Abstract
Sevelamer hydrochloride use in hemodialysis patients is complicated by metabolic acidosis aggravation and hyperkalemia. Rare reports about a short-term correction of this complication have been published. The current authors investigated the long-term correction of metabolic acidosis and hyperkalemia in sevelamer hydrochloride-treated patients at doses adequate to achieve serum phosphate levels within K/DOQI™ recommendations. The authors followed 20 hemodialysis patients for 24 months in an open-label prospective study. The dialysate bicarbonate concentration was increased stepwise to a maximum 40 mEq/L and adjusted to reach patient serum bicarbonate levels of 22 mEq/L, according to K/DOQI™ recommendations. Laboratory results for serum bicarbonate, potassium, calcium, phosphate, albumin, alkaline phosphatase, iPTH, cholesterol (HDL-LDL), triglycerides, Kt/V, systolic-diastolic arterial pressure were recorded. Sevelamer hydrochloride-induced metabolic acidosis aggravation and hyperkalemia in hemodialysis patients were corrected, on the long-term, by an increase in dialysate bicarbonate concentration. Further improvement in bone biochemistry was noted with this adequate acidosis correction and parallel sevelamer hydrochloride administration, in sufficiently large doses to achieve K/DOQI™ phosphate recommendations.
INTRODUCTION
Hyperphosphatasemia is considered the “silent killer” in chronic renal disease patients, and its successful management remains a challenge. Dietary phosphate (P) intake restriction and 4–5 hours of intermittent hemodialysis (HD) are insufficient therapeutic measures. Better phosphate removal by long nocturnal or short daily dialysis sessions is not widely accepted and may have practical limitations. Subsequently, adequate phosphate binder administration is essential for achieving phosphate balance. Aluminum- and calcium-containing phosphate-binding agents are effective in lowering serum phosphate levels to K/DOQI™ recommendations target values (p = 3.5–5.5 mg/dL and Ca × P < 55 mg2/dL2). Aluminum- containing phosphate binders cannot be used as primary therapy because they are associated with neurological, skeletal, and hematological toxicity, while calcium-containing phosphate binders with valvular and vascular calcifications.Citation[1]
In the past several years, sevelamer hydrochloride (sevelamer) has been introduced as a new aluminum- and calcium-free phosphate binder in hemodialysis. Advantages include efficacy on lowering serum phosphate and intact parathyroid hormone (iPTH) levels, a lack of hypercalcemia episodes, a decrease of Ca × P product values, and attenuation of the progression of coronary and aortic calcifications, with the added beneficial influence on lipid profile and inflammation.Citation[2–5] However, whether sevelamer is an ideal phosphate binder is still under investigation. Its low affinity for phosphate at pH >7.0 and gastrointestinal side effects may lead to low efficiency and poor patients' compliance.Citation[3] Concern is raised about metabolic complications from sevelamer use, such as metabolic acidosis aggravation and hyperkalemia found in some reports.Citation[6–10]
The efficient long-term correction of sevelamer treatment metabolic complications could result in drug safety improvement and larger dose administration with subsequent better phosphate control. For this purpose, the authors investigated the correction of sevelamer-induced metabolic acidosis aggravation and hyperkalemia by increasing dialysate bicarbonate concentration (DHCO3) in an open-label prospective study with a 24 month follow-up of a group of stable hemodialysis patients.
METHODS
Patients
Twenty-three out of 40 anuric dialysis patients treated in the A. Fleming General Hospital renal unit were selected and included in the study. Inclusion criteria were a stable medical condition, no diabetes mellitus or gastrointestinal abnormalities, and sevelamer treatment for at least three months. Two out of 23 patients died during the study period from infection and cardiovascular disease, respectively. A third patient switched to peritoneal dialysis. Thus, 20 patients completed the two-year study.
The etiology of their renal disease was as follows: glomerulonephritis (n = 7), interstitial nephritis (n = 2), polycystic kidney disease (n = 4), nephroangiosclerosis (n = 4), and unknown (n = 3). Patients had been exclusively treated with sevelamer as a phosphate binder (Renagel® 800 mg) since a median period of 20 (3–26) months before the start of the study. Calcitriol or alphacalcidol was administered intermittently in 7/20 patients during the study period. Patient characteristics are seen in .
