Abstract
Contrast-induced nephropathy (CIN) is associated with increased morbidity and mortality, as well as increased costs for medical care, particularly in patients with diabetes mellitus and chronic renal failure. A key step to safer CIN is to identify patients at risk and applying proven preventive interventions. Extracellular volume expansion, minimizing the dose of contrast media, using low-osmolar non-ionic contrast media, stopping the intake of nephrotoxic drugs, and avoiding short intervals between procedures have all been shown to be effective in reducing CIN. The aim of the present review is to summarize the knowledge about the risk factors and prophylactic treatments of CIN.
Introduction
Radiologic procedures utilizing intravascular contrast media injections are being widely applied for diagnostic and therapeutic purposes. These led to a parallel increase in the incidence of contrast-induced nephropathy (CIN). Renal failure requiring dialysis after contrast media administration is associated with a 40% in-hospital mortality rate and has a two-year mortality rate of 80%. Approximately $180 million are spent annually to manage CIN in the United States.Citation[1] The large number of patients who are severely affected by CIN underscores the importance of addressing known risk factors and preventions for CIN. Patients at risk for CIN can often be identified with a routine medical history, physical examination, and laboratory analysis before the procedure. Two of the major risk factors of CIN are pre-existing renal failure and diabetes mellitus.Citation[1–4] After the high-risk patient population has been identified and risk factors addressed, the next step in preventing CIN is the use of different prophylactic therapies. The following review provides an overview of current risk factors and preventive methods of CIN.
Method
The comprehensive search of the literature was performed in Medline, Embase, and PubMed and covered the period between 1966 and 2005. The search for literature included only articles written in English. An article was rejected if it was clearly a letter or case report. Two comprehensive search themes were derived and then combined using the Boolean operator “and.” The first theme used a recommended, highly sensitive, randomized controlled trial filter and systematic review filter method. The second theme, contrast-induced nephropathy, was created by using the Boolean search term “or” to search for the following terms appearing as both exploded medical subject headings (MeSH) or text words: “contrast media,” “radiocontrast,” “contrast nephropathy,” “radiocontrast nephropathy,” “iodinated-contrast media,” or “contrast media-induced nephropathy.” The authors independently evaluated identified articles for eligibility on the basis of three inclusion criteria:
study design
target population (patients undergoing coronary angiography and coronary interventions)
outcome (trials with clearly definition of CIN).
The authors independently extracted data from all primary studies that met eligibility criteria. Any discrepancies in extracted data were resolved by consensus. Data extracted included identifying details of study protocol and demographic data. The primary outcome measures were the incidence of CIN and change in serum creatinine. The secondary outcome measure was the requirement for renal replacement therapy. The authors independently assessed the methodological quality of individual studies. Items used to assess study quality were methods of randomization, blinding, use of a placebo, reporting of losses to follow-up or missing outcome assessments, and evidence of important baseline differences between the groups.
Definition and Incidence of CIN
In the literature, different definitions of CIN are given. CIN is commonly defined as the impairment of renal function (the elevation of serum creatinine by ≥ 0.5 mg/dL or ≥25%) occurring within 48 hours after administration of contrast media. The Iohexol Cooperative Study defined CIN as an increase in serum creatinine of ≥1 mg/dL 48 to 72 hours post-contrast.Citation[5] The Nephric Trial has two separate primary endpoints, including 0.5 and 1.0 mg/dL rises in serum creatinine from day 0 to day 7.Citation[6] According to the guidelines of European Society of Urogenital Radiology, the term CIN indicates an impairment of renal function (the elevation of serum creatinine by ≥ 0.5 mg/dL, or ≥25%) occurring within three days following the intravascular administration of contrast media, as well as the absence of an alternative etiology.Citation[7] This definition may overlook a large group of patients in whom nephropathy develops up to a week after the administration of contrast media. However, more conservative definitions have a lower sensitivity because they require greater increases in the serum creatinine level. The definition, which requires smaller increases in serum creatinine (≥25% or ≥0.5 mg/dL), is more sensitive for the diagnosis of CIN associated with clinically important adverse short- and long-term outcomes.Citation[8],Citation[9]
The incidence of CIN is usually <2% in patients who do not have any risk factor for CIN, but the incidence can be increased to 90% in high-risk patients for CIN.Citation[10–12]
Pathophysiology of CIN
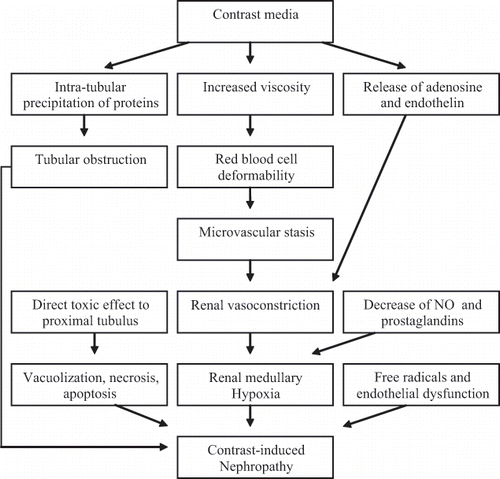
The pathogenesis of CIN is unclear. The proposed mechanisms are medullary hypoxia due to decreased renal blood flow secondary to renal artery vasoconstriction, tubular obstruction, and direct tubular toxicity of the contrast media due to apoptosis and oxidative damage. Endothelial dysfunction and renal microcirculatory alterations also may play a role.Citation[8],Citation[13–16] The major classes of potential pathophysiologic mechanisms of CIN are summarized in and below.
Renal Hemodynamic Changes
Contrast agents were associated with epithelial cell necrosis, primarily in the thin ascending limb in the renal medulla. The renal medulla is uniquely susceptible to ischemic injury, and contrast media may cause medullar hypoxia by shunting blood flow to the renal cortex.Citation[15] The development of CIN is affected by changes in renal hemodynamics due to the effects of the contrast media on the action of many substances, including increased activity of renal vasoconstrictors and decreased activity of renal vasodilators.Citation[4],Citation[8],Citation[13],Citation[15] Other factors that may decrease renal blood flow include increased viscosity of contrast media and erythrocyte aggregation induced by contrast media, which results in diminished oxygen delivery.Citation[17]
Direct Toxic Effect on Renal Cells and Reactive Oxygen Species
Contrast agents have been found to reduce antioxidant enzyme activity, and direct cytotoxic effects mediated by oxygen free radicals have been found in experimental models of CIN.Citation[4],Citation[8],Citation[13] Reactive oxygen species formed as a result of post-ischemic oxidative stress can lead to acute renal failure through their direct effects on endothelial cells. Pathological changes induced by contrast media suggest a direct toxic effect of contrast media on renal tubular epithelial cells. Apoptosis is also involved as a result of cellular injury.
Role of Osmolality
Hyperosmolar contrast agents induce renal hemodynamic changes and have direct toxic effects on renal epithelial cells. These effects of hyperosmolality could be caused by osmolar-driven solute diuresis with the activation of tubuloglomerular feedback or an increase in tubular hydrostatic pressures, which may cause compression of the intrarenal microcirculation and a decreased glomerular filtration rate. In addition, DNA fragmentation was increased in cells exposed to hyperosmolar contrast media. Thus, there is evidence of a direct cytotoxic effect of contrast media that is independent of hypoxia and may be related to the hyperosmolality of the contrast media.Citation[4],Citation[8],Citation[16]
Clinical Course and Outcomes
CIN may range in severity from asymptomatic, nonoliguric, transient renal dysfunction to oliguric severe renal failure that necessitates permanent dialysis or kidney transplantation. CIN is reported to be the third leading cause of in-hospital acute renal failure after hypotension and surgery.Citation[1],Citation[2],Citation[10] Approximately $180 million is spent annually to manage CIN in the United States.Citation[1] Dangas et al. showed that in-hospital outcomes such as death (6.3% vs. 0.8%), cardiac death (4.0% vs. 0.5%), coronary artery bypass grafting (5.8% vs. 0.5%), major adverse cardiac event (9.3% vs. 1.1%), packed red cell transfusion (28% vs. 6%), vascular surgery of access site (5.6% vs. 2.6%), and post-procedure length of stay (6.8 ± 7.1 vs. 2.3 ± 2.5) were significantly higher in CIN developed patients compared with control (p < 0.0001). In the same study in a cumulative one-year outcome, death, out-of-hospital death, and major adverse cardiac events were significantly higher in CIN-developed patients (p < 0.0001).Citation[18] In a study of acute myocardial infarction patients undergoing primary angioplasty, it was found that CIN-developed patients have a significantly higher incidence of high-rate atrial fibrillation (p = 0.01), high-degree conduction disturbances requiring a permanent pacemaker (p = 0.04), acute pulmonary edema (p = 0.008), respiratory failure requiring mechanical ventilation (p < 0.0001), cardiogenic shock requiring an intra-aortic balloon (p < 0.0001), and acute renal failure requiring renal replacement therapy (p < 0.0001).Citation[19] In a retrospective analysis of 16,248 patients undergoing contrast media examinations, the in-hospital mortality rates were almost five-fold higher (34% vs 7%, OR = 6.5; p < 0.01) in CIN patients, and CIN was associated with sepsis, bleeding, coma, or respiratory failure.Citation[20] Renal failure requiring dialysis after coronary interventions is associated with a 36% in-hospital mortality rate and has a two-year mortality rate of 81%.Citation[1]
Risk Factors for Contrast-Induced Nephropathy
Specific factors that increase the risks for development of CIN are related to the patient, the contrast medium, and the procedure (see ).
