Abstract
Background. To establish the baseline cutoff value of C-reactive protein (CRP) that would predict increased overall and cardiovascular mortality in patients with end-stage renal disease (ESRD). Methods. A cohort of 270 prevalent hemodialysis patients treated at Rijeka University Hospital was eligible for the study. Monthly CRP measurements were performed for three consecutive months. Only the patients with CRP values varying <20% were included (n=256). During the follow-up, 24 patients were transplanted and therefore excluded from the analysis. The CRP cutoff point of 6.2 mg/L was established by Receiver Operating Characteristic curve. The patients were divided into four groups according to their CRP values. Group 1 included 80 (34.5%) patients with CRP <3.0 mg/L, group 2 included 23 (9.9%) patients with CRP 3.0–6.1 mg/L, group 3 consisted of 18 (7.7%) patients with CRP 6.2–10.0 mg/L, and group 4 included 111 (47.9%) patients with CRP >10.0 mg/L. The survival was evaluated by Kaplan-Meier curve. Results. During the two-year follow-up, 59 patients died. The major cause of death was cardiovascular disease (64%). Significantly higher overall and cardiovascular mortality was observed in group 3 when compared with groups 1 and 2 (χ2=11.97; P < 0.001) and in group 4 when compared with groups 1 and 2 (χ2=14.40; P<0.001). Compared with survivors, non-survivors had a higher median CRP value (19.0 [1.5–99.7] mg/L vs. 2.3 [0.1–49.1] mg/L, respectively; P<0.001). Conclusion. Serum concentration of CRP above 6.2 mg/L is a strong predictor of overall and cardiovascular mortality in patients with ESRD.
INTRODUCTION
Cardiovascular disease (CVD) remains the major cause of morbidity and mortality in patients with end-stage renal disease (ESRD).Citation[1] However, its unacceptably high incidence in these patients cannot be fully explained by traditional risk factors, such as hypertension or dyslipidemia;Citation[2] other risk factors related to uremic milieu also play a role.Citation[3] Recent improvements in dialysis technology have not decreased the mortality of maintenance dialysis patients, which remains very high, even higher than that of patients with malignant diseases.Citation[4] Large multicentric clinical trials, such as ADEMEX and HEMO studies, have failed to show that the increased dialysis dose and use of high-flux dialysis improve the survival of dialysis patients.Citation[5],Citation[6]
Thus, the focus of research here has shifted on novel cardiovascular risk factors in these patients. One of them is chronic inflammation, considered a putative “secret killer” and suspected to promote atherosclerosis.Citation[7] The role of inflammation in the atherosclerotic disease process has become well-recognized over the past decade.Citation[8],Citation[9] The hemodialysis procedure itself contributes to the inflammatory response. The possible contributing factors are the presence of catheter, graft, and fistula infections, contamination of dialysis fluid, back-filtration and back-diffusion of contaminated dialysate, and the use of bio-incompatible membranes. The inflammatory response strongly promotes the production of acute phase proteins and proinflammatory cytokines. When combined with malnutrition, which is more common in patients with chronic inflammation, it predicts poor outcome. Thus, malnutrition, inflammation, and atherosclerosis (MIA) syndrome is the major risk factor for premature death in patients with ESRD.Citation[10]
Proinflammatory risk factors that can be measured include oxidized low-density lipoproteins; proinflammatory cytokines (i.e., interleukin [IL]-1 and tumour necrosis factor [TNF]-α); adhesion molecules (i.e., intercellular adhesion molecule-1 and selectins); inflammatory stimuli with hepatic effects (i.e., IL-6); or the products of the hepatic stimulation, such as serum amyloid A, C-reactive protein (CRP), and many other acute-phase reactants. Finally, other indicators of cellular response to inflammation, such as increased white blood cell (WBC) count, are also possible to evaluate.Citation[11] Many of these inflammatory biomarkers, such as CRP, IL-6, or WBC count, are robust predictors of the outcome in patients with ESRD.Citation[12]
Impaired immune response coupled with persistent immune stimulation maintains low-grade systemic inflammation and altered cytokine balance, which characterize the uremic state and may translate into increased CVD risk. The roles of cytokines are quite different. Whereas the IL-10 has antiinflammatory effects, the main proinflammatory cytokines, IL-6 and TNF-α, have proatherogenic properties. The fact that IL-6 promotes the production of CRP may be the reason why IL-6 has been reported to be a proatherogenic and its plasma concentration correlates with poor outcome in patients with ESRD.Citation[13] Although these cytokines, acute phase reactants, and cellular responses to inflammatory stimuli might be predictive of the clinical course of the disease, the laboratory tests to assess inflammation are limited to those useful in clinical settings, depending on commercially available assays that can be standardized and adequate precision. Therefore, it seems most reasonable to limit current assays of inflammatory markers to high-sensitivity (hs) CRP.Citation[11] CRP baseline concentration correlates well with mortality in patients with ESRD and is independent of serum albumin and other possible confounding factors.Citation[14],Citation[15] However, the CRP cutoff value that may indicate high overall and cardiovascular mortality risk in these patients has not yet been defined.Citation[14],Citation[16] The aim of this study was to determine the possible baseline cutoff value of CRP as a predictor for increased mortality in patients with ESRD.
