Abstract
Chronic renal failure (CRF) is increasingly prevalent in solid-organ-transplant patients. This is in part related to the long-term use of calcineurin inhibitor (CNI) agents. However, in orthotopic liver-transplant (OLT) patients, the effects of superimposed hepatitis C virus (HCV)-related renal lesions could also be a factor. The aim of this cohort study (February 2000 to September, 2003) was to identify the predictive factors at one year post-transplantation for CRF in OLT patients associated with induction therapies. CRF was defined as having a creatinine clearance (CC) lower than 60 mL/min. Of the 97 transplants performed during that period, 72 were still functioning after one year. Of these, 33 patients (45.8%) had CRF. In univariate analysis, the predicting factors for CRF were recipient sex (female), initial liver disease (HCV-related cirrhosis), pre-transplant CC (<80 mL/mn), and post-transplant serum creatinine >130 μmol/L at day 3 and months (M) 1, 3, and 6. In multivariate analysis, the independent predictive factors for CRF included female sex [OR: 11.5 (2.3–58.3); p = 0.003], HCV infection [OR: 5.01 (1.1–22.7); p = 0.03], pre-OLT CC <80 mL/mn [OR: 5.4 (1.2–23.7); p = 0.025], and serum creatinine at M6 greater than 130 μmol/L [OR: 19.6 (3.7–102.5); p = 0.0004]. Among all of the predictive factors for post-OLT CRF, only one is modifiable: post-transplant serum creatinine, which could be, to some extent, related to the long-term use of CNIs.
INTRODUCTION
Orthotopic liver transplantation (OLT) is a well-established therapy for end-stage liver disease (ESLD). However, it requires long-term therapy with immunosuppressants, among which are calcineurin inhibitors (CNIs), and these agents may induce chronic renal failure in the long term. Recent registry studies have clearly shown that chronic renal failure (CRF) is an emerging and serious problem in solid non-renal organ-transplant recipients, with a particularly high prevalence in OLT patients.Citation[1] In addition to the chronic nephrotoxicity of CNI agents, the impairment of renal function prior to transplantation might contribute to the development of CRF. This is particularly true in hepatitis C virus (HCV)-positive OLT patients because chronic HCV infection can result in specific renal lesions.Citation[2] Moreover, the occurrence of acute renal failure after liver transplantation, which is a fairly common feature, might also contribute to the development of CRF.Citation[3] It is now well known that there is a significant decrease in survival of OLT patients with CRF;Citation[4],Citation[5] therefore, it is important to identify those factors that might contribute to the development of CRF. Some studies have shown that pre-transplant renal function,Citation[6] as well as the occurrence of early post-OLT renal dysfunction,Citation[3],Citation[7] are predictive factors for long-term CRF in OLT patients.
In the era of modern immunosuppression, with treatments that include both induction therapy (to delay the introduction of CNIs) and the use of calcineurin-sparing agents (e.g., mycophenolate mofetil), the current authors decided to evaluate the prevalence of one-year post-OLT chronic renal failure and identify its predictive factors in a cohort of 88 consecutive patients who had received a liver transplant between February 2000 and September 2003.