Table 1. Risk factors for the development of contrast-induced nephropathy
Kidney-Related Risk Factors
Pre-Existing Renal Disease
The major risk factor for CIN is a GFR<60 mL/min/1.73 m2.Citation[1],Citation[21–23] McCullough et al. noted that with an estimated GFR greater than 60 mL/min, the chance of CIN was less than 5%.Citation[9] This was corroborated by Khanal et al.Citation[24] Chronic kidney disease is associated with a decreased vasodilatory response, which is important in developing CIN, and in patients with renal insufficiency, the clearance of contrast media is slower than in normal subjects. In a study of 7586 patients undergoing coronary intervention, CIN developed in 22.4% of those who had serum creatinine levels of 2.0 to 2.9 mg/dL and in 30.6% of those with serum creatinine levels of 3.0 mg/dL or higher, compared with 2.4% of patients with serum creatinine levels <1.1 mg/dL.Citation[12] Moore et al.Citation[25] and Barrett et al.Citation[26] both reported that the incidence of CIN increased from 4% to 20% as the baseline serum creatinine increased from 1.2 to 2.9 mg/dL. In another study, the incidence of CIN increased from 8% to 92% as the serum creatinine increased from 1.5 to 6.8 mg/dL. Furthermore, the probability of CIN requiring dialysis increases from 0.04% to 48% as baseline GFR decreases from 50 to 10 mL/min.Citation[9]
Diabetic Nephropathy
Diabetic patients constitute an important high-risk group for CIN. Nitrovasodilation is characteristically altered in diabetes. Chronic kidney disease and DM are associated with endothelial dysfunction and decreased vasodilatory responses.Citation[15] Diabetic nephropathy has been identified as a powerful and independent risk factor for CIN.Citation[1–3] Patients with diabetic nephropathy and a mean serum creatinine of 6.8 mg/dL had a 92% incidence of CIN after coronary angiography.Citation[27] Patients with diabetes who have advanced chronic renal failure due to causes other than diabetic nephropathy have a significantly higher risk of developing CIN-like diabetic nephropathy. On the other hand, studies have shown that when preexisting renal disease is present, patients with and without diabetes have the same risk of CIN, which correlates with the degree of renal disease.Citation[9],Citation[28] Some authors have suggested that DM in the absence of nephropathy, particularly in insulin-dependent diabetics, is associated with an increased risk of CIN. In a study by Lautin et al., the incidence of CIN was rather low (2%) in patients with neither diabetes nor azotemia, significantly higher (16%) in individuals with diabetes but preserved renal function, and much higher (38%) in patients who had both diabetes and azotemia.Citation[29] In a study by Rihal et al., the incidence of CIN was 2% in non-diabetic patients and 3.7% in diabetics with a baseline creatinine ≤1.1 mg/dL (OR = 1.86, p = 0.005). When renal function is mildly impaired (serum creatinine level 1.2 to 1.9 mg/dL), the risk of CIN in patients with diabetes mellitus increased to 4.5% (OR = 2.42, p < 0.001).Citation[12] Other studies have failed to corroborate this connection. For example, Parfrey et al. showed that none of 85 patients with diabetes and normal renal function developed clinically significant CIN.Citation[30] In general, patients with diabetes alone were found to be at slightly higher risk of CIN than the general population.Citation[2],Citation[21]
Nephrotoxic Drugs
Nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors decrease the generation of vasodilatory prostaglandins in the kidney and potentiate the vasoconstrictive effect of contrast media.Citation[31] Sulfonamides, aminoglycosides, and their combination with furosemide are particularly potent. Cyclosporin A may intensify medullary hypoxia, and cisplatin can attach to sulfhydryl groups. Mannitol can increase the metabolic workload in the kidney, and amphotericin B can effect the combination of mannitol and cyclosporine A. Note, though, that all of these medications' individual roles as independent risk factors of CIN have not been determined in large clinical trials.Citation[2],Citation[32]
Hypovolemia
The reduction of the effective intravascular volume has been reported as contributing to prerenal reduction in renal perfusion, thus enhancing the ischemic insult of contrast media.Citation[33–35] Hypovolemia increases neuro-humoral vasoconstrictive stimuli that might compromise medullary oxygenation.Citation[15],Citation[35] At the injection of contrast media, fluid shifts from tissues into the vascular system, leading to a decreased circulating volume. Volume expansion reduces the activity of the renin-angiotensin system, minimizes increases in blood viscosity and osmolality, and increases medullary perfusion.Citation[13],Citation[36] Before the angiography, the volume status of the high-risk patients can be examined by the inferior vena cava index, mean atrial pressure, or non-invasive pulmonary-capillary wedge pressure.Citation[37]
Cardiovascular System-Related Risk Factors
Congestive Heart Failure and Reduced Left Ventricular Ejection Fraction
Studies have shown that reduced left ventricular ejection fraction (LVEF) and advanced congestive heart failure (New York Heart Association class III or IV) are independent risk factors for CIN. In one study, Dangas et al. found that LVEF< 40% is an independent predictor of CIN.Citation[18] The current authors have previously reported that if the LVEF >30%, this condition will not show any significant effect on the development of CIN.Citation[38] Advanced heart failure and reduced left ventricular ejection fraction are characterized by effective volume depletion due to low cardiac output, increased neuro-humoral vasoconstrictive stimuli, and impaired nitrovasodilation, which might compromise medullary oxygenation.Citation[3],Citation[8],Citation[15] In one study, it was shown that congestive heart failure is an independent risk for CIN (OR = 1.53, p = 0.007).Citation[12] In a derivation and validation cohort study, congestive heart failure was a risk for CIN in patients undergoing coronary intervention (OR = 2.2, p < 0.0001).Citation[39]
Acute Myocardial Infarction
In a study by Rihal et al, acute myocardial infarction within 24 hours before administration of the contrast media was found to be a risk factor for CIN (OR = 1.85, p = 0.0006).Citation[12] This study demonstrates that CIN is a frequent complication in acute myocardial infarction, even in patients with normal baseline renal function. Marenzi et al. showed that in 208 acute myocardial infarction patients undergoing primary coronary interventions, anterior acute myocardial infarction was significantly higher in CIN-developed patients (p = 0.0015), but in multivariate analysis, anterior acute myocardial infarction (OR = 2.17, p = 0.09) was not a risk predictor of CIN.Citation[19] Sadeghi et al. reported a more than seven-fold (3.2% vs. 23.3%) increase in one-year mortality in acute myocardial infarction patients who developed CIN.Citation[40]
Hypertension
Impaired nitrovasodilation is prevalent in the hypertensives. Hypertension has been categorized as a risk factor for CIN in some papers. In a study of 8,628 patients who underwent percutaneous interventions, hypertension was found as an independent predictor of CIN (OR = 1.2, p = 0.0035).Citation[41] In a cohort study in which patients were subjected to cardiac catheterization, hypertension was found a risk factor for CIN (OR = 2.0, p = 0.0001).Citation[39]
Hypotension and Cardiac Shock
Decreased effective circulating volume and reduced renal perfusion potentiate renal vasoconstriction following the administration of intravascular CM. Systolic blood pressure of less than 80 mmHg for at least one hour requiring inotropic support with medications is a risk factor for CIN.Citation[1] Dangas et al. found that peri-procedural hypotension was an independent predictor of CIN in patients with chronic kidney disease (OR = 2.50, p < 0.00001).Citation[18] Preprocedure shock was an independent risk factor for CIN (OR = 1.19, p = 0.05) in a study by Rihal et al.Citation[12]
Use of an Intra-Aortic Balloon Pump
The use of an intra-aortic balloon pump might signify a very high-risk population due to very severe coronary atherosclerosis and/or indicate a role of atheroembolism. Marenzi et al. showed that in 208 consecutive acute myocardial infarction patients undergoing percutaneous coronary intervention, the use of an intra-aortic balloon pump was a risk predictor of CIN (OR = 15.51, p < 0.0001).Citation[19] Dangas et al. have demonstrated that intra-aortic balloon pump use is an independent predictor of CIN in patients with chronic kidney disease (OR = 2.27, p = 0.004).Citation[18] Also, Gruberg et al. found that the use of an intra-aortic balloon pump was a risk factor for CIN requiring dialysis after percutaneous coronary interventions (OR = 1.94).Citation[42] In another derivation and validation cohort study, Bartholomew et al. found that intra-aortic balloon pump use was a risk for CIN in patients undergoing coronary intervention (OR = 5.1, p < 0.0001).Citation[39]
Peripheral Vascular Disease
Factors related to accelerated or diffuse atherosclerosis are linked to the development of CIN.Citation[18] In a cohort study by Bartholomew et al.Citation[33] and another by Rihal et al.Citation[12], it was shown that peripheral vascular disease is a risk for CIN in patients undergoing percutaneous coronary intervention (OR = 1.9, p < 0.0001 and OR = 1.71, p = 0.001, respectively).
