Abstract
Melatonin, the chief secretory product of the pineal gland, is a direct free radical scavenger. In addition to a direct scavenging effect on nitric oxide (NO), its inhibitory effect on nitric oxide synthase (NOS) activity has been also reported. L-arginine is the substrate for both NOS and arginase. It has been suggested that there is a competition between arginase and NOS and that they control each other's level. NO plays a crucial role in the pathogenesis of myoglobinuric acute renal failure (ARF). In this study, the authors aimed to investigate the effect of melatonin on arginase activity, ornithine, and NO levels on the myoglobinuric ARF formed by intramuscular (im) injection of hypertonic glycerol. Forty rats were randomly divided into four groups. Rats in SHAM were given saline, and those in groups ARF, ARF-M5, and ARF-M10 were injected with glycerol (10 mL/kg) im. Concomitant and 24 hours after glycerol injection for the ARF-M5 and ARF-M10 groups, melatonin—5 mg/kg and 10 mg/kg, respectively—was administrated intraperitoneally. Forty-eight hours after the glycerol injection, kidneys of the rats were taken under anesthesia. Arginase activity, ornithine, and NO levels in the kidney tissue were determined. Melatonin had an increasing effect on kidney tissue arginase activities and ornithine levels while decreasing NO concentration. It is possible that besides the direct scavenging effect, the stimulatory effect of melatonin on arginase activity may result in an inhibition of NOS activity and, finally, a decrease in the kidney NO level.
INTRODUCTION
The term rhabdomyolysis refers to the disintegration of skeletal muscle, which results in the release of muscular cell constituents into the extracellular fluids and the circulation. In general, about 10–40% of patients undergoing significant rhabdomyolysis develop some degree of acute renal failure (ARF).Citation[1] Rhabdomyolysis is associated with both traumatic (natural disasters, such as earthquakes, auto accidents, and mine collapse) and non-traumatic (e.g., hyperthermia, muscle ischemia, exposure to toxins such as alcohol or drug overdose) cases. Citation[2],Citation[3] The most commonly used in vivo model of myoglobinuric ARF is produced by intramuscular injection of hypertonic glycerol, which causes myolysis, hemolysis, and intravascular volume depletion and exposes the kidney to a large burden of heme proteins, myoglobin, and hemoglobin. It has been suggested that heme proteins or their degradation products (including hematin and iron) display tubular nephrotoxic properties, partially mediated by the generation of free oxygen radicals, and induce vasoconstriction.Citation[2],Citation[3] NO scavenging induced by heme proteins could directly contribute to renal hypoperfusion and tissue injury in the setting of rhabdomyolysis. Myoglobinuric renal injury is largely secondary to the ischemic as well as toxic renal insults induced by heme proteins.Citation[2]
Melatonin, the main secretory product of pineal gland, is a direct free radical scavenger and indirect antioxidant. Melatonin has been reported to scavenge hydrogen peroxide, hydroxyl radical, NO, peroxynitrite anion, hypochlorous acid, singlet oxygen, superoxide anion, and peroxyl radical, although the validity of its ability to scavenge superoxide anion and peroxyl radical is debatable.Citation[4],Citation[5] Additionally, antioxidant actions of melatonin probably derive from its stimulatory effect on superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase, glucose-6-phosphate dehydrogenase, and its inhibitory action on nitric oxide synthase (NOS).Citation[4],Citation[5]
Arginine is classified as a semiessential or conditionally essential amino acid because the ability of the body to synthesize sufficient quantities to meet its needs varies according to development age and incidence of disease or injury. L-arginine can be catabolized by four sets of enzymes in mammalian cells: NOSs, arginases, arginine: glycine amidino transferase, and arginine decarboxylase.Citation[6] NOS are the enzymes responsible for NO generation and catalyze the oxidation of L-arginine to NO and L-citrulline.Citation[7] It has been reported that the three NOS isoforms have been found to be expressed in the kidney. The endothelial NOS (eNOS) is important in the maintenance of glomerular filtration rate regional vascular tone and renal blood flow. The neuronal NOS (nNOS) is expressed primarily in the macula densa and participates in the control of glomerular hemodynamics via tubulo-glomerular feedback and renin release. The inducible NOS is expressed in the kidney under pathological conditions in the glomerular mesangium, infiltrating macrophages and tubules.Citation[8] Moreover, NO may also produce mitochondria via the activity of mitochondrial NOS (mt NOS).Citation[9] The arginines catalyze the divalent cation-dependent hydrolysis of L-arginine to form the nonprotein amino acid L-ornithine and urea. In the liver, this reaction constitutes the final step in urea biogenesis. The urea cycle arginase (arginase I or liver arginase) is cytosolic and the best characterized of the mammalian arginases. A second isozyme, arginase II or kidney arginase, is mitochondrial in location.Citation[10] Arginase II is located primarily in the production of L-ornithine as a precursor to proline, glutamate, or polyamines such as spermine, spermidine, and putrescine, which are essential for cell growth.Citation[11]
In view of the above findings, the present study examined the effects of exogenous melatonin supplementation in different doses on arginase activity, ornithine, and NO levels in glycerol-induced ARF.