PATIENTS AND METHODS
From February 2000 to September 2003, 97 orthotopic liver transplants were performed at the University Hospital, CHU Rangueil, Toulouse, in 88 patients, including nine re-transplantations. Within the first year of follow-up, 16 patients died, two of whom had received second transplants: seven died of sepsis, three died of HCV-related fibrosing cholestatic hepatitis, two died of a primary non-functioning graft, two died of chronic rejection, and two died of a recurrence of hepatocarcinoma. Thus, data were analyzed from 72 patients who were still alive with the same graft at one year, seven of which had previously undergone a re-transplantation. The following data were recorded for all patients:
donor and recipient sex
age and body mass index
etiology and severity of hepatic failure
history of diabetes or hypertension
cold ischemia time
occurrence of hemodynamic instability during procedure (defined as a mean arterial blood pressure of <65 mmHg for >15 min)
pre-transplant requirement for blood or fresh frozen plasma transfusions (number of red packed or plasma units)
post-transplant hemodynamic instability (assessed by the measurement of mean blood pressure and pulse at 0, 6, and 12 hours after completion of the OLT procedure and by the requirement for vasopressive drugs)
duration of mechanical ventilation
hourly urine output (diuresis) during the first 24 hours post-OLT
peak and time to peak of aspartate (AST) and alanine (ALT) aminotransferase levels
time to reach a pre-accelerin value greater than 50%
cause of any post-transplant abdominal surgery
type, dosage, and trough levels of immunosuppressants at days 7, 14, and 30, and at months 3, 6, 9, and 12 post‐OLT
occurrence of biopsy—proven acute—rejection episodes
length of stay of hospitalization for OLT
occurrence of sepsis
early mortality (within the first year of post-transplantation)
With respect to renal function, the authors took into account pre-transplant serum creatinine and calculated creatinine clearance (CC) according to the Cockcroft and Gault formula, the need for post-transplant renal-replacement therapy (i.e., hemodialysis or hemodiafiltration sessions), the occurrence of a doubling or trebling of serum creatinine within the first month post-OLT (i.e., acute renal dysfunction with a RIFLE score [www.adqi.net] of, respectively, ≥2 [Injury] and ≥3 [Failure]), and serum creatinine levels and calculated CC values at 1, 3, 6, and 12 months post-OLT. Chronic renal failure was defined by a CC value lower than 60 mL/min at one year post-OLT because, according to K/DOQI guidelines, after creatinine clearance decreases to less than 60mL/min/1.73 m2, the patient is classified as having chronic kidney disease regardless of evidence of kidney damage.Citation[8]
Patients
Data were analyzed from 72 patients at one year after liver transplantation. There were 52 men and 20 women, with a mean age of 52 (22–66) years. All patients underwent orthotopic whole-liver transplantation with the preservation of the recipient's vena cava. Indications for OLT included post-alcoholic cirrhosis (40%), post-hepatitis C cirrhosis (24%), cirrhosis related to both alcohol and hepatitis C (7%), chronic rejection of a first graft (7%), primary hepatocarcinoma (4%), auto-immune-related cirrhosis (4%), post-hepatitis B cirrhosis (3%), and miscellaneous reasons (10%). Of those patients with cirrhosis (n = 64), 53% were Child-Pugh C, 27% were Child-Pugh B, and 20% were Child-Pugh A. The latter were transplanted because of the concomitant presence of hepatocarcinoma. Type I hepatorenal syndrome was only present in three cases. Concerning the medical history of the patients, 22% had diabetes mellitus and 14% had hypertension prior to transplantation. Renal function before transplantation was defined by a median serum creatinine of 72 μmol/L (range 38–202) and a creatinine clearance (Cockcroft and Gault) of 107 mL/min (range 37–230).
Immunosuppression
Immunosuppression was based on CNIs and changed with time—that is, the beginning of the cohort received cyclosporine A-based therapy (20%), aiming at trough levels between 150–250 ngmL/mL until month 3, then tapered to 100–150 ngmL/mL in association with steroids and induction therapy with antithymocyte globulins. Afterward, the rest of the cohort received tacrolimus-based therapy (80%), aiming trough levels between 10 to 15 ngmL/mL until month 3, then 5 to 10 ngmL/mL, associated with steroids and induction therapy with anti-CD25 monoclonal antibodies (basiliximab 20mg at post-operative days 0 and 4, or daclizumab at 2mg/kg at day 0 and 1mg/kg at day 7). Therapy allowed the introduction of CNIs by post-operative days 1 to 3. In the case of hepatitis C, mycophenolate mofetil was also given (i.e., in 36% of cases. which was usually withdrawn at 1 month). Acute rejection rate was 17.5% at one month and 25.8% at 12 months posttransplantation.
Statistical Analysis
Variables are expressed as means ± standard deviations, or as the median (ranges) when appropriate. A univariate analysis was first performed on a priori defined variables in order to determine the predictive factors for chronic renal failure at one year. Qualitative variables were compared by chi-square test or Fisher's exact test. Quantitative variables were analyzed by Student's t-test or the Wilcoxon test. Quantitative variables, for which the p value was lower than 0.25, were grouped into classes and then analyzed qualitatively (chi-square or Fisher tests). To determine the significant variables, an odds ratio (OR) was calculated with a 95% confidence interval (CI). A multivariate analysis was also performed to determine the independent factors predicting CRF at one year post-OLT. Those factors where the p value was <0.25 in the univariate analysis, as well as any potential confounding factors, were taken into account. Thus, a logistic regression analysis was performed, and risk factors were identified by means of a step-by-step analysis. The statistical analyses were performed using SAS version 8.2 software.