Bypass Graft Intervention and Time to Reperfusion
Procedures with bypass angiography and intervention may be associated with higher complexity, longer duration, and limited success, thus indicating an unstable post-procedural period with impaired cardiac output. Gruberg et al. showed that the risk of CIN requiring dialysis after percutaneous coronary intervention was increased with bypass graft intervention (OR = 4.94).Citation[42] Marenzi et al. showed that in 208 acute myocardial infarction patients undergoing primary percutaneous coronary intervention, the risk of CIN was increased if the time-to-reperfusion is ≥6 h (OR = 2.51, p = 0.04).Citation[19]
Demographic Risk Factors
Advanced Age
Possible reasons of the high incidence of CIN in the elderly include age-related changes in renal function, such as the dominance of renal vasoconstrictive forces, as opposed to renal vasodilatory forces; more difficult vascular access following tortuosity; the calcification of the vessels, requiring greater amount of contrast; defective prostaglandin synthesis; a decline in prostaglandin E2; and the presence of renovascular disease.Citation[2],Citation[14],Citation[43] In a prospective study in which elderly patients (≥70 years) were subjected to cardiac catheterization, 11% developed CIN.Citation[11] In another study, CIN incidence was 17% in the elderly patients (>60 years) as compared with 4% in the younger group.Citation[44] In a study of 208 acute myocardial infarction patients who underwent coronary intervention, age ≥75 years was found an independent risk of CIN (OR = 5.28, p = 0.0009).Citation[19]
Female Gender
Ovarian hormones can affect the renin-angiotensin system and renal blood flow.Citation[45] In a retrospective study of 8,628 patients who underwent percutaneous coronary intervention, female sex was found as an independent predictor of CIN (OR=1.4, p < 0.0001), And one-year outcome analyses by gender showed a higher mortality among females than males in the CIN patients (14% vs. 10%, p = 0.05).Citation[41] The findings of this study contradict those of a previous randomized controlled trial of ionic vs. non-ionic contrast media, in which a multivariate analysis identified male gender as an independent risk factor for CIN.Citation[5] Whether female gender is an independent predictor of CIN will require further studies.
Contrast Media-Related Risk Factors
Increased Dose of Contrast Medium
According to different sources, the relatively safe cutoff point of contrast amount varies from 70 ml up to 220 ml.Citation[23],Citation[46],Citation[47] However, doses as low as 20 to 30 ml are capable of inducing CIN. In a study of patients undergoing coronary angiography, each 100 ml of contrast medium administered was associated with a significant increase of 12% in the risk of CIN (OR = 1.12, p= 0.02).Citation[12] Marenzi et al. showed that contrast volume >300 ml is an independent risk for CIN (OR = 2.80, p = 0.02).Citation[19] In another study, patients with preexisting renal failure revealed a ten-fold risk of CIN when more than 125 ml of contrast media was administered.Citation[48]
High-Osmolar and Ionic Contrast Media
Most side effects attributable to contrast medias are related to hypertonicity. Currently, four main types of contrast media are used in routine practice today: nonionic low-osmolar, ionic low-osmolar, nonionic iso-osmolar, and ionic high-osmolar contrast media.Citation[49] In a large study comparing the non-ionic low-osmolality agent iohexol to the ionic high-osmolality agent meglumine/sodium diatrizoate in patients with pre-existing renal dysfunction undergoing angiography, patients with renal insufficiency receiving diatrizoate were 3.3 times as likely to develop CIN compared to those receiving iohexol.Citation[5] However, the risk of CIN in patients with healthy renal function is equal to either low-osmolar or high-osmolar contrast media. NEPHRIC trial is a randomized, prospective study comparing the nonionic iso-osmolar contrast media iodixanol with the nonionic low-osmolar contrast media iohexol in 129 renal impairment patients with diabetes undergoing coronary or aorta-femoral angiography. The incidence of CIN was 3% in the iodixanol group and 26% in the iohexol group (p = 0.002).Citation[6] In another randomized study, the renal tolerance of iodixanol and iohexol was compared in 124 patients with creatinine >1.7 mg/dL. The incidence of CIN was 3.7% in the iodixanol group and 10% in the iohexol group (p > 0.05).Citation[50] The available data do not provide clear evidence that the whole iso-osmolar contrast media class offers an improvement over the low-osmolar CM class. Other studies with iodixanol in renal failure patients have shown a higher incidence of CIN than that observed in the NEPHRIC study (21% in the RAPPID trial, 30% in the CONTRAST trial).Citation[51],Citation[52] In an animal study, the decline in medullary oxygen tension was similar in both low- and iso-osmolar contrast media.Citation[28],Citation[53]
In addition to their osmolarity, contrast media are characterized as ionic versus non-ionic. Small clinical trials of low-risk patients undergoing coronary angiography have shown little difference in the risk of CIN between the two types of contrast media. However, a randomized trial of 1196 patients undergoing coronary angiography showed that non-ionic contrast media reduced the incidence of CIN in patients with preexisting renal disease with or without diabetes.Citation[5] In addition, symptomatic or hemodynamic adverse drug events have been shown to occur less often with non-ionic, low-osmolality contrast media compared with ionic, high-osmolality contrast media. In an experimental study, non-ionic contrast media was less nephrotoxic in rat renal cortical slices in vitro.Citation[54] In high-risk patients, it is reasonable to not use the high-osmolar and ionic contrast media to minimize the risk of CIN.Citation[14]
Multiple Infusions of Contrast Media within a Short Period of Time and Urgent Procedure
In those who have no risk factors for CIN, angiography should be delayed more than 48 hours after a previous exposure to intravascular contrast media. In patients with diabetes or preexisting renal disease, this time interval should be increased to more than 72 hours.Citation[2],Citation[7],Citation[8] In a cohort study by Bartholomew et al., the urgent/emergency procedure was found as a predictor of CIN (OR = 4, p < 0.0001).Citation[39] The higher risk of developing CIN in patients with urgent status was irrespective of baseline renal function.
Other CIN Risk Factors
Hyperuricemia
It has been suggested that tubular obstruction by uric acid plays a role in the pathogenesis of CIN. There are only two studies examining the relationship between the contrast media and uric acid. According to these studies, contrast agents have a uricosuric effect, which appears to be caused by enhanced renal tubular secretion of uric acid.Citation[55],Citation[56] Furthermore, hyperuricemia is accompanied by the enhanced synthesis of reactive oxygen species, an activated renin-angiotensin-aldosterone system, the increased endothelin-1, and the inhibited nitric oxide system, which plays a role in the pathogenesis of CIN.Citation[57]
Hypercholesterolemia
Altered nitrovasodilation, which is important in the pathogenesis of CIN, is prevalent in hypercholesterolemia. In the literature, there are only two experimental studies of the relationship between hypercholesterolemia and CIN.Citation[58],Citation[59] According to these studies, hypercholesterolemia aggravates CIN through the reduced production of nitric oxide.
Intra-Arterial Administration of the Contrast Media
Intra-arterial contrast administration is a risk for CIN.Citation[2],Citation[60] This effect is thought to be due to the fact that the acute intrarenal concentration of contrast media is much higher after intra-arterial rather than intravenous injection.