Table 1. Patient characteristics
Methods
Dialysis was performed using synthetic, 1.5–1.8 m2 surface dialysis membranes, with blood flow at 300 and dialysate flow at 500 mL/min, in three 4–5-hour-duration sessions per week. Dialysate calcium concentrations were variable (2.5–3.5 mEq/L) according to vitamin D administration and mid-week pre-dialysis serum calcium (Ca) levels. DHCO3 was at 34 mEq/L for the three months prior to inclusion in the study (control period) and then increased stepwise up to a maximum of 40 mEq/L, adjusted monthly according to serum bicarbonate (SHCO3) target value, which was set at ≥ 22 mEq/L and measured before the mid-week dialysis session.
Three monthly measurements of SHCO3, potassium (K), Ca, P, Ca × P products, alkaline phosphatase activity (ALP), albumin (ALB), total cholesterol (TC), and triglycerides (TG), as well as two measurements of iPTH, HDL-cholesterol (HDL) and LDL-cholesterol (LDL) prior to the midweek dialysis session and Kt/V, were recorded in the three months before the start of the study (control period T0 values: the averages of these three consecutive monthly measurements). Systolic and diastolic blood pressure (SBP, DBP) values prior to mid-week dialysis sessions were also recorded during the same period.
These measurements were repeated during the 24 months of the study on monthly intervals, except for iPTH (measured every three months); HDL, LDL, and Kt/V (every six months); and SBP-DBP (every week). In the two-year course, every parameter was expressed as the mean average of six monthly values for each of the four six-month intervals (T6, T12, T18, T24) of the study. Sevelamer daily dose was adjusted in each patient in order to achieve serum phosphate levels below 5.5 mg/dL.
Blood samples were analyzed by standard laboratory methods. SHCO3 was evaluated without delay in a local laboratory by electrode TCO2 quantification. iPTH was measured by luminoelectric assay. Single-pool Kt/V was calculated by urea kinetic modeling using the post-dialysis BUN value obtained 20 seconds after slowing the blood pump to 50 mL/min. Serum Ca corrected for albumin was calculated using the formula:
Statistics
Data were expressed as mean ± standard deviation except for iPTH graphic, where mean ± standard error was used. Changes in parameter values were analyzed using paired student's t-test. Wherever data were not normally distributed, median values and ranges were given (age, HD time, and sevelamer dose) and were analyzed using non-parametric statistical methods (Wilcoxon matched pair test). Correlations between parameters were performed using Pearson's method; whereas Spearman's non-parametric method was used for correlations between values not normally distributed.
RESULTS
The daily prescribed sevelamer dose at the end was similar to that at the start of the study: 5.4 (2–14.4) g versus 4.8 (0.8–16) g. The average adherence with therapy (determined by pill counts) was 86%.
Results are shown in .
Table 2. Averages of the control period (T0) and the study's four six-month intervals (mean ± SD)
Dialysate Bicarbonate
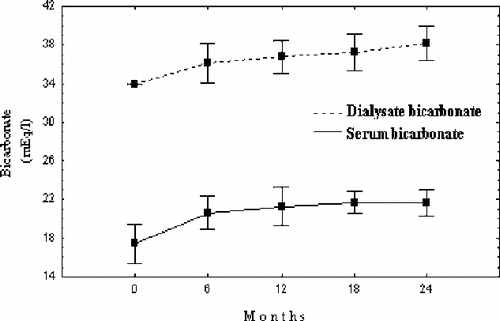
Mean DHCO3 values achieved in all the four six-month intervals were significantly higher versus initial values. At the end of the two-year period, one patient was still dialyzed against DHCO3 of 34 mEq/L, four patients against 36 mEq/L, five patients against 38 mEq/L, and ten patients against 40 mEq/L. Mean DHCO3 increase was 4.4±1.9 (median 5, range 0–6) mEq/L.