PATIENTS AND METHODS
Patients and Study Design
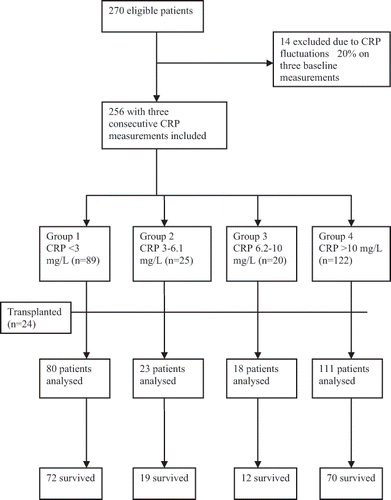
A cohort of 270 prevalent hemodialysis patients treated at Rijeka University Hospital, Croatia, between December 2002 and November 2005 were found eligible for the study. All patients were on chronic hemodialysis for a minimum of three months and clinically stable without any overt active inflammatory state, infection, or infectious disease. None of them had a fever or was hospitalized within 30 days before the study. Patients with autoimmune disease or malignancy and those taking immunosuppressive therapy were excluded. Basic nutritional and inflammatory markers were determined on a monthly basis for three consecutive months. Monthly CRP measurements for three consecutive months were performed in all patients. Only the patients with CRP values varying <20% were included in the study (see ). The final sample consisted of 256 patients aged between 22 and 86 years.
Data were collected on demographic characteristics, vascular access, underlying renal diseases, and the baseline clinical status of the patients (see ). Dialysis was performed for 12 hours, three times a week, by using a steam-sterilized, low-flux polysulfone dialyser membrane (Fresenius Polysulfone®, Fresenius Medical Care, Bad Homburg, Germany) with 1.4 m2 and 1.8 m2 surface area, and the bicarbonate-based dialysate at a delivered bicarbonate concentration of 33 mEq/L. The blood flow rate was maintained at 300–400 mL/min and the dialysate flow rate at 500 mL/min. Concomitant medications, when indicated, included antihypertensive medications (except angiotensin-converting enzyme inhibitors), iron gluconate, calcium-based phosphate binders, aspirin, active oral vitamin D supplementation, and human recombinant erythropoietin therapy to maintain the hemoglobin concentration above 110 g/L.