Sepsis, Shock, Cirrhosis, and Pulmonary Edema
Pulmonary edema was an independent predictor of CIN in patients with chronic kidney disease in a study by Dangas et al. (OR = 2.56, p = 0.001).Citation[18] Through the direct damage by bacterial toxins to renal tubules and impairment of circulation, sepsis has also been reported as a risk factor.Citation[2] The reduction of effective intravascular volume due to liver cirrhosis has been reported as contributing to prerenal reduction in renal perfusion, thus enhancing the ischemic insult of contrast media.Citation[2] Preprocedure shock was an independent predictor of CIN (OR = 1.19, p = 0.05) in a study by Rihal et al.[12]
Multi-Vessel Coronary Involvement and Renal Artery Stenosis
Factors related to accelerated or diffuse atherosclerosis are linked to the development of CIN.Citation[18] The treatment of multi-vessel disease, challenging chronic total occlusions and extensively diseased coronary segments, may require high doses of contrast media to provide an optimal image quality, thus enhancing the potential toxic effects on the renal function. If a patient has multi-vessel coronary involvement, the other vessels in the body, such as the renal artery, can be involved. If the renal artery is involved, the renal blood supply may decrease, and the kidneys may be more susceptible for CIN. In a study of 177 patients undergoing cardiac catheterization, subjects were also evaluated for renal artery stenosis. Coronary artery disease was detected in 110 patients (62%) and significant renal artery stenosis in 19 patients (11%). Using multivariate analysis, the authors found that the extent of coronary artery disease was an independent predictor of renal artery stenosis.Citation[61] We found only one study that evaluated multi-vessel coronary involvement as a risk predictor of CIN in addition to the well-known CIN risk factors. A total of 5571 patients who underwent percutaneous coronary intervention were evaluated for CIN risk factors, and the authors found that multi-vessel coronary involvement was only a univariate predictor of CIN (p= 0.003).Citation[22]
Renal Transplantation
Patients with renal transplantation may be at a higher risk of CIN due to concomitant use of cyclosporine and the higher prevalence of diabetes and renal insufficiency. In a study by Ahuja et al., 33 patients with functioning renal allograft who underwent different contrast studies were studied, and the incidence of CIN was 21.2%.Citation[62]
Multiple Myeloma
Multiple myeloma has been suggested as a potential risk factor for CIN on the basis of initial reports. The pathomechanism of this process has been explained by the precipitation of CM molecules together with Tamm-Horsfall proteins and other abnormal proteins, tubular epithelial cells damaged and desquamated as a result of ischemia, direct contrast toxicity, or the disturbed function of integrins. In animal studies, intratubular light chains, particularly in the setting of intravascular volume depletion, augment the nephrotoxic potential of contrast media.Citation[33],Citation[63] Larger studies have since shown that the observed risk is linked to coexisting risk factors, such as preexisting renal insufficiency, low circulating volume, proteinuria, amyloidosis, hyperuricemia, and hypercalcemia rather than to the myeloma itself.Citation[2] Retrospective studies showed an incidence of CIN of only 0.6–1.25% in patients with myeloma if dehydration is avoided.Citation[64]
Hypoalbuminemia
Hypoalbuminemia impairs endothelial function, enhances renal vasoconstriction, impairs the synthesis and release of nitric oxide, and decreases antioxidant enzyme activity.Citation[65] In the literature, only one study of the association between serum albumin and CIN was found. In this study, low serum albumin (< 3.5gr/dL) was identified as a risk factor for CIN in patients 70 years of age or older who underwent cardiac catheterization.Citation[11]
Metformin
The oral antidiabetic agent metformin is not itself nephrotoxic, but it has been known that patients who are receiving metformin may develop lactic acidosis as a result of CIN. A decline in renal function after contrast exposure could adversely affect the clearance of metformin. The complication was almost always observed in diabetic patients with decreased renal function before injection of contrast media.Citation[7],Citation[28],Citation[66]
Low Hematocrit Level
The anemia-induced deterioration of renal ischemia may be one plausible explanation of the higher incidence of CIN in patients with low hematocrit. The relationship between low hematocrit and CIN has been investigated by Nikolsky et al. in a prospective study of 6,773 patients undergoing percutaneous coronary intervention. A lower baseline hematocrit was an independent predictor of CIN; each 3% decrease in baseline hematocrit resulted in a significant increase in the odds of CIN in patients with and without chronic kidney disease (11% and 23%, respectively).Citation[67] Dangas et al. showed that baseline hematocrit was an independent predictor of CIN in patients with chronic kidney disease (OR = 0.95, p < 0.00001).Citation[18]
Prevention Strategies for Contrast-Induced Nephropathy
The treatment of established CIN is limited to supportive measures and dialysis. For this reason, screening for high-risk patients before contrast media, including cardiac procedures, and taking the appropriate prophylactic regimens are important in reducing CIN. The main prevention strategies for CIN are extracellular volume expansion with intravenous saline or sodium bicarbonate; minimizing the dose of contrast media; using low-osmolar non-ionic contrast media instead of high-osmolar ionic contrast media; stopping the intake of nephrotoxic drugs, such as diuretics, nonsteroidal anti-inflammatory drugs, and COX-2 inhibitors; and avoiding short intervals between procedures requiring contrast media. Alternatives to ordinary contrast media, such as carbon dioxide or gadolinium chelates, can be used in patients at high risk of CIN (see ).Citation[36],Citation[49],Citation[68],Citation[69]
Table 2. Prevention strategies for contrast-induced nephropathy in high-risk patients
Volume Expansion
Volume expansion is the single most important documented measure that is beneficial in preventing CIN.Citation[68],Citation[69] A standardized saline hydration protocol has been proven effective in reducing the risk of CIN and should be used routinely. The most widely accepted protocol is administering isotonic saline at 1 to 1.5 mL/kg/h beginning 6 to 12 hours prior to the procedure and continuing for up to 12 hours following contrast administration.Citation[1–3] Three prospective randomized studies evaluated different hydration protocols in patients undergoing procedures requiring contrast media. In a randomized trial, two different hydration regimens were compared in 1620 patients undergoing coronary interventions. They showed that the incidence of CIN was significantly lower among patients given an isotonic saline solution than among those given a hypotonic saline solution (0.7% vs. 2.0% respectively, p = 0.04).Citation[70] It seems reasonable to advocate the use of isotonic saline compared with half-isotonic saline; however, adequate hydration is mandated regardless of whether isotonic or half-isotonic saline is used.
In the second trial, a total of 119 patients with serum creatinine exceeding 1.1 mg/dL were randomized to receive isotonic solution of sodium bicarbonate (n=59) or isotonic saline (n=60) at a rate of 3 mL/kg/h for 1 hour before and 1 mL/kg/h for 6 hours after contrast administration. CIN developed in only 1 patient (1.7%), compared with 8 patients (13.6%) in the saline group (p = 0.02).Citation[71] The authors postulated that a reduction in oxidative injury may have conferred protection against CIN. However, further studies are required to clarify the role of hydration with sodium bicarbonate in preventing CIN. In a recent prospective study, the effect of combination intravenous and oral volume supplementation on the development of CIN was studied in 425 patients undergoing percutaneous coronary intervention. Patients were randomly assigned to receive hydration with either isotonic or half-isotonic. In addition, patients were encouraged to drink plenty of fluids (at least 1500 ml). They found that applying the combination of intravenous and oral volume supplementation results in a very low incidence of CIN (1.4%).Citation[72]
Most studies have found that hydration alone is better than hydration combined with a diuretic. In a study, 78 patients with serum creatinine >1.6 mg/dL were randomized to three groups: hydration alone, hydration with mannitol, and hydration with furosemide. Half-isotonic saline was used for hydration. CIN occurred in 11%, 28%, and 40% of patients in the three groups, respectively (p = 0.02), thus showing that forced diuresis is of no benefit in preventing CIN.Citation[73]
Antioxidants
N-acetylcysteine
It had been postulated that antioxidant N-acetylcysteine (NAC) might scavenge oxygen free radicals and thus attenuate the cytotoxic effects of contrast media. NAC may also have direct vasodilating effects on the kidneys through an increase in the biologic effects of nitric oxide, which is a potent and stable vasodilator contributing to improved renal hemodynamics.Citation[74],Citation[75]
Tepel et al. first evaluated the effects of NAC (600 mg orally twice daily) in 83 patients undergoing computed tomography. Two percent of the patients in the NAC group had CIN versus 21% in the placebo group (p = 0.01).Citation[74] Since then, a number of trials have been published, though the results from these trials have been inconsistent. These findings by Tepel et al. were confirmed by Diaz-Sandoval et al. in a randomized, placebo-controlled study that also found NAC to be protective against CIN. Fifty-four patients were randomized to receive either 600 mg of NAC twice daily for 4 doses or placebo. The incidence of CIN was 8% in the NAC group versus 45% in the placebo group (p = 0.005).Citation[76]
In addition to oral administration, intravenous administration of NAC to protect against CIN has also been evaluated. In another study, Baker et al. randomly assigned 80 patients to receive either NAC infusion (n = 41) versus saline infusion (n = 39). CIN developed in only 2 (5%) of patients in the NAC group compared with 8 (21%) in the saline group (p = 0.04).Citation[51] The authors concluded that NAC infusion protects against CIN. A meta-analysis by Birck et al. evaluating more than 800 patients at high risk of developing CIN also documented a positive impact of NAC prophylaxis on CIN.Citation[77] In another recent meta-analysis, nine randomized controlled trials were included, and the difference in mean change in creatinine between the NAC-treated group and controls was -0.27 mg/dL. The relative risk of developing CIN was 0.43 in subjects randomized to NAC.Citation[78] They suggest that NAC helps prevent declining renal function and CIN.