Serum Bicarbonate
During the study, SHCO3 increased to significantly higher levels compared to those at the control period. DHCO3 and SHCO3 changes during the study are shown in .
Potassium
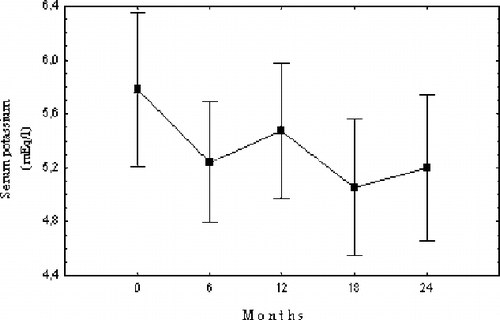
During the study, serum K levels decreased significantly (see ).
Phosphate, Calcium, Ca × P
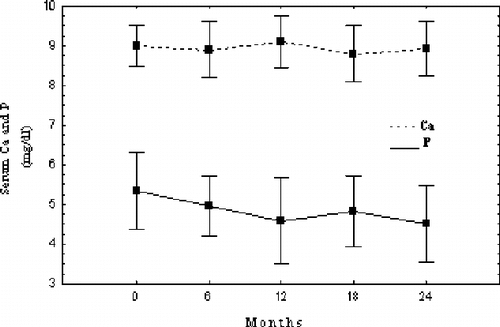
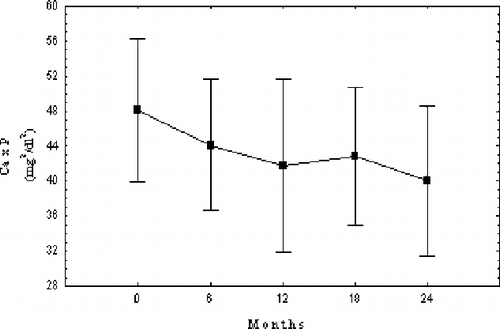
Mean serum P concentration decreased significantly in the course of the study compared to initial values. Serum Ca remained stable throughout the study. Serum Ca and P changes during the study are shown in . Ca × P product values followed P changes and significantly decreased in comparison to the beginning (see ).
Alkaline Phosphatase Activity
ALP levels remained overall stable.
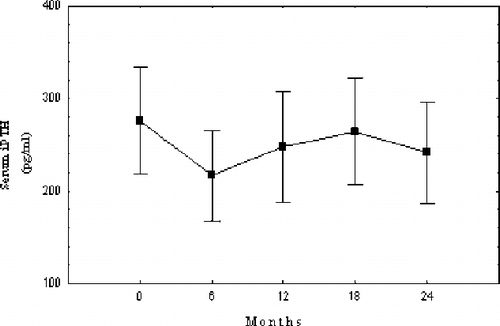
iPTH
After increasing DHCO3, serum iPTH levels remained stable during the study, with no statistically significant differences. However, a slight tendency to decrease was noted (see ).
Other Parameters
TC, TG, HDL, and LDL did not change significantly over the study period. Kt/V values were stable all over the study. Mean SBP and DBP remained also unchanged during the two-year period.
Correlations
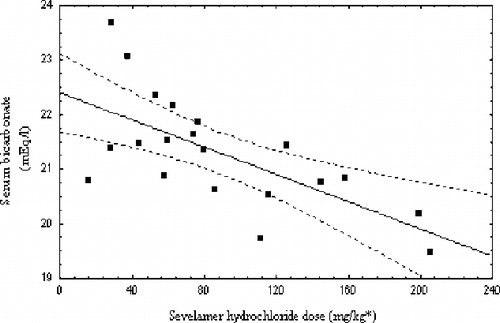
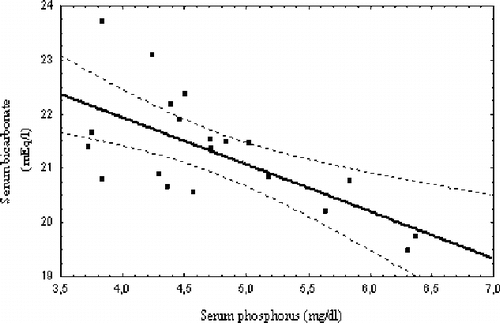
A significant negative correlation was found between sevelamer dose and SHCO3 over the whole study period (R = −0.67, p = 0.001; see ). Mean SHCO3 throughout the two-year study was also found negatively correlated to P (R = −0.66, p = 0.001; see ).