MATERIALS AND METHODS
Induction of ARF and Melatonin Treatment
Forty male Wistar-Albino rats weighing between 230 and 280 g were included in this study. All animal experiments were approved by the Trakya University School of Medicine Animal Care and Use Committee. The rats were maintained on standard rat chow and were dehydrated for 16 h. The rats were randomly divided into four groups in an equal number (n = 10): SHAM, serving as the control, was intramuscularly (im) applied 10 mL/kg of saline, and the ARF, ARF-M5, and ARF-M10 groups were administered 10 mL/kg of glycerol (50% v/v in sterile saline) im. Concomitantly, the SHAM, ARF, ARF-M5, and ARF-M10 groups were intraperitoneally applied saline, alcoholic saline (vehicle), 5 mg/kg melatonin, and 10 mg/kg melatonin, respectively. The intraperitoneal injections were repeated 24 hours later. The SHAM group received saline intraperitoneally in an equivalent volume, like the other groups. The dose of glycerol was divided into each hind limb equally. Melatonin was dissolved in absolute ethanol, and further dilutions were made in saline (7.5% ethanol).
The rats were followed up for 48 h without any diet or water restriction. The animals were sacrificed after 48 h;, at the time of sacrifice, the rats were anesthetized with 10 mg/kg xylazine and 50 mg/kg ketamine. Bilateral nephrectomy was performed. The renal tissue samples were stored at −70°C.
Determination of Tissue Arginase Activity and Ornithine Levels
Arginase activities and ornithine levels were determined in the all four groups of rats. The frozen tissues were separately weighted and homogenized in 10 volumes of cold 0.05 M Tris/HCl buffer (pH 8.05) using an automatic homogenizator. Samples then were centrifuged at 11,000 g for 20 minutes at 4°C. For the determination of tissue arginase activity and ornithine level, the methods of Geyer and DabichCitation[12] and ChinardCitation[13] were used, respectively.
For the measurement of arginase activity, 10 mM MnCl2 in 100 mM carbonate buffer (pH 9.7) was added to each tissue sample, and the enzyme was activated by heating at 55°C for 20 minutes. The substrate 50 mM L-arginine was then added and incubated at 37°C for 15 minutes. The reaction was terminated by the addition of an acid mix, (FeCl3+H3PO4+H2SO4). The urea formed was then assessed spectrophotometrically at 520 nm after the addition of color reagent (thiosemicarbazide and diacetylmonoxime) and heating at 100°C for ten minutes. One unit of arginase was defined as the amount of enzyme that released 1 μmol of urea for one minute at 37°C.
The measurement of ornithine levels was carried out by adding 10% TCA (1:1) to the tissue samples. The mixture then was centrifuged and glacial acetic acid added. Ninhidrine reagent was also added, and ornithine levels were determined spectrophotometrically at 515 nm after the incubation at 100°C for 30 minutes. Tissue ornithine levels were expressed as μmol/mg protein.
The tissue protein concentrations were determined according to Lowry et al.Citation[14]
Nitrite and Nitrate Assay
Nitrite and nitrate are the primary oxidation products of NO subsequent to reaction with oxygen, and therefore the nitrite/nitrate concentrations in kidney were used as an indicator of NO synthesis. The quantitation of nitrate and nitrite was based on the Griess reaction, in which a chromophore with a strong absorbance at 545 nm is formed by the reaction of nitrite with a mixture of naphthyl ethylenediamine and sulphanilamide.Citation[15] Samples were deproteinized with Somogyi reagent. The nitrate was reduced to nitrite by copper-coated cadmium in glycine buffer (pH 9.7). Total nitrite/nitrate concentrations were calculated by using standard of sodium nitrate. Results were expressed as μmol/mg protein.