Table 1 Characteristics of 256 chronic hemodialysis patients enrolled in the study
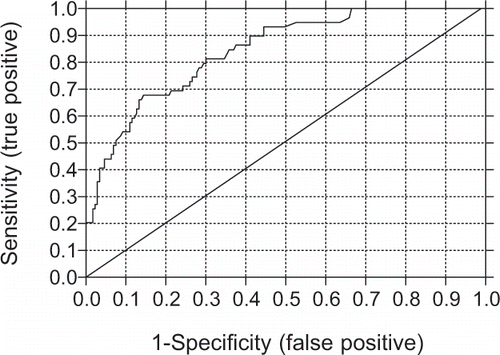
Chronic inflammation was defined as CRP >6.2 mg/L on the basis of the receiver operating characteristics (ROC) curve, which showed a threshold value of CRP > 6.2 mg/L as predictive of death (see ). Using this CRP value as a cutoff point, the patients were divided into four groups according to the mean value of three consecutive CRP measurements during the screening period. Group 1 included patients with a normal CRP value range, according to the laboratory standard (CRP <3.0 mg/L). Group 2 included patients with increased CRP values but below the cutoff point (CRP 3.0–6.1 mg/L). Group 3 consisted of patients with CRP values above the cutoff point but below the common limit value in patients with ESRD (CRP 6.2–10.0 mg/L), whereas group 4 included patients with CRP values above that common limit (CRP >10.0 mg/L).Citation[15–17]
Figure 2 Receiver operating characteristics (ROC) curve for C-reactive protein (CRP) as a predictor of death. Area under curve was 0.843. Data for C-reactive protein values were collected from 232 maintenance hemodialysis patients. The CRP cutoff value where the sensitivity and specificity were the highest (80% and 70%, respectively) was 6.2 mg/L.
Laboratory Testing
Blood samples (10 mL) for laboratory testing were drawn from the venous part of a vascular access at the beginning of the hemodialysis session, centrifuged within two hours, and stored on ice (4°C).
The CRP values were determined by turbidimetric immunoassay method on Olympus AU 400 autoanalyzer (Olympus, Hamburg, Germany) using Olympus Diagnostics reagents, according to the hsCRP application protocol for Olympus instruments.Citation[18] The detection interval for CRP is 0.08–160.0 mg/L, and the reference interval of serum CRP value is <1 mg/L.Citation[19]
The baseline serum albumin was measured by the bromcresol green methodCitation[20] on Olympus AU 400 autoanalyzer using Olympus Diagnostics reagents, according to the application protocol for Olympus instruments.Citation[18] The detection interval for serum albumin is 15–60 g/L, and the normal range is 35–52 g/L.Citation[21]
Dialysis adequacy (Kt/V) was analyzed at baseline by the Daugirdas equation.Citation[22]
Follow-Up
After the three baseline CRP measurements, the patients were followed-up at 24 months. Date and cause of patient's death as an outcome measure was recorded, reviewed, and assigned an underlying cause by a panel of three physicians. Autopsy reports were obtained in as many cases as possible. As part of the review process, all available medical information on each death was collected, always including study and hospitalization records. In the case of an out-of-hospital death, family members were interviewed by telephone to ascertain the circumstances of death. Causes of death were classified as cardiovascular events (myocardial infarction, congestive heart failure, or stroke), infection (sepsis or pneumonia), or other causes (malignant tumors or hyperkalemia). Twenty-four patients who received a kidney transplant during the follow-up were excluded from the analysis.
Statistical Analysis
Data were recorded using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), and expressed either as means with standard deviation (±SD) or median with range. A Mann-Whitney U-test was used to compare the groups. Kaplan-Meier curves were used for an analysis of survival. The CRP cutoff value predictive of increased mortality was determined by Receiver Operating Characteristic (ROC) curve. The cutoff point for CRP, as a predictor of clinical outcome, reached 80% sensitivity and 70% specificity (see ). The area under the ROC curve (mean±SEM) was 0.843 ± 0.028, with 95% confidence interval of 0.788–0.898 and p<0.001. A p value <0.05 was considered statistically significant. Statistical analysis was performed with Prism® 4 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
We analyzed a total of 232 prevalent hemodialysis patients. CRP concentration above the cutoff point of >6.2 mg/L was found in as many as 129 (55.6%) patients (see ). Baseline serum concentrations of albumin were within the normal laboratory limits or slightly below the lower range, indicating a relatively acceptable nutritional status of our patients at the beginning of the study (see ).
During the two-year follow-up, 59 patients died: 8 patients in group 1 (10%), 4 patients in group 2 (17%), 6 patients in group 3 (35%), and 41 patients in group 4 (37%). The most frequent cause of death was cardiovascular event (see ). Over two-thirds of patients who died during the follow-up belonged to groups 3 and 4, i.e., they had the mean baseline CRP concentration above the cutoff point of 6.2 mg/L. In these patients, cardiovascular events were the predominant causes of death (see ). The proportion of non-cardiovascular death events was higher in groups 1 and 2—that is, in patients with lower mean baseline CRP value (see ).