DISCUSSION
Long-term correction of sevelamer-induced metabolic acidosis aggravation in hemodialysis patients by adjustment of dialysis bicarbonate concentration was successful in our study. The two-year follow-up of 20 HD patients showed that increasing dialysate bicarbonate concentration up to 40 mEq/L resulted in a significant rise in serum bicarbonate with a parallel decrease of previously high serum potassium levels, while renal osteodystrophy biochemical parameters were kept within the K/DOQI™-recommended range by the administration of adequate sevelamer doses and acidosis correction.
The role of sevelamer hydrochloride on metabolic acidosis aggravation in hemodialysis patients is now established. Initial works on sevelamer did not refer to any alteration of treated patients' acid-base status. Chertow et al. reported a trend toward a lower serum bicarbonate concentration under sevelamer treatment that was not statistically significantCitation[2] and even a slight increase in serum bicarbonate levels.Citation[3] Gallieni et al. first reported a transient serum bicarbonate decrease in patients on sevelamer.Citation[6] This finding was later confirmed by other investigators but considered transient and was attributed to the discontinuation of calcium carbonate or aluminum-containing alkali phosphate binders.Citation[6],Citation[7] In contrast, a serum bicarbonate decrease was permanent in a short-term study of sevelamer treatment.Citation[8] The current findings were similar to those of a longer follow-up study, where a sustained serum bicarbonate decrease with a parallel serum potassium increase was established for the first time in patients receiving sevelamer for more than two years.Citation[9]
Additionally, the present findings strongly support a significant negative correlation of serum bicarbonate levels with sevelamer dosage, indicating the existing direct relationship between the drug dose and metabolic acidosis aggravation.
Sevelamer is a cationic polymer-chain with multiple amines, 40% of which are in the form of amine hydrochloride, so that sevelamer contains 17% chloride by weight. This polymer acts as an anion exchange resin that binds monovalent phosphate via ionic and hydrogen bonding under the release of anion chloride. For every mol of phosphate bound in the gut by sevelamer, one mol of HCl is liberated.Citation[11] However, sevelamer binds not only phosphate but also any other anion present in the gastrointestinal lumen. In the small intestine, it binds bile acid-lowering serum cholesterolCitation[3] similarly to cholestyramine, as well as bicarbonate anions in exchange for chloride liberation, favoring bicarbonate loss in the stool similarly to chronic diarrhea.Citation[12] In the large intestine, it binds short-chain fatty acid anions (SCFAA) that are HCO3 precursors. Every mol of SCFAA lost by sevelamer is equal to one mol of HCl reabsorbed and one mol of HCO3 lost.Citation[12] The complete displacement of chloride by all of the above anions would lead to a potential net acid load of ∼4mEq HCl for each 800mg-containing sevelamer tablet. These mechanisms can explain the metabolic acidosis aggravation under sevelamer that has been found in clinical and experimental studies.Citation[6–9]
Dialysis patients, already on metabolic acidosis due to renal failure, are challenged by a further bicarbonate loss from the gastrointestinal tract due to this sevelamer action. Thus, satisfactory control of their acid-base status is essential, as metabolic acidosis is a factor of increased morbidity and mortality in these patients. In an analysis of data from the Dialysis Outcomes & Practice Pattern Study (DOPPS) concerning 7000 patients from Europe, Japan, the United States, the association between predialysis bicarbonate levels and risk of mortality and hospitalization was found to be best represented by a U curve. Patients with mid-week predialysis bicarbonate levels between 201 and 22 mEq/L faced the lowest risk for mortality and hospitalization, whereas mortality risk was markedly elevated among patients with pre-dialysis bicarbonate ≤17 and >27 mEq/L.Citation[13] Similar findings were reported by Lowrie et al. with an increasing risk of death under a serum total CO2 below 17.5 or above 25 mmol/L.Citation[14]