In contrast to these reports, a study by Briguori et al. failed to find a significant effect of NAC on the occurrence of CIN. A total of 183 patients with preexisting renal insufficiency undergoing contrast study were randomly assigned to receive NAC at a dose of 600 mg twice daily on the day before and the day of the contrast study plus saline infusion or saline alone. The incidence of CIN was 6.5% in the NAC group versus 11% in the control group (p = 0.22).Citation[79] In a recent multicenter double-blind clinical trial, 156 patients undergoing coronary angiography or percutaneous coronary intervention with creatinine clearance <50 mL/min were randomly assigned to receive N‐acetylcysteine 600 mg orally twice daily for two days or placebo. Sixteen patients developed CIN, eight of 77 patients (10.4%) in the NAC group and eight of 79 patients (10.1%) in the placebo group (p = 1.00). No difference was observed in the change in endogenous creatinine clearance (p = 0.28). They concluded that oral NAC did not prevent CIN in patients at low to moderate risk undergoing cardiac catheterization with ionic low osmolality contrast medium.Citation[80]
In another recent study, 50 patients undergoing elective diagnostic coronary angiography with serum creatinine values above 1.3 mg/dL were included, and CIN was detected in 3 of 25 patients (12%) in the NAC group and 2 of 25 patients (8%) in the control group (p > 0.05). It was determined that in patients who were scheduled to undergo elective diagnostic coronary angiography with renal dysfunction, oral NAC and hydration before the procedure was not more effective than hydration alone in the prevention of CIN.Citation[81] A direct renoprotective effect of NAC remains questionable. To date, only a few trials described the effects of NAC not only on serum creatinine but also on clinical end points. The serum creatinine can be decreased in the administration of NAC without a renoprotective effect.Citation[82]
In a prospective study, NAC was given at a dose of 600 mg every 12 h for a total of four doses to the volunteers with a normal renal function who did not receive contrast agent. There was a significant decrease of the mean serum creatinine (p < 0.05) and a significant increase of the GFR (p < 0.02), whereas the cystatin C concentration did not change significantly.Citation[83] In patients undergoing emergency diagnostic procedures and in whom a full hydration protocol is not possible, an abbreviated hydration regimen plus oral or intravenous administration of NAC can be recommended. NAC may be of benefit mostly in high-risk patients. Furthermore, before renal protective effects of NAC in CIN are proposed, any direct effects of NAC on creatinine should be addressed, the GFR should be measured directly, and/or additional markers of the renal function and hard end points should be considered in the design of studies of CIN. If NAC is to be used as a preventative measure, it should be given at a dose of 600 mg orally twice daily on the day before and day of the procedure.Citation[8],Citation[68],Citation[69]
Ascorbic Acid
Prophylactic oral administration of ascorbic acid may protect against CIN. A recent randomized, placebo-controlled trial in 231 patients with serum creatinine concentration ≥1.2 mg/dL who underwent coronary angiography showed that the use of ascorbic acid was associated with a significant reduction in the rate of CIN. CIN occurred in 11 of the 118 patients (9%) in the ascorbic acid group and 23 of the 113 patients (20%) in the placebo group (OR = 0.38; p = 0.02).Citation[84] Further prospective studies are needed to validate these preliminary results.
Vasodilators
Fenoldopam
Fenoldopam mesylate is a selective dopamine-1 receptor agonist that produces systemic, peripheral, and renal arterial vasodilatation. Several investigators have reported a positive impact of fenoldopam against CIN in small studies.Citation[85],Citation[86] In a placebo-controlled, double-blind, multicenter trial, 315 patients with creatinine clearance of less than 60 mL/min were randomized to receive fenoldopam infusion [0.05 μg/kg/min titrated to 0.1 μg/kg/min (n = 157)] or matching placebo (n = 158). CIN occurred in 33.6% of patients in the fenoldopam group compared with 30.1% of patients in the placebo group (p = 0.61).Citation[52] The authors concluded that fenoldopam did not protect against CIN. In two other large studies comparing fenoldopam plus NAC treatment with fenoldopam alone, it was found that both had a similar, non-significant effect to that of NAC or were inferior to it.Citation[87],Citation[88] Therefore, the routine use of fenoldopam cannot be recommended at the present time.
Adenosine Antagonists
Contrast media stimulate the intrarenal secretion of adenosine, which binds to the renal adenosine receptor and acts as a potent vasoconstrictor, reducing renal blood flow and increasing the generation of oxygen free radicals as it is metabolized to xanthine and hypoxanthine. Theophylline and aminophylline, adenosine antagonists, have also been studied in the prevention of CIN in a number of trials. Studies with these agents have used varying doses and dosage forms and yielded conflicting results. Based on the conflicting information found in clinical studies, adenosine antagonists should not be routinely used in patients as a preventative measure at this time.Citation[89],Citation[90]
Calcium Channel Blockers
The calcium channel antagonists verapamil and diltiazem have been found to attenuate the renal vasoconstrictor response after exposure to contrast media. However, when the efficacy of the felodipine, nitrendipine, and nifedipine was evaluated, results were inconsistent. Two small studies examined the use of sublingual nifedipine given prior to contrast administration. Patients (n=20) who received sublingual nifedipine did not have a significant increase in serum creatinine, while those in the placebo group did.Citation[91] In another study, patients (n = 30) who received nifedipine showed an increase in renal plasma flow following the administration of contrast media, while patients in the placebo group had a decrease in renal flow.Citation[92] One other study that examined the effect of nitrendipine on CIN showed that after contrast administration, the GFR decreased in the placebo group, but there was no decrease in GFR in the nitrendipine group.Citation[93] In another study, 27 patients with normal to moderately reduced renal function underwent femoral angiography randomized to receive either oral felodipine or placebo. Patients in the felodipine group had a significant increase in serum creatinine from baseline, while patients in the placebo group did not demonstrate a similar increase.Citation[94] More large-scale trials are needed before calcium channel blockers can be routinely recommended in patients prior to contrast administration.
ACE Inhibitors
In some studies, ACE inhibitors have been identified as a risk factor for CIN due to their potential to reduce renal function. Some studies have shown that the nephrotoxicity of contrast media may be reduced due to decreased renal vasoconstriction by inhibition of angiotensin II. Caldicott et al. showed that renal vasoconstriction occurs after the contrast media administration, and the renin-angiotensin system is responsible for this vasoconstriction.Citation[95] In another animal study, after contrast media administration, there were no significant changes in angiotensin II and renin levels. They found that the suppression of prostacyclin contributed to renal function changes rather than the activation of the renin–angiotensin system.Citation[96] In a controlled study with 71 diabetic patients undergoing coronary angiography randomized to either captopril or control, captopril 25 mg was given three times daily. There was a significant decrease in CIN in the patients who received captopril as compared with the control group (6% vs 29%, respectively, p < 0.02).Citation[97] The current authors have performed a randomized controlled study in 80 patients with serum creatinine below 2 mg/dL undergoing coronary angiography. Captopril was administered in 48 patients before coronary angiography. Five patients (10.4%) in the captopril group developed CIN, compared with only one patient (3.1%) in the control group (p = 0.02).Citation[98] Until further studies are able to characterize the pathophysiologic and clinical effect of ACE inhibitors on contrast media, no firm recommendations can be made regarding their use or discontinuation.
Prostaglandin E1 (PGE1)
PGE1 has vasodilatory effects that may be beneficial in preventing CIN. In one study, 130 patients were randomly assigned to receive either placebo or one of three doses of PGE1. The increase in serum creatinine level was smaller in all of the three PGE1 groups than in the placebo group, but the difference was significant only in the medium-dose (20 ng/kg/min) of PGE1 group.Citation[99] More studies need to be done to better understand the role of prostaglandin E1, but results from this pilot study appear promising.
Atrial Natriuretic Peptide (ANP)
ANP may prevent CIN by increasing renal blood flow. Clinical studies have failed to support this hypothesis. In one study, ANP was included in one of the four arms in which dopamine, mannitol, and ANP caused an increase in CIN in diabetic patients as compared to saline alone.Citation[100] In another randomized, double-blind, placebo-controlled trial, 257 patients underwent a radiographic procedure with contrast media. Patients were randomized to one of four treatment arms: fluid alone or one of three doses of ANP. Results showed no statistically significant differences in the incidence of CIN between any of the four treatment arms.Citation[101] Based on these results and the limited clinical data, ANP cannot be advocated in the prevention of CIN.
Endothelin Antagonists
Endothelin-1 is a potent endogenous vasoconstrictor that is thought to play a role in the development of CIN. Endothelin-1 has two primary receptors: endothelin-A is found in vascular smooth muscle and causes vasoconstriction, while endothelin-B is found primarily in endothelial cells and causes the release of prostacyclin and nitric oxide, leading to vasodilatation. In animal studies, endothelin-A antagonists were shown to reduce the incidence of CIN.Citation[102] However, in a randomized study of 158 patients, the use of a mixed endothelin-A and -B antagonist was associated with a significantly higher incidence of CIN than was placebo (56% vs. 29%, p = 0.002).Citation[103] Endothelin antagonists currently have no role in prevention of CIN.
Low Dose of Dopamine
At low doses (1–3 mcg/kg/min), dopamine activates two types of dopamine (DA) receptors, DA-1 and DA-2. The activation of the DA-1 receptor results in an increase in natriuresis and renal blood flow. Because dopamine at low doses is believed to be more selective for the DA-1 receptors, it has been investigated in the prevention of CIN.
Kapoor et al. randomized 40 patients with diabetes scheduled to undergo a coronary angiography to either dopamine or placebo control. None of the patients in the dopamine group developed CIN, compared to 50% of patients receiving placebo.Citation[104] In another prospective, randomized trial, Hans et al. evaluated 55 patients (40% had diabetes) with chronic renal insufficiency. Patients were randomized to receive dopamine or an equal volume of saline. The group receiving dopamine had a significantly lower incidence of CIN as compared to the control group.Citation[105] In contrast to the trials showing a potential benefit of dopamine, other studies have failed to demonstrate this benefit. Abizaid et al. performed a randomized, prospective study involving patients with renal insufficiency that underwent coronary angioplasty. Patients were randomized to continue with the saline, receiving aminophylline in addition to the saline or dopamine plus saline. In the dopamine plus saline group, 50% of patients developed CIN, while only 30% of the patients in the saline-alone group developed CIN. This difference did not reach statistical significance, but it appeared that use of dopamine might worsen outcomes.Citation[106]
Looking at the results from the studies, low-dose dopamine use cannot be supported at this time.