Table 2 Causes of death in 59 chronic hemodialysis patients during two-year follow-up
Table 3 Distribution of cardiovascular and non-cardiovascular causes of death according to the baseline serum concentration of C-reactive protein (CRP) in 59 chronic hemodialysis patients who died during two-year follow-up
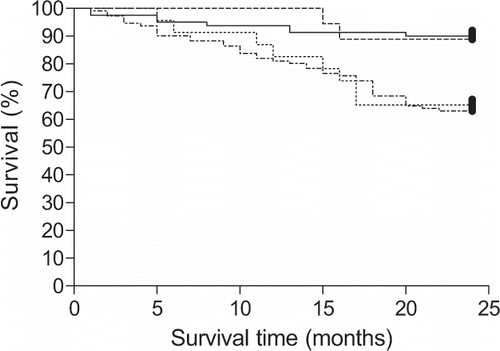
The four groups of patients significantly differed in survival rate (χ2=19.67; p<0.001; see ). There was no significant difference in the survival rate between groups 1 and 2, which both had a mean baseline CRP value below the cutoff point of 6.2 mg/L. Nor was there a significant difference in survival rate between the groups 3 and 4. However, a significantly higher mortality was found in group 3 when compared with groups 1 and 2 (χ2=11.97; p<0.001). Also, group 4 had a significantly higher mortality when compared with groups 1 and 2 (χ2=14.40; p<0.001).
Figure 3 Kaplan-Meier estimates of survival of hemodialysis patients during two-year follow-up with respect to all-cause mortality and serum concentrations of C-reactive protein (CRP). Significantly higher mortality was observed in patients with CRP >6.2 mg/L. Dashed line: group 1 (CRP <3.0 mg/L); full line: group 2 (CRP 3.0–6.1 mg/L); dotted line: group 3 (CRP 6.2–10.0 mg/L); dot-dash line: group 4 (CRP >10.0 mg/L). p<0.001 for group 3 vs. groups 1 and 2 and for group 4 vs. groups 1 and 2.
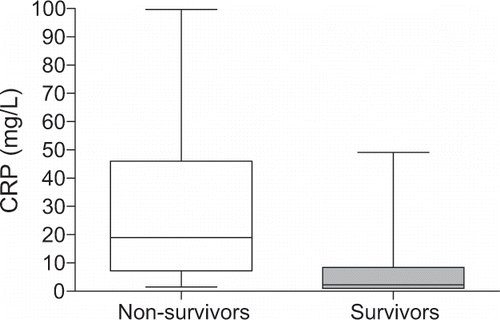
In comparison with survivors, non-survivors had a significantly higher median concentration of CRP (2.3 mg/L vs. 19.0 mg/L, respectively) and wider range of CRP values, which were up to twice the values of CRP found in survivors (see ).
Figure 4 Mean values and distribution of C-reactive protein (CRP) in 173 survivors and 59 non-survivors at the beginning of hemodialysis treatment. Median serum concentration of CRP in non-survivors was significantly higher (open box) than CRP concentration in survivors (full box): 19.0 (1.5–99.7) mg/L vs. 2.3 (0.1–49.1) mg/L, respectively. p<0.001, Mann-Whitney U test.
DISCUSSION
The CRP cutoff point of 6.2 mg/L was found to predict increased mortality in these patients with ESRD.