Several investigators have studied metabolic acidosis treatment in renal patients. Alkali has usually been provided either by increasing dialysate bicarbonate concentration, oral bicarbonate administration, or a combination of both.Citation[15–19] Oral administration of sodium bicarbonate may result in volume overload and possibly diminish the effectiveness of sevelamer, as the alkali may compete with phosphate for binding to the resin. Additionally, reported studies refer to hemodialysis and peritoneal dialysis patients not receiving sevelamer and treated by alkali for short periods ranging from four weeks to 19 months.Citation[15–20]
The present study is the first detailed report of a successful correction of sevelamer-induced metabolic acidosis aggravation with a long follow-up. In a preliminary report, the current authors showed a significant rise of serum bicarbonate levels in a short follow-up study of 23 hemodialysis patients under sevelamer who were treated with progressively increased dialysate bicarbonate concentration.Citation[21] In contrary findings, though, a cross-sectional retrospective study of 30 hemodialysis patients on sevelamer with selection bias had determined that using a dialysate bicarbonate concentration of 40 mEq/L led to suboptimal mean serum bicarbonate levels.Citation[22]
Hyperkalemia under sevelamer administration has been previously reported and attributed to metabolic acidosis aggravation.Citation[9] During the 24-month follow-up to the present study, serum predialysis potassium levels decreased progressively. High dialysis bicarbonate concentration is known to cause intracellular potassium shift that might even result in hypokalemia by increasing insulin sensitivity and secretion; therefore, this could be the mechanism of the serum potassium decrease observed in the present study.Citation[23] Lin et al. have reported similar findings with pre-dialysis serum bicarbonate increase coupled with significant serum potassium decrease.Citation[17]
However, contrasting our findings on potassium, no difference in serum potassium levels was observed in other studies during acidosis correction in hemodialysis, perhaps because the correction was not adequate enough.Citation[16],Citation[19] Brady et al. performed a relatively short study with dialysate bicarbonate increased to 40 mEq/L for the first two weeks and then administering supplemental oral NaHCO3 for 16 weeks if serum bicarbonate levels remained below 22 mEq/L.Citation[19] They actually achieved amelioration of acid-base status but with an increase of serum bicarbonate only to approximately 20 mEq/L, which could explain the lack of a significant serum potassium effect.
Serum phosphate and Ca × P product significantly decreased during the 24-month follow- up of our study. Treatment goals recently recommended by K/DOQI™ guidelines were attained in our patient group.Citation[1] Sevelamer efficacy as a phosphate binder is already established, though its potency to achieve the K/DOQI™ serum phosphate target levels is questioned by some authors.Citation[2],Citation[3],Citation[6–9],Citation[24] High doses of the polymer were necessary in order to achieve this target in our patients.
Negative correlations of serum bicarbonate with serum phosphate levels have been reported in some studies of acidosis correction and were attributed to a possible beneficial effect on protein catabolism.Citation[25] The current authors also found a negative correlation between mean serum phosphate and serum bicarbonate values. This correlation probably reflects the effect of higher sevelamer doses administered to these patients with more severe hyperphosphatemia, resulting in a more significant acidosis.