Statistical Analysis
The results are expressed as mean ± standard deviation. The Mann-Whitney U tests were used for statistical analysis and p < 0.05 was considered statistically significant.
RESULTS
Ten rats—one, three, and six rats from ARF, ARF-M5, and ARF-M10 groups, respectively—died during the follow-up period and were substituted with new ones.
Kidney Tissue Arginase Activity and Ornithine Levels
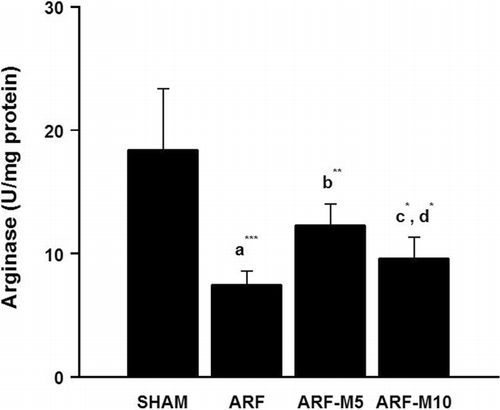
Kidney tissue arginase activities and ornithine levels were measured for the four experimental groups (SHAM, ARF, ARF-M5, and ARF-M10, n = 10). The mean values of arginase activities were 18.4 ± 5.2, 7.4 ± 1.2, 12.3 ± 1.8, and 9.6 ± 1.8 U/mg protein, respectively (see ). There was a significant difference between SHAM and ARF groups (p < 0.001) and kidney tissue arginase activities were significantly increased with two different doses of melatonin treatment (p < 0.01 and p < 0.05) when they were compared to ARF group. There was also a significant difference between ARF-M5 and ARF-M10 groups arginase activities (p < 0.05).
Figure 1 Kidney tissue arginase activities in SHAM, ARF, ARF-M5, and ARF-M10 groups: (a) the comparison between SHAM and ARF, (b) the comparison between ARF and ARF-M5, (c) the comparison between ARF and ARF-M10, (d) the comparison between groups (*p < 0.05, **p < 0.01, and ***p < 0.001). SHAM: Sham control; ARF: Acute renal failure treated with saline; ARF-M5: Acute renal failure treated with melatonin (5 mg/kg); ARF-M10: Acute renal failure treated with melatonin (10 mg/kg).
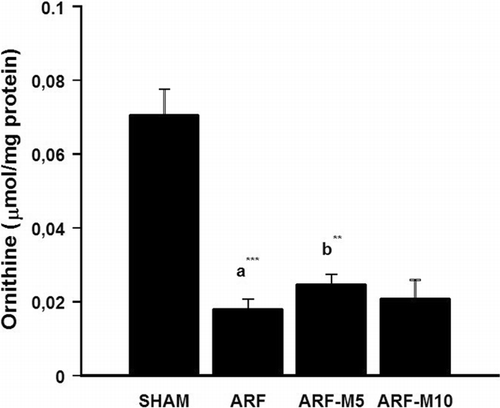
Kidney tissue ornithine levels were significantly decreased in the ARF group when compared to the SHAM group (p < 0.001), and ornithine levels were significantly increased in ARF-M5 group when compared to ARF group (p < 0.01). There was no other statistically significant difference between the other groups. The mean values of SHAM, ARF, ARF-M5, and ARF-M10 group ornithine levels were 0.07 ± 0.007, 0.018 ± 0.002, 0.024 ± 0.003 and 0.020 ± 0.005 μmol/mg protein, respectively (see ).
Figure 2 Kidney tissue ornithine levels in SHAM, ARF, ARF-M5, and ARF-M10 groups: (a) the comparison between SHAM and ARF, (b) the comparison between ARF and ARF-M5 groups (**p < 0.01 and ***p < 0.001). SHAM: Sham control; ARF: Acute renal failure treated with saline; ARF-M5: Acute renal failure treated with melatonin (5 mg/kg); ARF-M10: Acute renal failure treated with melatonin (10 mg/kg).