Considering the fact that the median CRP concentration is about 1.5 mg/L in the general population, the majority of patients with ESRD could be considered to have chronic inflammation.Citation[23] Because it is common in that population of patients, chronic inflammation has recently become intensively investigated as a major novel cardiovascular risk factor. However, different studies have determined different cutoff points of CRP that were predictive of increased overall and cardiovascular mortality. Analyzing the data from the Modification of Diet in Renal Disease Study, Menon et al.Citation[24] identified higher mortality in chronic renal patients with CRP concentration above 3 mg/L. The patients with chronic renal disease but not yet on dialysis are also at an increased mortality risk, but their CRP concentration is lower than that in patients with ESRD.Citation[24] In the current study, increased mortality risk was found only for the patients with CRP concentration above the cutoff point of 6.2 mg/L. Owen et al.Citation[25] also used 3 mg/L as a cutoff point in their study and found that more than one-third of their patients had CRP values above that level. However, they found no significant correlation with mortality. Obviously, it was not an appropriate CRP cutoff point to predict increased mortality risk (i.e., it was too low), which is in agreement with our results. Chauveau et al.Citation[26] used 5 mg/L as a cutoff point and found that 69% of their patients had CRP concentration above that value. On the other hand, Tellingen et al.Citation[27] used 8 mg/L, and Iseki et al.Citation[15] used 10 mg/L as their cutoff values. In all of these studies, patients with CRP values above the chosen cutoff point were at an increased risk of overall and cardiovascular mortality. Different cutoff points obtained by different authors could be rather confusing. The current authors' data showed that a possible cutoff point could be in the middle of those reported previously (i.e., closer to the lower values). Similarly to these data, Nascimento et al.Citation[16] found a lower CRP cutoff point to be predictive of increased mortality in patients with ESRD.
Three consecutive measurements of CRP value were taken in the current study because a single measurement could not be sufficiently accurate for assessment of inflammation, considering possible intra-individual variations. A recent Brazilian studyCitation[16] also confirmed that fluctuating CRP values could lead to false interpretation of inflammatory state, as only persistent inflammation is an important predictor of death. Data from the study of Tsirpanlis et al.Citation[28] showed that average of two consecutive CRP measurements was a reliable indicator of inflammation in about 70% of study patients. A greater number of measurements (up to five) in a healthy population improved the CRP assessment, but only slightly.Citation[29]
The present authors found the patients with increased CRP values to be at increased risk of cardiovascular death, which is in accordance with results of Zimmermann et al.Citation[30] On the other hand, Korevaar et al.Citation[31] found that the mortality risk increased with an increase in the CRP level during a single dialysis session in 25% of the patients, but it was not associated with increased CRP values before the dialysis session. The impact of these findings underlies the importance of understanding which factors might cause inflammatory response. However, the precise mechanisms still remain unclear. A possible contribution of the hemodialysis procedure per se on inflammatory response, especially choice of membrane and dialysis fluid quality, still needs to be confirmed.
This study had several limitations. First, only CRP was analyzed, whereas other inflammatory markers were not assessed. Second, in the nutritional evaluation of our patients, the Subjective Global Assessment (SGA) was not used, as was proposed by Dialysis Outcome Quality Initiative (DOQI) guidelines.Citation[32] Only a single albumin measurement at the beginning of the study was used. Third, only the initial three consecutive CRP levels were analyzed, but not the levels at the time of the overall and cardiovascular death event. However, CRP level at that time of death event cannot be predictive, as the CRP levels may vary widely depending on the cause of death.
In conclusion, the current study's findings suggest that CRP as a prototypical acute phase protein in humans is a strong predictor of overall and cardiovascular mortality in patients with ESRD. A standard CRP cutoff point in patients with ESRD should be further investigated and confirmed in multicentric large clinical studies, including possible interventional strategies to control the CRP values below the cutoff point.