Serum calcium levels in the current study's patients were also within the recommended by K/DOQI™ range and did not change during the study. This is in agreement with other sevelamer treatment studies as well as with studies on metabolic acidosis correction.Citation[3]
iPTH values remained stable in our study, which is similar to other reports.Citation[15],Citation[16],Citation[19] The role of acidosis on PTH levels is still under investigation with conflicting results. Metabolic acidosis has a catabolic action on bone that is both physicochemical and cell-mediated.Citation[26],Citation[27] Metabolic acidosis in hemodialysis patients is frequently associated with increased iPTH levels, leading to a worsening of hyperparathyroidism.Citation[11],Citation[15],Citation[26] The present authors also made this observation in a previous report on metabolic acidosis aggravation under sevelamer treatment.Citation[9]
Metabolic acidosis correction increases parathyroid gland sensitivity to ionized Ca, resulting in iPTH suppression, favoring vitamin D action, and thus retarding osteodystrophy progression.Citation[17],Citation[28] On the contrary, though, other studies found that metabolic acidosis correction by alkali administration had no effect on PTH.Citation[16],Citation[19] In a short-term study, Lefebvre et al. found increased serum PTH levels in patients treated with standard (low) dialysate bicarbonate, whereas in patients treated by a higher dialysate bicarbonate concentration, serum PTH remained stable over time, with a tendency to decrease. This is in agreement with the current authors' findings.Citation[15] Sadec et al. have even found an iPTH rise under high dialysate bicarbonate concentration in all patients, those treated by CaCO3 (and a low dialysate calcium concentration) or by sevelamer (and a high dialysate calcium or alphacalcidol administration).Citation[8] Further investigation is needed on the acidosis-hyperparathyroidism link, as secondary hyperparathyroidism is a known catabolic state that promotes metabolic acidosis, which aggravates pre-existing bone disease.
No significant drop in serum cholesterol levels was noted, which is usually observed at the beginning of sevelamer treatment (here, 3–24 months before the start of the present study).Citation[3]
Under high dialysate bicarbonate concentrations, patient blood pressure did not change, which is similar to reports of other short-term studies.Citation[19] Higher bicarbonate administration through dialysis did not result in thirst increase, fluid overload, or significant interdialytic weight gain variation, which are common undesirable effects observed during the oral administration of alkali salts.Citation[19]
CONCLUSION
The current study reports a long-term correction of sevelamer-induced acidosis aggravation and associated hyperkalemia in hemodialysis patients. Under increasing dialysate bicarbonate concentrations of up to 40 mEq/L, while maintaining adequate sevelamer dosage in order to achieve K/DOQI™-recommended serum phosphate levels, the patients' serum bicarbonate was maintained at 22 mEq/L, within the guidelines range recommended by K/DOQI™.
A beneficial effect on all bone biochemical parameters maintained in the K/DOQI™ guidelines range was also noted. Sevelamer hydrochloride treatment was well tolerated, without any demonstrable side effects. The present study established that sevelamer-induced acidosis and hyperkalemia can be successfully managed on the long-term, making the polymer quite a safe treatment for hemodialysis patients, even administered in high daily doses, which is essential for the achievement of K/DOQI™ guideline targets on serum phosphate and Ca × P values.
REFERENCES
- National Kidney Foundation. K/DOQI™ clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(Suppl. 3)51–52, [CSA]
- Chertow GM, Burke SK, Lazarus JM, et al. Poly [allylamine hydrochloride] (renagel): a noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis 1997; 29(1)66–71, [INFOTRIEVE], [CSA]
- Chertow GM, Burke SK, Dillon MA, Slatopolski EA, for the Renagel® Study Group. Long-term effects of sevelamer hydrochloride on the calcium × phosphate product and lipid profile of hemodialysis patients. Nephrol Dial Transplant 1999; 14: 2907–2914, [INFOTRIEVE], [CSA], [CROSSREF]