RESULTS
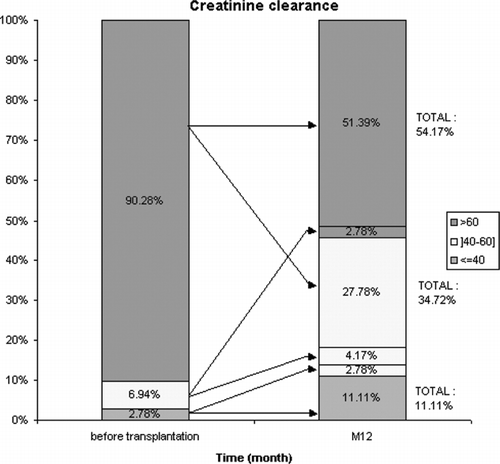
shows the outcome of creatinine clearance at M12 according to pre-OLT creatinine clearance. Of note, seven patients (9.7%) had a calculated creatinine clearance (CC) lower than 60 mL/min before OLT, the median CC being 46.9 mL/min. Thirty-three patients (i.e., 45.8%) developed CRF (as defined by CC <60 mL/min) at one year post-OLT. Of these, 18 (54.5%) were men and 15 (45.5%) women, with a mean age of 56 (33–66) years. Considering sex, 15 (75%) women developed CRF at one year post-OLT, compared to 18 (35%) men (p = 0.002). Patients with CRF were significantly older at OLT than those without CRF (55.4 ± 8 versus 48.8 ± 9 years; p = 0.0027). A total of 57% of patients aged over 50 years developed CRF compared to 24% of those aged less than 50 years (p = 0.006). The number of OLTs is not correlated with the occurrence of CRF (9.1% CRF in re-transplant patients vs. 5.1% in patients who had only received one transplant, p = 0.6).
Ten patients had a history of hypertension before OLT. Of these, 7 (70%) developed CRF, compared to 26 (42%) of those without hypertension (p = 0.17). Pre-OLT diabetes mellitus was reported in 16 patients. There was no significant difference in the incidence of CRF between patients with (62.5%) or without (41%) this pathology (p = 0.13).
Considering the etiology of liver disease, there is a correlation between HCV infection and CRF: of the 17 HCV-positive patients, 12 (71%) developed CRF, compared to 21 (38%) of the 55 patients with another type of liver disease (p = 0.02).
With respect to pre-OLT renal function, serum creatinine was not significantly different in patients with or without subsequent CRF (77 vs. 71 μmol/L, p = NS), but pre-transplant creatinine clearance was significantly lower in patients who developed CRF [80 mL/min (range 36–182) vs. 118 mL/min (range 40–230), p = 0.0026]. Thus, a pre-transplant CC of less than 80 mL/min was significantly associated with the genesis of CRF (p = 0.0001).
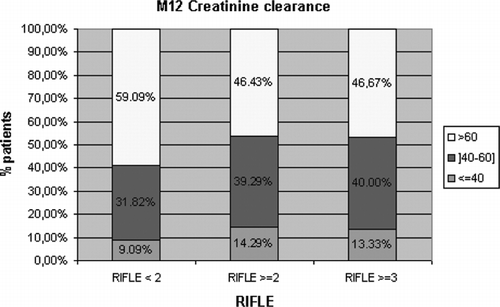
Considering post-OLT renal function, the occurrence of acute renal dysfunction within the first month post-OLT, be it mild (RIFLE score ≥2) (41.5% of the patients) or severe (RIFLE score ≥3 or need for renal-replacement therapy; 22.3% of the patients) was not significantly associated with the development of CRF (see ). Nevertheless, serum creatinine levels were significantly higher by days 3 (p = 0.044) and 10 (p = 0.018) and months 3 (p = 0.034) and 6 (p = 0.004) in patients who presented with CRF at one year post-OLT.
Figure 2 The outcome of creatinine clearance at M12 according to the occurrence of acute renal dysfunction within the first month post-OLT, be it mild (RIFLE score ≥2) or severe (RIFLE score ≥3).