REFERENCES
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal failure. Am J Kidney Dis 1998; 32(Suppl 5)S112–119, [INFOTRIEVE], [CSA]
- Cheung AK, Sarnak MJ, Guofen Y, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 2000; 58: 353–362, [INFOTRIEVE], [CSA], [CROSSREF]
- Racki S, Zaputovic L, Vujicic B, Mavric Z, Grzetic M, Ravlic-Gulan J. Cardiovascular risk factors and diseases strongly predict hemodialyis treatment outcome in maintenance hemodialysis patients. Croat Med J 2005; 46(6)936–941, [INFOTRIEVE], [CSA]
- Stenvinkel P. Inflammation in end-stage renal disease—a fire that burns within. Cardiovascular Disorders in Hemodialysis, C Ronco, A Brendolan, NW Levin. Karger, Basel 2005; 185–199
- Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13: 1307–1320, [INFOTRIEVE], [CSA]
- Eknoyan G, Beck GJ, Cheung AK, , for the Hemodialysis (HEMO) Study Group, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–2019, [INFOTRIEVE], [CSA], [CROSSREF]
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002; 62: 1524–1538, [INFOTRIEVE], [CSA], [CROSSREF]
- Tracy RP. Inflammation in cardiovascular disease. Circulation 1998; 97: 2000–2002, [INFOTRIEVE], [CSA]
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340: 115–126, [INFOTRIEVE], [CSA], [CROSSREF]
- Yao Q, Lindholm B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodialysis Int 2004; 8: 118–129, [CSA], [CROSSREF]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practise. A statement for healthcare professionals from the Centres for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511, [INFOTRIEVE], [CSA], [CROSSREF]
- Stenvinkel P, Alvestrand P. Inflammation in end-stage renal disease: Sources, consequences and therapy. Semin Dial 2002; 15: 329–337, [INFOTRIEVE], [CSA], [CROSSREF]
- Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNFα: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 2005; 67: 1216–1233, [INFOTRIEVE], [CSA], [CROSSREF]
- Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000; 35: 469–476, [INFOTRIEVE], [CSA]
- Iseki K, Tozawa M, Yoshi S, Fukiyama K. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant 1999; 14: 1956–1960, [INFOTRIEVE], [CSA], [CROSSREF]
- Nascimento MN, Pecoits-Filho R, Qureshi AR, et al. The prognostic impact of fluctuating levels of C-reactive protein in Brazilian haemodialysis patients: a prospective study. Nephrol Dial Transplant 2004; 19: 2803–2809, [INFOTRIEVE], [CSA], [CROSSREF]
- Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition and atherosclerosis in chronic renal patients. Kidney Int 1999; 55: 1899–1911, [INFOTRIEVE], [CSA], [CROSSREF]
- Baudner S, Dali F. Standardization of the measurement of 14 proteins in human serum based on the new IFCC/BCR/CAP international reference material CRM 470. J Lab Med 1996; 20: 145–152, [CSA]
- Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 1997; 43: 52–58, [INFOTRIEVE], [CSA]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 1971; 31: 87–96, [INFOTRIEVE], [CSA], [CROSSREF]
- Painter PC, Cope JY, Smith JL. Reference information for the clinical laboratory. Tietz Textbook of Clinical Chemistry, CA Burtis, ER Ashwood. WB Saunders Company, Philadelphia 1999; 1800
- Depner T, Beck B, Daugirdas J, Kusek J, Eknoyan G. Lessons from the hemodialysis (HEMO) study: an improved measure of the actual hemodialysis dose. Am J Kidney Dis 1999; 33: 142–149, [INFOTRIEVE], [CSA]
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347: 1615–1617, [CSA], [CROSSREF]
- Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int 2005; 68: 766–772, [INFOTRIEVE], [CSA], [CROSSREF]
- Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int 1998; 54: 627–636, [INFOTRIEVE], [CSA], [CROSSREF]
- Chauveau P, Level C, Lasseur C, et al. C-reactive protein and procalcitonin as markers of mortality in hemodialysis patients: a two-year prospective study. J Ren Nutr 2003; 13: 137–143, [INFOTRIEVE], [CSA], [CROSSREF]
- Tellingen A, Grooteman MPC, Schoorl M, et al. Intercurrent clinical events are predictive of plasma C-reactive protein levels in hemodialysis patients. Kidney Int 2002; 62: 632–638, [INFOTRIEVE], [CSA], [CROSSREF]
- Tsirpanlis G, Bagos P, Ioannou D, et al. The variability and accurate assessment of microinflammation in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 150–157, [INFOTRIEVE], [CSA], [CROSSREF]
- Ockene IR, Matthews CE, Rifai N, et al. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem 2001; 47: 444–450, [INFOTRIEVE], [CSA]
- Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 1999; 55: 648–658, [INFOTRIEVE], [CSA], [CROSSREF]
- Korevaar JC, Manen JG, Dekker FW, Waart DR, Boeschoten EW, Krediet RY, for the NECOSAD study group. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J Am Soc Nephrol 2004; 15: 2916–2922, [INFOTRIEVE], [CSA], [CROSSREF]
- National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6, Suppl. 2)1–140, [CSA]