- Yamada K, Fujimoto S, Nishiura R, et al. Effects of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. J Am Soc Nephrol 2004; 14: 168A, [abstract][CSA], [CROSSREF]
- Chertow GM, Burke SK, Raggi P, et al. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 63: 245–252, [CSA], [CROSSREF]
- Gallieni M, Cozzolino M, Brancaccio D. Transient decrease of serum bicarbonate levels with sevelamer hydrochloride as the phosphate binder. Kidney Int 2000; 57(4)1776–1777, [INFOTRIEVE], [CSA], [CROSSREF]
- Marco MP, Muray S, Betriu A, et al. Treatment with sevelamer decreases bicarbonate levels in hemodialysis patients. Nephron 2002; 92: 499–500, [INFOTRIEVE], [CSA], [CROSSREF]
- Sadec T, Mazouz H, Bahloul H, et al. Sevelamer hydrochloride with or without alphacalcidol or higher dialysate calcium vs. calcium carbonate in dialysis patients: an open label, randomized study. Nephrol Dial Transplant 2003; 18: 582–589, [CSA], [CROSSREF]
- Sonikian M, Pani I, Iliopoulos A, et al. Metabolic acidosis aggravation and hyperkalemia in hemodialysis patients treated by sevelamer hydrochloride. Renal Failure 2005; 27: 143–147, [INFOTRIEVE], [CSA]
- Mitch WE. Metabolic acidosis stimulates protein metabolism in uraemia. Miner Electolyte Metab 1996; 22: 62–65, [CSA]
- Brezina B, Qunib WY, Ndan CR. Acid loading during treatment with sevelamer hydrochloride: mechanisms and clinical implications. Kidney Int 2004; 66(Suppl. 90)39–45, [CSA], [CROSSREF]
- Wrong OM, Harland CE. Sevelamer-induced acidosis. Kidney Int 2005; 67(2)776, [INFOTRIEVE], [CSA], [CROSSREF]
- Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44(4)661–671, [INFOTRIEVE], [CSA], [CROSSREF]
- Lowrie EG, Lew N. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990; 15: 458–482, [INFOTRIEVE], [CSA]
- Lefebvre A, De Vernejoul MC, Queris J, et al. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int 1989; 36: 1112–1118, [INFOTRIEVE], [CSA]
- Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in hemodialysis patients: effects on acidosis and nutritional status. Nephrol Dial Transplant 1997; 12: 2633–2637, [INFOTRIEVE], [CSA], [CROSSREF]
- Lin S-H, Lin Y-F, Chin H-M, Wu C-C. Must metabolic acidosis be associated with malnutrition in hemodialysis patients?. Nephrol Dial Transplant 2002; 17: 2006–2010, [INFOTRIEVE], [CSA], [CROSSREF]
- Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on albumin in chronic hemodialysis patients. Am J Kidney Dis 1998; 31: 35–40, [INFOTRIEVE], [CSA]
- Graham KA, Reaich D, Channon SM, et al. Correction of acidosis in CAPD decreases whole body protein degradation. Kidney Int 1996; 49: 1396–1400, [INFOTRIEVE], [CSA]
- Movilli E, Zani R, Carli O, et al. Correction of metabolic acidosis increases albumin concentrations and decreases kinetically evaluated protein intake in hemodialysis patients: a prospective study. Nephrol Dial Transplant 1998; 13: 1719–1722, [INFOTRIEVE], [CSA], [CROSSREF]
- Sonikian M, Pani I, Koutala K, et al. Management of Acidosis Aggravation and Hyperkalaemia Associated with Sevelamer Hydrochloride Treatment in Dialysis Patients. Book of Abstracts of the XLIst Congress of the European Renal Association, European Dialysis and Transplant Association 2004; 104, Abstract SP264[CSA]
- Ciampi MA, Reilly RF. Long-term sevelamer use is associated with a higher serum phosphate, higher Ca × P product and lower serum bicarbonate than calcium-containing binders. J Am Soc Nephrol 2002; 13: 586A, [CSA]
- Heguilen RM, Sciurano C, Bellusci AD, Fried P, et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic hemodialysis patients. Nephrol Dial Transplant 2005; 20: 591–597, [INFOTRIEVE], [CSA], [CROSSREF]
- Nolan CR. Phosphate binder therapy for attainment of K/DOQI™ bone metabolism guidelines. Kidney Int 2005; 68(Suppl. 96)7–14, [CSA]
- Uribarri J, Levin NW, Delmez J, et al. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis 1999; 34: 493–499, [INFOTRIEVE], [CSA]
- Mehrotra R, Kopple JD, Wolfson M. Metabolic acidosis in maintenance dialysis patients: clinical considerations. Kidney Int 2003; 64(Suppl. 88)13–25, [CSA], [CROSSREF]
- Busevelamerinski DA. Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol 1989; 256: F836–F842, [CSA]
- Graham KA, Hoenich NA, Tarbit M, et al. Correction of acidosis in hemodialysis patients increases the sensitivity of the parathyroid glands to calcium. J Am Soc Nephrol 1997; 8: 627–632, [INFOTRIEVE], [CSA]