With respect to the immunosuppressive regimen, 59 (82%) patients received an induction therapy, either by anti-thymocyte globulins (17 patients) or anti-CD-25 (42 patients). CRF occurred in 26 (44%) patients with induction therapy and 7 (54%) patients without induction therapy (p = 0.83). Also, there was no significant difference in the occurrence of CRF in those patients who received tacrolimus (49%) compared to those who received cyclosporine A (36.7%). CNI dosages and trough levels were accounted for on days 3, 5, 7, and 15, and months 1, 3, and 6. No correlation was found between the development of CRF at one year and CNI dosage or trough levels at any time during the first six months of post-OLT. Moreover, there was no significant difference in the proportion of patients developing CRF that had or had not received MMF in the early stages of post-OLT.
summarizes the only factors that were significantly associated in the univariate analysis with the development of CRF. These included a recipient over 50 years of age [OR 4.3 (1.44–12.65); p = 0.006], female sex [OR 5.67 (1.8–18.1); p = 0.002], having a hepatitis C virus infection [OR 3.8 (1.2–12.6); p = 0.019], pre-OLT creatinine clearance <80 mL/mn [OR 4.85 (1.59–14.77); p = 0.004], serum creatinine at M1 >130 μmol/L [OR 3.98 (1.11–14.25); p = 0.027], serum creatinine at M3 >130 μmol/L [OR 3.77 (1.16–12.25); p = 0.022], and serum creatinine at M6 >130 μmol/L [OR 7.29 (2.1–25.22); p = 0.0007]. In multivariate analysis, the factors independently associated with the development of CRF are female sex (p = 0.003), hepatitis C virus infection (p = 0.03), a calculated CC at pre-OLT of <80 mL/min (p = 0.025), and a serum creatinine higher than 130 μmol/L at six months post-OLT (p = 0.0004). These predictive factors are summarized in .
Table 1 Factors associated in the univariate analysis with the development of chronic renal failure at one-year post-liver transplantation
Table 2 Factors associated in the multivariate analysis with the development of chronic renal failure at post-liver transplantation
DISCUSSION
In this study, the present authors have evaluated the incidence of chronic renal failure at post-OLT and determined its predictive risk factors. The occurrence of CRF is a frequent complication after OLT: thus, almost 50% of OLT patients show a decrease in creatinine clearance in the first six months after procedure,Citation[9–11] and the cumulative incidence of CRF after OLT ranges from 4 to 28% for severe renal dysfunctionCitation[10],Citation[12] and from 1.4 to 9% for end-stage renal disease.Citation[2],Citation[13],Citation[14] The lack of a standard definition of post-transplantation CRF, the differences in immunosuppressive regimens, and the variable periods of follow-up may account for the variability in these incidences. The development of CRF after liver transplantation leads to an increase of morbidity, mortality, and cost of medical management.Citation[1],Citation[7]
In this study, the incidence of CRF, as defined by creatinine clearance lower than 60 mL/min at one year post-OLT, was 45.8%. This high value may be related to the cutoff chosen for the definition of CRF. As previously shown, it was found that age, female sex, pre-transplantation, and hepatitis C virus infection were associated with an increased risk of CRF.Citation[1],Citation[5],Citation[10] As reported here and previously,Citation[3],Citation[5],Citation[10] a diminution in creatinine clearance before procedure was an independent risk factor for the development of post-OLT CRF. Even if a significant link between CRF and post-OLT acute renal failure, as defined by the RIFLE criteria, could not be shown, it was found that serum creatinine levels were significantly higher by days 3 and 10 post-OLT in patients who presented with CRF at one year. Moreover, in this study, serum creatinine levels were significantly higher by months 3 and 6 post-OLT in those patients who developed CRF, and a serum creatinine level of >130 μmol/L at six months was an independent predictive factor for CRF at one year, underscoring the fact that CRF may be predicted early after OLT. In contradiction to previous studies,Citation[1],Citation[2] no influence of a history of hypertension or of diabetes mellitus with the development of renal dysfunction was found, though this may be related to the low incidences of these pathologies in our study population.
