to proximal tubular injury in diabetic nephropathy and not to chronic renal failure because the serum Cr levels were not elevated. Another possible cause of the reduction in serum GAA levels is pancreatic dysfunction in streptozotocin-induced diabetic rats. GAA concentrations in the renal cortex were reduced at 1, 2, and 3 weeks after streptozotocin injection in rats. Because GAA synthesis occurs mainly in the proximal tubules, the decrease may reflect metabolic disturbance of the proximal renal tubules induced by a high serum glucose level in diabetic nephropathy. In contrast, increased urinary NAG activity may reflect tubular cell membrane destruction.
In summary, a high serum glucose level may affect GAA synthesis in the renal cortex. Urinary GAA may prove to be a useful indicator for renal tubular metabolic disturbance in diabetic nephropathy.
REFERENCES
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structure–functional relationships in diabetic nephropathy. J Clin Invest 1984; 74: 1143–1155, [PUBMED], [INFOTRIEVE]
- Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 1991; 187: 251–259, [PUBMED], [INFOTRIEVE]
- Jung K, Pergande M, Schimke E, Ratzmann KP, Ilius A. Urinary enzymes and low-molecular-mass proteins as indicators of diabetic nephropathy. Clin Chem 1988; 34: 544–547, [PUBMED], [INFOTRIEVE]
- Watts GF, Vlitos MA, Morris RW, Price RG. Urinary N-acetyl‐beta-D-glucosaminidase excretion in insulin-dependent diabetes mellitus: relation to microalbuminuria, retinopathy and glycemic control. Diabetes Metab 1988; 14: 653–658
- Margi E, Baldoni G, Grazi E. On the biosynthesis of creatine. Intramitochondrial localization of transamidinase from rat kidney. FEBS Lett 1975; 55: 91–93, [CROSSREF]
- Takeda M, Kiyatake I, Koide H, Jung KY, Endou H. Biosynthesis of guanidinoacetic acid in isolated renal tubules. Eur J Clin Chem Clin Biochem 1992; 30: 325–333, [PUBMED], [INFOTRIEVE]
- Sasaki M, Takahara K, Natelson S. Urinary guanidinoacetate/ guanidino-succinate ratio: an indicator of kidney dysfunction. Clin Chem 1973; 19: 315–321, [PUBMED], [INFOTRIEVE]
- Marescau B, Nagels G, Possemiers I, et al. Guanidino compounds in serum and urine of non-dialyzed patients with chronic renal insufficiency. Metabolism 1997; 46: 1024–1031, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tanaka A, Takahashi Y, Mizokuchi M, Shimada N, Koide H. Plasma, urinary, and erythrocyte concentrations of guanidino compounds in patients with chronic renal failure. Ren Fail 1999; 21: 499–514, [PUBMED], [INFOTRIEVE]
- Kiyatake I, Nakamura T, Koide H. Urinary guanidinoacetic acid excretion as an indicator of gentamicin nephrotoxicity in rats. Ren Fail 2004; 26: 339–344, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hyvarinen A, Nikkila EA. Specific determination of blood glucose with o-toluidine. Clin Chim Acta 1962; 7: 140–143, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ellis EN, Brouhard BH, LaGrone L. Urinary N-acetyl-beta-D-glucosaminidase in streptozotocin-induced diabetic rats. Biochem Med 1984; 31: 303–310, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Patrick AW, Oliver MD, Howie AF, Dawes J, Macintyre CC, Frier BM. Urinary excretion of beta-thromboglobulin and N-acetyl-beta-D-glucosaminidase in type 1 diabetes: potential indicators of early nephropathy?. Diabetes Metab 1990; 16: 441–447
- Kordonouri O, Hartmann R, Muller C, Danne T, Weber B. Predictive value of tubular markers for the development of microalbuminuria in adolescents with diabetes. Horm Res 1998; 50(suppl 1)23–27, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Walker JB. Formamidine group transfer in extracts of human pancreas, liver, and kidney. Biochim Biophys Acta 1963; 73: 241–247, [PUBMED], [INFOTRIEVE], [CROSSREF]
- McGuire DM, Gross MD, Elde RP, van Pilsum JF. Localization of L-arginine-glycine amidinotransferase protein in rat tissues by immunofluorescence microscopy. J Histochem Cytochem 1986; 34: 429–435, [PUBMED], [INFOTRIEVE]
- Gerber GB, Gerber G, Koszalka TR, Miller LL. The rate of creatine synthesis in the isolated, perfused rat liver. J Biol Chem 1962; 237: 2246–2250, [PUBMED], [INFOTRIEVE]
- Natelson S, Sherwin JE. Proposed mechanism for urea nitrogen re-utilization: relationship between urea and proposed guanidine cycles. Clin Chem 1979; 25: 1343–1344, [PUBMED], [INFOTRIEVE]
- Takeda M, Koide H, Jung KY, Endou H. Intranephron distribution of glycine-amidinotransferase activity in rats. Ren Physiol Biochem 1992; 15: 113–118, [PUBMED], [INFOTRIEVE]