Hemofiltration and Hemodialysis
Currently available data do not support the use of prophylactic hemodialysis for prevention of CIN and may even increase the risk of CIN. A study of 113 patients reported that CIN occurred in 24% of the hemodialysis group as compared with 16% of non-hemodialysis group. Clinically relevant events also were not different in two groups.Citation[107] Further trials are needed to clarify the strategy of performing hemodialysis immediately after the administration of contrast media. Only continuous venovenous hemofiltration has been shown to protect against CIN. In a study, 114 patients with chronic renal failure undergoing percutaneous coronary intervention were divided in two groups: 56 patients received normal saline and 58 patients underwent hemofiltration at a rate of 1000 mL/h. Hemofiltration seems to have a protective effect, including a significant reduction in in-hospital and one-year mortality compared with routine hydration (90% reduction in the incidence of CIN, 88% reduction in requirement of dialysis, 86% reduction in in-hospital mortality, and 67% reduction in one-year mortality).Citation[108] The mechanisms of this benefit are not clear. Further studies are needed to confirm the results of this trial and elucidate the mechanisms involved.
New Types of Contrast Media
Gadolinum-based contrast agents are believed to be safe. Therefore, it has been suggested that gadolinium-based contrast media could be used in place of iodinated contrast agents for radiological examinations in patients with significant renal impairment.Citation[109] In a study by Hoffmann et al., the effect of gadopentetate dimeglumine (iodine-based contrast agent) was studied in 181 patients with normal renal function, and the effect of gadolinium was studied in 198 patients with pre-existing renal failure. There was no statistically significant change in serum creatinine concentration after the administration of gadopentetate dimeglumine. In contrast, serum creatinine levels decreased significantly after the administration of gadolinium (p < 0.01). The high diagnostic value of gadolinium contrast media was associated with a very small risk of adverse reactions.Citation[110] But other studies have different results. In a recent retrospective study, the safety of gadolinium was evaluated in 91 patients with stage 3 and 4 renal failure who underwent angiographic MRI procedures. Eleven of 91 patients developed CIN (12.1%). According to the study, CIN can occur after gadolinium-based contrast agents in patients with moderate to severe chronic renal failure.Citation[111] In another study, gadobutrol, a gadolinium-based contrast media, was compared with standard iohexol, an iodinated contrast media, in 21 patients with renal dysfunction in a randomized clinical study. In both groups, GFR decreased significantly two days after the contrast exposure. The incidence of CIN was 50% in gadobutrol group and 45% in iohexol group (p = 0.70). No patient demonstrated other adverse effects of gadobutrol or iohexol administration. In this study, gadolinium-based angiography showed no benefit over iohexol in patients with severely impaired renal function.Citation[112] More studies need to be done to better understand the role of gadolinium-based contrast agents on CIN.
Ultrasound contrast agents are micro-bubbles that produce acoustic enhancement. They are pharmacologically almost inert and safe.Citation[113]
Conclusions
The development of CIN is associated with adverse outcomes, including prolonged hospitalization, the potential need for renal replacement therapy, and, most importantly, increased mortality. A careful evaluation of each patient who is to receive intravascular contrast media for the presence of underlying risk factors for CIN is essential. Pre-existing renal dysfunction, especially when secondary to diabetic nephropathy, is the most important risk factor. Extracellular volume expansion and the use of low osmolar contrast media are the two most effective measures to prevent CIN. Acetylcysteine may be used in high-risk patients, but this finding has not been uniform or always demonstrated by currently available trials.
References
- McCullough PA, Soman SS. Contrast-induced nephropathy. Crit Care Clin. 2005; 21: 261–280, [INFOTRIEVE], [CSA]
- Gleeson TG, Bulugahapitiya S. Contrast-induced nephropathy. Am J Roentgenol. 2004; 183: 1673–1689, [CSA]
- Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004; 79: 211–219, [INFOTRIEVE], [CSA]
- Itoh Y, Yano T, Sendo T, Oishi R. Clinical and experimental evidence for prevention of acute renal failure induced by radiographic contrast media. J Pharmacol Sci. 2005; 97: 473–488, [INFOTRIEVE], [CSA], [CROSSREF]
- Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, Hill JA, Winniford M, Cohen MB, VanFossen DB. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995; 47: 254–261, [INFOTRIEVE], [CSA]
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348: 491–499, [INFOTRIEVE], [CSA], [CROSSREF]
- Morcos SK, Thomsen HS. European Society of Urogenital Radiology guidelines on administering contrast media. Abdom Imaging. 2003; 28: 187–190, [INFOTRIEVE], [CSA], [CROSSREF]
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005; 172: 1461–1471, [INFOTRIEVE], [CSA]
- McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention; incidence, risk factors, and relationships to mortality. Am J Med. 1997; 103: 368–375, [INFOTRIEVE], [CSA], [CROSSREF]
- Bettmann MA. Contrast medium-induced nephropathy: critical review of the existing clinical evidence. Nephrol Dial Transplant 2005; 20(Suppl.)12–17, [CSA], [CROSSREF]
- Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older: a prospective study. Arch Intern Med. 1990; 150: 1237–1242, [INFOTRIEVE], [CSA], [CROSSREF]
- Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR, Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105: 2259–2264, [INFOTRIEVE], [CSA], [CROSSREF]
- Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005; 68: 14–22, [INFOTRIEVE], [CSA], [CROSSREF]
- Detrenis S, Meschi M, Musini S, Savazzi G. Lights and shadows on the pathogenesis of contrast-induced nephropathy: state of the art. Nephrol Dial Transplant. 2005; 20: 1542–1550, [INFOTRIEVE], [CSA], [CROSSREF]
- Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal hemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005; 20(Suppl. 1)6–11, [CSA], [CROSSREF]
- Rudnick MR, Goldfarb S. Pathogenesis of contrast-induced nephropathy: experimental and clinical observations with an emphasis on the role of osmolality. Rev Cardiovasc Med. 2003; 4(Suppl. 5)28–33, [CSA]
- Nygren A, Ulfendahl HR. Effects of high- and low-osmolar contrast media on renal plasma flow and glomerular filtration rate in euvolaemic and dehydrated rats. Acta Radiol. 1989; 30: 383–389, [INFOTRIEVE], [CSA]
- Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005; 95: 13–19, [INFOTRIEVE], [CSA], [CROSSREF]
- Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004; 44: 1780–1785, [INFOTRIEVE], [CSA], [CROSSREF]
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA 1996; 275: 1489–1494, [INFOTRIEVE], [CSA], [CROSSREF]
- Toprak O, Cirit M, Bayata S, Yesil M. Review of the radiocontrast nephropathy risk profiles and risk stratification. Anadolu Kardiyol Derg. 2004; 4: 331–335, [INFOTRIEVE], [CSA]
- Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004; 44: 1393–1399, [INFOTRIEVE], [CSA]
- Nikolsky E, Aymong ED, Dangas G, Mehran R. Radiocontrast nephropathy: identifying the high-risk patient and the implications of exacerbating renal function. Rev Cardiovasc Med. 2003; 4(Suppl.1)7–14, [CSA]
- Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O'Donnell MJ, Moscucci M. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005; 118: 843–849, [INFOTRIEVE], [CSA], [CROSSREF]
- Moore RD, Steinberg EP, Powe NR, Brinker JA, Fishman EK, Graziano S, Gopalan R. Nephrotoxicity of high-osmolarity vs low-osmolarity contrast media: randomized clinical trial. Radiology 1992; 182: 649–655, [INFOTRIEVE], [CSA]
- Barrett BJ, Parfrey PS, Vavasour HM, McDonald J, Kent G, Hefferton D, O'Dea F, Stone E, Reddy R, McManamon PJ. Contrast nephropathy in patients with impaired renal function: high versus low osmolar media. Kidney Int. 1992; 41: 1274–1279, [INFOTRIEVE], [CSA]