Calcineurin-inhibitor therapy has been implicated as one of the major causes of post-OLT renal dysfunction.Citation[15],Citation[16] In a retrospective study of more than 800 OLT patients, Fisher et al.Citation[7] showed that CsA trough levels at one month post-OLT and cumulative CsA dosages were risk factors for the development of late CRF (by one year or more post-OLT) but not early CRF (before the first year post-OLT). Some authorsCitation[1],Citation[6] have pointed out a difference in renal function in OLT patients when comparing the effects of CsA and tacrolimus. A very recent study evaluated the six-month impact on renal function of delayed posttransplant introduction of tacrolimus (i.e., by day 4–6 in association with MMF and daclizumab induction therapy). The renal function evaluated by a calculated creatinine clearance was statistically significantly better (+6mL/mn/1.73m2) in the arm where induction therapy was used as compared to the control arm (i.e., no induction therapy, immediate tacrolimus and MMF).Citation[17] In this study, even with the use of induction therapy, CNIs were introduced as early as days 1–3. Moreover, no significant relationship was found between CNI dosages or trough levels at any time post-OLT and the occurrence of CRF, nor was any difference found in renal outcome in patients treated with CsA compared with those receiving tacrolimus.
Some authors have suggested that diminution or even withdrawal of CNIs in OLT patients with CRF may contribute to an improvement in renal function. This has already been shown in lung, heart, and renal transplant patients.Citation[18–20] Nevertheless, previous reports are discordant concerning the safety for the liver graft and the impact upon renal function. In two studies on CNI withdrawal and replacement by azathioprine (AZA), Chan et al.Citation[21] reported that this therapeutic change was safe and effective, whereas Sandborn et al.'sCitation[22] study showed no sustained improvement in renal function, but instead an increased incidence of chronic liver rejection.
The result of the replacement of CNIs by MMF also remains controversial: in three small studies of patients presenting with renal function impairment, CNI withdrawal and its replacement by MMF was safe (i.e., no acute rejection) and was associated with a substantial improvement in renal function.Citation[23–25] However, in these studies, the follow-up was short. Conversely, CNI withdrawal and its replacement by MMF were associated with a high rate of acute rejection in 12 to 21%.Citation[26–28] Nonetheless, in all of these studies, CNI withdrawal was associated with a significant improvement in renal function, even in long-term OLT patients.Citation[29] Two recent studies have evaluated the beneficial effect of a significant reduction in the dosage of CNIs under the umbrella of MMF therapy in OLT patients with renal dysfunction and observed an improvement in renal function.Citation[30],Citation[31]
More recently, the use of non-nephrotoxic drugs (i.e., mTOR inhibitors, such as sirolimus [SRL]) in OLT patients with CRF has been suggested. The benefits of the conversion from CNIs to SRL on renal function have already been shown in renal transplant patientsCitation[32],Citation[33] and, more recently, in heart transplant patients.Citation[34] Two recent studies in long-term OLT patients with renal dysfunction have shown that conversion from CNI to SRL was associated with a significant improvement in renal function without an increased risk of acute rejection.Citation[35],Citation[36]
Are CNIs the culprit in the deterioration of renal function in OLT patients? Although chronic nephrotoxicity is a well-known and documented side effect of CNIs in OLT as well as other transplant patients, in most studies, there is no histological proof of this assumption. However, a recent study on renal biopsies in OLT patients with a chronic impairment of renal function evidenced the fact that besides CNI-related nephropathy, there were numerous other causes of nephropathy. Therefore, a renal biopsy might be considered in cases of progressive degradation of GFR when contemplating major changes in CNI-based therapies, especially in patients who are HCV positive, as HCV is known to induce specific renal lesions.Citation[37],Citation[38]
CONCLUSION
Chronic renal failure (CRF) is a frequent complication in OLT patients and contributes to increased morbidity. In this study, the prevalence of CRF, as defined by a CC lower than 60 mL/min at one year post-OLT, was 45.8%. Factors contributing to the development of CRF are age, female sex, chronic HCV infection, pre-transplant renal failure, and early (within six months) post-transplantation reduction of GFR. Although a direct correlation between CNI doses or their trough levels and CRF was not shown, previous clinical and histopathological studies show that CNIs are partly responsible for chronic nephrotoxicity in OLT patients. The beneficial effects of CNI reduction or withdrawal have still to be studied. Moreover, new strategies, including RAS blockade agents, should be evaluated.
REFERENCES
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931–940, [INFOTRIEVE], [CSA]
- Shokouh-Amiri MH, Egidi MF, Lo A, et al. The importance of early prevention of renal dysfunction in liver transplant recipients. Transplant Proc 2001; 33: 1399–1400, [INFOTRIEVE], [CSA], [CROSSREF]
- Velidedeoglu E, Crawford MD, Desai NM, et al. Predictors of late kidney dysfunction post-liver transplantation. Transplant Proc 2002; 34: 3315–3316, [INFOTRIEVE], [CSA], [CROSSREF]
- Gonwa TA, Mai ML, Melton LB, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation 2001; 72: 1934–1939, [INFOTRIEVE], [CSA], [CROSSREF]
- Moreno JM, Cuervas-Mons V, Rubio E, et al. Chronic renal dysfunction after liver transplantation in adult patients: prevalence, risk factors, and impact on mortality. Transplant Proc 2003; 35: 1907–1908, [INFOTRIEVE], [CSA], [CROSSREF]
- Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation 1995; 59: 361–365, [INFOTRIEVE], [CSA]
- Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation 1998; 66: 59–67, [INFOTRIEVE], [CSA], [CROSSREF]
- Eknoyan G. Meeting the challenges of the new K/DOQI guidelines. Am J Kidney Dis 2003; 41: 3–10, [INFOTRIEVE], [CSA], [CROSSREF]
- Poplawski S, Gonwa T, Goldstein R, Husberg B, Klintmalm G. Long-term nephrotoxicity in liver transplantation. Transplant Proc 1989; 21: 2469–2471, [INFOTRIEVE], [CSA]
- Gayowski T, Singh N, Keyes L, et al. Late-onset renal failure after liver transplantation: role of post-transplant alcohol use. Transplantation 2000; 69: 383–388, [INFOTRIEVE], [CSA], [CROSSREF]
- Gonwa TA, Poplawski SC, Husberg BS, Nery JR, Klintmalm GB. Cyclosporine nephrotoxicity in orthotopic liver transplantation. Transplant Proc 1988; 20: 401–404, [INFOTRIEVE], [CSA]
- Wilkinson AH, Cohen DJ. Renal failure in the recipients of nonrenal solid organ transplants. J Am Soc Nephrol 1999; 10: 1136–1144, [INFOTRIEVE], [CSA]
- Gonwa TA, Mai ML, Melton LB, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation 2001; 72: 1934–1939, [INFOTRIEVE], [CSA], [CROSSREF]
- Gonwa TA, Mai ML, Klintmalm GB. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 2563–2565, [INFOTRIEVE], [CSA], [CROSSREF]
- Iwatsuki S, Esquivel CO, Klintmalm GB, Gordon RD, Shaw BW, Jr., Starzl TE. Nephrotoxicity of cyclosporine in liver transplantation. Transplant Proc 1985; 17: 191–195, [INFOTRIEVE], [CSA]
- Puschett JB, Greenberg A, Holley J, McCauley J. The spectrum of ciclosporin nephrotoxicity. Am J Nephrol 1990; 10: 296–309, [INFOTRIEVE], [CSA]
- Yoshida EM, Marotta PJ, Greig PD, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transpl 2005; 11: 1064–1072, [INFOTRIEVE], [CSA], [CROSSREF]
- Soccal PM, Gasche Y, Favre H, Spiliopoulos A, Nicod LP. Improvement of drug- induced chronic renal failure in lung transplantation. Transplantation 1999; 68: 164–165, [INFOTRIEVE], [CSA], [CROSSREF]
- Aleksic I, Baryalei M, Busch T, et al. Improvement of impaired renal function in heart transplant recipients treated with mycophenolate mofetil and low-dose cyclosporine. Transplantation 2000; 69: 1586–1590, [INFOTRIEVE], [CSA], [CROSSREF]
- Schrama YC, Joles JA, van Tol A, Boer P, Koomans HA, Hene RJ. Conversion to mycophenolate mofetil in conjunction with stepwise withdrawal of cyclosporine in stable renal transplant recipients. Transplantation 2000; 69: 376–383, [INFOTRIEVE], [CSA], [CROSSREF]
- Chan CY, DasGupta K, Baker AL. Cyclosporin A: drug discontinuation for the management of long-term toxicity after liver transplantation. Hepatology 1996; 24: 1085–1089, [INFOTRIEVE], [CSA]