- Weinrauch LA, Healy RW, Leland OS, Jr., Goldstein HH, Kassissieh SD, Libertino JA, Takacs FJ, D'Elia JA. Coronary angiography and acute renal failure in diabetic azotemic nephropathy. Ann Intern Med 1977; 86: 56–59, [INFOTRIEVE], [CSA]
- Asif A, Preston RA, Roth D. Radiocontrast-induced nephropathy. Am J Ther. 2003; 10: 137–147, [INFOTRIEVE], [CSA], [CROSSREF]
- Lautin EM, Freeman NJ, Schoenfeld AH, Bakal CW, Haramati N, Friedman AC, Lautin JL, Braha S, Kadish EG, Sprayregen S. Radiocontrast-associated renal dysfunction: incidence and risk factors. AJR Am J Roentgenol. 1991; 157: 49–58, [INFOTRIEVE], [CSA]
- Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989; 320: 143–149, [INFOTRIEVE], [CSA]
- Jabs K, Zeidel ML, Silvia P. Prostaglandin E2 inhibits Na-K ATPase activity in the inner medullary collecting duct. Am J Physiol. 1989; 257: 424–430, [CSA]
- Kolonko A, Kokot F, Wiecek A. Contrast-associated nephropathy—old clinical problem and new therapeutic perspectives. Nephrol Dial Transplant. 1998; 13: 803–806, [INFOTRIEVE], [CSA], [CROSSREF]
- Weisbord SD, Palevsky PM. Radiocontrast-induced acute renal failure. J Intensive Care Med. 2005; 20: 63–75, [INFOTRIEVE], [CSA], [CROSSREF]
- Dudzinski PJ, Petrone AF, Persoff M, Callaghan EE. Acute renal failure following high dose excretory urography in dehydrated patients. J Urol. 1971; 106: 619–621, [INFOTRIEVE], [CSA]
- Katzberg RW. Contrast medium-induced nephrotoxicity: which pathway?. Radiology. 2005; 235: 752–755, [INFOTRIEVE], [CSA]
- Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004; 44: 1763–1771, [INFOTRIEVE], [CSA], [CROSSREF]
- Toprak O, Cirit M. Investigating the volume status before contrast nephropathy studies. Nephrol Dial Transplant. 2005; 20: 464, [INFOTRIEVE], [CSA], [CROSSREF]
- Toprak O, Cirit M, Bayata S, Aslan SL, Sarioglu F, Cetinkaya GS. Is there any relationship between left ventricul ejection fraction and contrast induced nephropathy?. Turkiye Klinikleri J Med Sci. 2003; 23: 104–107, [CSA]
- Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O'Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004; 93: 1515–1519, [INFOTRIEVE], [CSA], [CROSSREF]
- Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Fahy M, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Stuckey TD, Turco M, Carroll JD. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003; 108: 2769–2775, [INFOTRIEVE], [CSA], [CROSSREF]
- Iakovou I, Dangas G, Mehran R, Lansky AJ, Ashby DT, Fahy M, Mintz GS, Kent KM, Pichard AD, Satler LF, Stone GW, Leon MB. Impact of gender on the incidence and outcome of contrast-induced nephropathy after percutaneous coronary intervention. J Invasive Cardiol. 2003; 15: 18–22, [INFOTRIEVE], [CSA]
- Gruberg L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, Pichard AD, Satler LF, Wu H, Leon MB. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001; 52: 409–416, [INFOTRIEVE], [CSA], [CROSSREF]
- Prasad PV, Epstein FH. Changes in medullary pO2 during water diuresis as evaluated by blood oxygen level-dependent magnetic resonance imaging. Kidney Int. 1999; 55: 294–298, [INFOTRIEVE], [CSA], [CROSSREF]
- Kohli HS, Bhaskaran MC, Muthukumar T, Thennarasu K, Sud K, Jha V, Gupta KL, Sakhuja V. Treatment-related acute renal failure in the elderly: a hospital-based prospective study. Nephrol Dial Transplant. 2000; 15: 212–217, [INFOTRIEVE], [CSA], [CROSSREF]
- Kisley LR, Sakai RR, Flanagan-Cato LM, Fluharty SJ. Estrogen increases angiotensin II-induced c-Fos expression in the vasopressinergic neurons of the paraventricular nucleus in the female rat. Neuroendocrinology. 2000; 72: 306–317, [INFOTRIEVE], [CSA], [CROSSREF]
- McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003; 4(Suppl. 5)3–9, [CSA]
- Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989; 86: 649–652, [INFOTRIEVE], [CSA], [CROSSREF]
- Taliercio CP, Vlietstra RE, Fisher LD, Burnett JC. Risks for renal dysfunction with cardiac angiography. Ann Intern Med. 1986; 104: 501–504, [INFOTRIEVE], [CSA]
- Lin J, Bonventre JV. Prevention of radiocontrast nephropathy. Curr Opin Nephrol Hypertens. 2005; 14: 105–110, [INFOTRIEVE], [CSA]
- Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999; 72: 701–703, [INFOTRIEVE], [CSA]
- Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-inducted renal dysfunction: the RAPPID Study. J Am Coll Cardiol. 2003; 41: 2114–2118, [INFOTRIEVE], [CSA]
- Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O'Neill WW. CONTRAST Investigators. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003; 290: 2284–2291, [INFOTRIEVE], [CSA]
- Liss P. Effects of contrast media on renal microcirculation and oxygen tension: an experimental study in the rat. Acta Radiol 1997; 409(Suppl.)1–29, [CSA]
- Soejima K, Uozumi J, Kanou T, Fujiyama C, Masaki Z. Nonionic contrast media are less nephrotoxic than ionic contrast media to rat renal cortical slices. Toxicol Lett. 2003; 143: 17–25, [INFOTRIEVE], [CSA], [CROSSREF]
- Mudge GH. Uricosuric action of cholecystographic agents: possible nephrotoxicity. N Engl J Med. 1971; 284: 929–933, [INFOTRIEVE], [CSA]
- Postlethwaite AE, Kelley WN. Uricosuric effect of radiocontrast agents: a study in man of four commonly used preparations. Ann Intern Med. 1971; 74: 845–852, [INFOTRIEVE], [CSA]
- Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, Hollenberg NK, Fisher ND. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int. 2004; 66: 1465–1470, [INFOTRIEVE], [CSA], [CROSSREF]
- Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of arginine. Kidney Int. 1998; 53: 1736–1742, [INFOTRIEVE], [CSA], [CROSSREF]
- Yang DW, Jia RH, Yang DP, Ding GH, Huang CX. Dietary hypercholesterolemia aggravates contrast media-induced nephropathy. Chin Med J. 2004; 117: 542–546, [INFOTRIEVE], [CSA]
- Byrd L, Sherman RL. Radiocontrast-induced acute renal failure: a clinical and pathophysiologic review. Medicine. 1979; 58: 270–279, [INFOTRIEVE], [CSA]
- Weber-Mzell D, Kotanko P, Schumacher M, Klein W, Skrabal F. Coronary anatomy predicts presence or absence of renal artery stenosis: a prospective study in patients undergoing cardiac catheterization for suspected coronary artery disease. Eur Heart J. 2002; 23: 1684–1691, [INFOTRIEVE], [CSA]
- Ahuja TS, Niaz N, Agraharkar M. Contrast-induced nephrotoxicity in renal allograft recipients. Clin Nephrol. 2000; 54: 11–14, [INFOTRIEVE], [CSA]
- Holland MD, Galla JH, Sanders PW, Luke RG. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985; 27: 46–50, [INFOTRIEVE], [CSA]
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology. 1992; 183: 519–521, [INFOTRIEVE], [CSA]
- Vuong TD, Braam B, Willekes-Koolschijn N, Boer P, Koomans HA, Joles JA. Hypoalbuminemia enhances the renal vasoconstrictor effect of lysophosphatidylcholine. Nephrol Dial Transplant. 2003; 18: 1485–1492, [INFOTRIEVE], [CSA], [CROSSREF]
- Nawaz S, Cleveland T, Gaines PA, Chan P. Clinical risk associated with contrast angiography in metformin treated patients: a clinical review. Clin Radiol. 1998; 53: 342–344, [INFOTRIEVE], [CSA], [CROSSREF]
- Nikolsky E, Mehran R, Lasic Z, Mintz GS, Lansky AJ, Na Y, Pocock S, Negoita M, Moussa I, Stone GW, Moses JW, Leon MB, Dangas G. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005; 67: 706–713, [INFOTRIEVE], [CSA], [CROSSREF]
- Ide JM, Lancelot E, Pines E, Corot C. Prophylaxis of iodinated contrast media-induced nephropathy: a pharmacological point of view. Invest Radiol. 2004; 39: 155–170, [INFOTRIEVE], [CSA]
- Asif A, Epstein M. Prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 2004; 44: 12–24, [INFOTRIEVE], [CSA], [CROSSREF]
- Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of two hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002; 162: 329–336, [INFOTRIEVE], [CSA], [CROSSREF]
- Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA III, Rittase RA, Norton HJ, Kennedy TP. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004; 291: 2328–2334, [INFOTRIEVE], [CSA], [CROSSREF]
- Mueller C, Seidensticker P, Buettner HJ, Perruchoud AP, Staub D, Christ A, Buerkle G. Incidence of contrast nephropathy in patients receiving comprehensive intravenous and oral hydration. Swiss Med Wkly. 2005; 135: 286–290, [INFOTRIEVE], [CSA]
- Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994; 331: 1416–1420, [INFOTRIEVE], [CSA], [CROSSREF]
- Tepel M, van Der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000; 343: 180–184, [INFOTRIEVE], [CSA], [CROSSREF]
- Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004; 19: 1803–1807, [INFOTRIEVE], [CSA], [CROSSREF]
- Diaz-Sandoval LJ, Kosowsky BD, Losordo DW. Acetylcysteine to prevent angiography-related renal tissue injury (The APART Trial). Am J Cardiol. 2002; 89: 356–358, [INFOTRIEVE], [CSA], [CROSSREF]
- Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003; 362: 598–603, [INFOTRIEVE], [CSA], [CROSSREF]
- Liu R, Nair D, Ix J, Moore DH, Bent S. N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis. J Gen Intern Med. 2005; 20: 193–200, [INFOTRIEVE], [CSA], [CROSSREF]
- Briguori C, Manganelli F, Scarpato P, Elia PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A, Ricciardelli B. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002; 40: 298–303, [INFOTRIEVE], [CSA], [CROSSREF]
- Gomes VO, Poli de Figueredo CE, Caramori P, Lasevitch R, Bodanese LC, Araujo A, Roedel AP, Caramori AP, Brito FS, Jr.Bezerra HG, Nery P, Brizolara A. N-acetylcysteine does not prevent contrast induced nephropathy after cardiac catheterization with an ionic low osmolality contrast medium: a multicentre clinical trial. Heart. 2005; 91: 774–778, [INFOTRIEVE], [CSA], [CROSSREF]
- Gulel O, Keles T, Eraslan H, Aydogdu S, Diker E, Ulusoy V. Prophylactic acetylcysteine usage for prevention of contrast nephropathy after coronary angiography. J Cardiovasc Pharmacol. 2005; 46: 464–467, [INFOTRIEVE], [CSA], [CROSSREF]
- Tietz NW. Clinical guide to laboratory tests. General Clinical Tests 3rd, NW Tietz. WB Saunders Company, Philadelphia 1995; 186–187
- Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004; 15: 407–410, [INFOTRIEVE], [CSA], [CROSSREF]
- Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, Voudris V, Pavlides G, Buller CE, Kremastinos D, Cokkinos DV. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004; 110: 2837–2842, [INFOTRIEVE], [CSA], [CROSSREF]
- Madyoon H, Croushore L, Weaver D, Mathur V. Use of fenoldopam to prevent radiocontrast nephropathy in high-risk patients. Catheter Cardiovasc Interv. 2001; 53: 341–345, [INFOTRIEVE], [CSA], [CROSSREF]
- Tumlin JA, Wang A, Murray PT, Mathur VS. Fenoldopam mesylate blocks reductions in renal plasma flow after radiocontrast dye infusion: a pilot trial in the prevention of contrast nephropathy. Am Heart J. 2002; 143: 894–903, [INFOTRIEVE], [CSA], [CROSSREF]
- Allaqaband S, Tumuluri R, Malik AM, Gupta A, Volkert P, Shalev Y, Bajwa TK. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv. 2002; 57: 279–283, [INFOTRIEVE], [CSA], [CROSSREF]
- Briguori C, Colombo A, Airoldi F, Violante A, Castelli A, Balestrieri P, Paolo Elia P, Golia B, Lepore S, Riviezzo G, Scarpato P, Librera M, Focaccio A, Ricciardelli B. N-Acetylcysteine versus fenoldopam mesylate to prevent contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2004; 18: 762–765, [CSA], [CROSSREF]
- Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Muller C, Osswald H, Risler T. Prevention of radiocontrast media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999; 14: 1146–1149, [INFOTRIEVE], [CSA], [CROSSREF]
- Kapoor A, Kumar S, Gulati S, Gambhir S, Sethi RS, Sinha N. The role of theophylline in contrast-induced nephropathy: a case-control study. Nephrol Dial Transplant. 2002; 17: 1936–1941, [INFOTRIEVE], [CSA], [CROSSREF]
- Rodicio JL, Morales JM, Ruilope LM. Calcium antagonists and the kidney. Nephrol Dial Transplant. 1990; 5: 81–86, [INFOTRIEVE], [CSA]
- Russo D, Testa A, Della Volpe L, Sansone G. Randomized prospective study on renal effects of two different contrast media in humans: protective role of a calcium channel blocker. Nephron. 1990; 55: 254–257, [INFOTRIEVE], [CSA]
- Neumayer HH, Junge W, Kufner A, Wening A. Prevention of radiocontrast-media-induced nephrotoxicity by the calcium channel blocker nitrendipine: a prospective randomized clinical trial. Nephrol Dial Transplant. 1989; 4: 1030–1036, [INFOTRIEVE], [CSA]
- Spangberg-Viklund B, Berglund J, Nikonoff T, Nyberg P, Skau T, Larsson R. Does prophylactic treatment with felodipine, a calcium antagonist, prevent low-osmolar contrast-induced renal dysfunction in hydrated diabetic and nondiabetic patients with normal or moderately reduced renal function?. Scand J Urol Nephrol. 1996; 30: 63–68, [INFOTRIEVE], [CSA]
- Caldicott WJ, Hollenberg NK, Abrams HL. Characteristics of response of renal vascular bed to contrast media: evidence for vasoconstriction induced by renin-angiotensin system. Invest Radiol. 1970; 5: 539–547, [INFOTRIEVE], [CSA]
- Workman RJ, Shaff MI, Jackson RV, Diggs J, Frazer MG, Briscoe C. Relationship of renal hemodynamic and functional changes following intravascular contrast to the renin-angiotensin system and renal prostacyclin in the dog. Invest Radiol. 1983; 18: 160–166, [INFOTRIEVE], [CSA]
- Gupta RK, Kapoor A, Tewari S, Sinha N, Sharma RK. Captopril for preventing of contrast-induced nephropathy in diabetic patients: a randomized study. Indian Heart J. 1999; 51: 521–526, [INFOTRIEVE], [CSA]
- Toprak O, Cirit M, Bayata S, Yesil M, Aslan LS. The effect of pre-procedural captopril on contrast media induced nephropathy who underwent coronary angiography. Anadolu Kardiyol Derg. 2003; 3: 98–103, [INFOTRIEVE], [CSA]
- Koch JA, Plum J, Grabensee B, Modder U. Prostaglandin E1: a new agent for the prevention of renal dysfunction in high risk patients caused by radiocontrast media?. Nephrol Dial Transplant. 2000; 15: 43–49, [INFOTRIEVE], [CSA], [CROSSREF]
- Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994; 45: 259–265, [INFOTRIEVE], [CSA]
- Kurnik BR, Allgren RL, Genter FC, Solomon RJ, Bates ER, Weisberg LS. Prospective study of atrial natriuretic peptide for the prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 1998; 31: 674–680, [INFOTRIEVE], [CSA]
- Liss P, Carlsson PO, Nygren A, Palm F, Hansell P. ET-A receptor antagonist BQ123 prevents radiocontrast media-induced renal medullary hypoxia. Acta Radiologica. 2003; 44: 111–117, [INFOTRIEVE], [CSA], [CROSSREF]
- Wang A, Holcslaw T, Bashore TM, Freed MI, Miller D, Rudnick MR, Szerlip H, Thames MD, Davidson CJ, Shusterman N, Schwab SJ. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000; 57: 1675–1680, [INFOTRIEVE], [CSA]
- Kapoor A, Sinha N, Sharma RK, Shrivastava S, Radhakrishnan S, Goel PK, Bajaj R. Use of dopamine in prevention of contrast induced acute renal failure: a randomized study. Int J Cardiol. 1996; 53: 233–236, [INFOTRIEVE], [CSA]
- Hans SS, Hans BA, Dhillon R, Dmuchowski C, Glover J. Effect of dopamine on renal function after arteriography in patients with preexisting renal insufficiency. Am Surg. 1998; 34: 1682–1688, [CSA]
- Abizaid AS, Clark CE, Mintz GS, Dosa S, Popma JJ, Pichard AD, Satler LF, Harvey M, Kent KM, Leon MB. Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol. 1999; 83: 260–263, [INFOTRIEVE], [CSA], [CROSSREF]
- Vogt B, Ferrari P, Schonholzer C, Marti HP, Mohaupt M, Wiederkehr M, Cereghetti C, Serra A, Huynh-Do U, Uehlinger D, Frey FJ. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001; 111: 692–698, [INFOTRIEVE], [CSA], [CROSSREF]
- Marenzi G, Marana I, Lauri G, Assanelli E, Grazi M, Campodonico J, Trabattoni D, Fabbiocchi F, Montorsi P, Bartorelli AL. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003; 349: 1333–1340, [INFOTRIEVE], [CSA], [CROSSREF]
- Thomsen HS. How to avoid CIN: guidelines from the European Society of Urogenital Radiology. Nephrol. Dial. Transplant. 2005; 20: 18–22, [CSA], [CROSSREF]
- Hoffmann U, Fischereder M, Reil A, Fischer M, Link J, Kramer BK. Renal effects of gadopentetate dimeglumine in patients with normal and impaired renal function. Eur J Med Res. 2005; 10: 149–154, [INFOTRIEVE], [CSA]
- Ergun I, Keven K, Uruc I, Ekmekci Y, Canbakan B, Erden I, Karatan O. The safety of gadolinium in patients with stage 3 and 4 renal failure. Nephrol Dial Transplant. 2006; 21: 697–700, [INFOTRIEVE], [CSA], [CROSSREF]
- Erley CM, Bader BD, Berger ED, Tuncel N, Winkler S, Tepe G, Risler T, Duda S. Gadolinium-based contrast media compared with iodinated media for digital subtraction angiography in azotaemic patients. Nephrol Dial Transplant. 2004; 19: 2526–2531, [INFOTRIEVE], [CSA], [CROSSREF]
- Jakobsen JA, Oyen R, Thomsen HS, Morcos SK, Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Safety of ultrasound contrast agents. Eur Radiol. 2005; 15: 941–945, [INFOTRIEVE], [CSA], [CROSSREF]