- Sandborn WJ, Hay JE, Porayko MK, et al. Cyclosporine withdrawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology 1994; 19: 925–932, [INFOTRIEVE], [CSA], [CROSSREF]
- Detry O, De Roover A, Honore P, Delwaide J, Jacquet N, Meurisse M. Mycophenolate mofetil monotherapy in stable liver transplant recipients with progressive renal failure. Transplant Proc 2002; 34: 782–783, [INFOTRIEVE], [CSA], [CROSSREF]
- Neau-Cransac M, Morel D, Bernard PH, et al. Renal failure after liver transplantation: outcome after calcineurin inhibitor withdrawal. Clin Transplant 2002; 16: 368–373, [INFOTRIEVE], [CSA], [CROSSREF]
- Barkmann A, Nashan B, Schmidt HH, et al. Improvement of acute and chronic renal dysfunction in liver transplant patients after substitution of calcineurin inhibitors by mycophenolate mofetil. Transplantation 2000; 69: 1886–1890, [INFOTRIEVE], [CSA], [CROSSREF]
- Moreno Planas JM, Cuervas-Mons Martinez V, Rubio Gonzalez E, et al. Mycophenolate mofetil can be used as monotherapy late after liver transplantation. Am J Transplant 2004; 4: 1650–1655, [INFOTRIEVE], [CSA], [CROSSREF]
- Papatheodoridis GV, O'Beirne J, Mistry P, Davidson B, Rolles K, Burroughs AK. Mycophenolate mofetil monotherapy in stable liver transplant patients with cyclosporine-induced renal impairment: a preliminary report. Transplantation 1999; 68: 155–157, [INFOTRIEVE], [CSA], [CROSSREF]
- Schlitt HJ, Barkmann A, Boker KH, et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet 2001; 357: 587–591, [INFOTRIEVE], [CSA], [CROSSREF]
- Pruvot F, Hazzan M, Roumilhac D, et al. Improvement of severe chronic renal dysfunction by replacement of anticalcineurins with mycophenolate mofetil in long term liver transplant recipients. Am J Transplant 2003; 3(suppl 5)376, [CSA]
- Cantarovich M, Tzimas GN, Barkun J, Deschenes M, Alpert E, Tchervenkov J. Efficacy of mycophenolate mofetil combined with very low-dose cyclosporine microemulsion in long-term liver-transplant patients with renal dysfunction. Transplantation 2003; 76: 98–102, [INFOTRIEVE], [CSA], [CROSSREF]
- Raimondo ML, Dagher L, Papatheodoridis GV, et al. Long-term mycophenolate mofetil monotherapy in combination with calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Transplantation 2003; 75: 186–190, [INFOTRIEVE], [CSA], [CROSSREF]
- Citterlo F, Scata MC, Violi P, et al. Rapid conversion to sirolimus for chronic progressive deterioration of the renal function in kidney allograft recipients. Transplant Proc 2003; 35: 1292–1294, [INFOTRIEVE], [CSA], [CROSSREF]
- Mota A, Arias M, Taskinen EI, et al. Sirolimus-based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at three years. Am J Transplant 2004; 4: 953–961, [INFOTRIEVE], [CSA], [CROSSREF]
- Groetzner J, Kaczmarek I, Meiser B, Muller M, Daebritz S, Reichart B. Sirolimus and mycophenolate mofetil as calcineurin inhibitor-free immunosuppression in a cardiac transplant patient with chronic renal failure. J Heart Lung Transplant 2004; 23: 770–773, [INFOTRIEVE], [CSA], [CROSSREF]
- Nair S, Eason J, Loss G. Sirolimus monotherapy in nephrotoxicity due to calcineurin inhibitors in liver transplant recipients. Liver Transplant 2003; 9: 126–129, [CSA], [CROSSREF]
- Fairbanks KD, Eustace JA, Fine D, Thuluvath PJ. Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus. Liver Transplant 2003; 9: 1079–1085, [CSA], [CROSSREF]
- Johnson RJ, Wilson R, Yamabe H, et al. Renal manifestations of hepatitis C virus infection. Kidney Int 1994; 46: 1255–1263, [INFOTRIEVE], [CSA]
- Stehman-Breen C, Willson R, Alpers CE, Gretch D, Johnson RJ. Hepatitis C virus-associated glomerulonephritis. Curr Opin Nephrol Hypertens 1995; 4: 287–294, [INFOTRIEVE], [CSA]