Kidney Tissue NO Levels
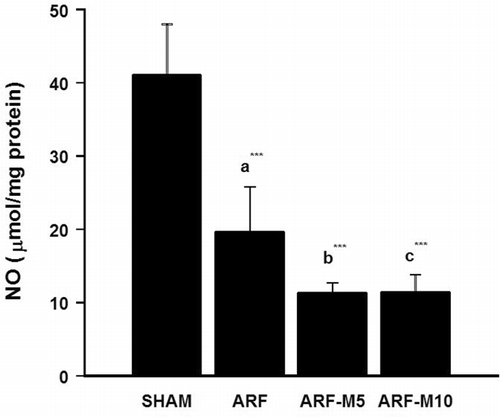
Kidney tissue NO levels were measured for the four experimental groups (n = 10). The mean values of SHAM, ARF, ARF-M5, and ARF-M10 group NO levels were 41.0 ± 7.3, 19.6 ± 6.4,11.3 ± 1.4, and 11.4 ± 2.5 μmol/mg protein, respectively (see ). Kidney tissue NO levels were significantly decreased in ARF groups when compared to the SHAM group (p < 0.001). NO levels were significantly decreased in both of ARF-M5 and ARF-M10 groups when they were compared to the ARF group (p < 0.001). There was no statistically significant difference between ARF-M5 and ARF-M10 groups (p > 0.05).
Figure 3 Kidney tissue NO levels in SHAM, ARF, ARF-M5, and ARF-M10 groups: (a) the comparison between SHAM and ARF; (b) the comparison between ARF and ARF-M5 groups; (c) the comparison between ARF and ARF-M10 (***p < 0.001). SHAM: Sham control; ARF: Acute renal failure treated with saline; ARF-M5: Acute renal failure treated with melatonin (5 mg/kg); ARF-M10: Acute renal failure treated with melatonin (10 mg/kg).
DISCUSSION
The pathophysiology of myoglobinuric acute ARF has been studied extensively in the animal model of glycerol-induced ARF.Citation[2],Citation[3] At the kidney level, the main pathophysiologic mechanisms in the genesis of myoglobinuric ARF have been identified as intense renal vasoconstriction, tubular obstruction by intraluminal cast formation, and direct heme protein-induced cytotoxicity.Citation[2] Several potential mechanisms may contribute to renal vasoconstriction/hypoperfusion in the setting of rhabdomyolysis. First, muscle necrosis causes dramatic fluid third spacing, leading to intravascular volume depletion. That third spacing effect causes hypovolemia and hypotension, which impair renal perfusion. Second, severe muscle injury can activate the endotoxin cytokine cascade, which may lead to renal vasoconstriction. Third, heme proteins scavenge NO, a potent renal vasodilator, causing renal vasoconstriction and, as a result, reducing renal blood flow.Citation[2]
In this study, the present authors have reported—perhaps for the first time—the relationship between melatonin and arginase activity and ornithine levels.
Arginase I is a cytosolic isoform that is highly expressed in the liver and constitutes >98% of the total body arginase activity. Arginase II is a mitochondrial isoform that contributes to the remaining 2% of the total body arginase activity. It is present in many nonhepatic tissues, such as the kidney, mammary gland, brain, intestine, and lung. Extrahepatic arginase may be involved in the regulation of cell growth and tissue repair. L-ornithine, the amino acid metabolite of arginase, may be converted to glutamate and proline by a mitochondrial enzyme ornithine aminotransferase.Citation[16] Ornithine is the common substrate for the synthesis of proline, glutamate, and polyamines in mammalian cells.Citation[17] Glutamate can be converted either to amino acids for protein synthesis or to α-ketoglutarate, and proline is an amino acid that is essential for the synthesis of many structural proteins, including collagen. Polyamines (putrescine, spermine, and spermidine) have also been shown to play an important role in cell proliferation and growth.Citation[16],Citation[17] An increase in the kidney tissue ornithine levels might suggest that melatonin could show protective effects, which as shown in several studies might be via this pathway.
NOSs and arginases can compete for their common substrate, L-arginine, and the interaction between these two enzymes represents a potentially important factor in the regulation of NO production. Increased arginase activity could limit NO synthesis by reducing L-arginine availability for NOS.Citation[18] It has been reported that arginase activity is five-fold greater than NOS activity, and arginase was found to be the major pathway of L-arginine metabolism in nephritic glomeruli.Citation[19] Likewise, it was found that while arginase activity increases, NOS activity decreases in the erythrocytes of the patients with chronic renal failure.Citation[20] In a previous study, the present authors have shown that there was a negative relation between arginase enzyme activities and NO levels in renal ischemia-reperfusion injury.Citation[21] Changes in the relative activities of these enzymes have been suggested to alter NO production in experimental glomerulonephritis.Citation[22] Zhang et al.Citation[23] previously showed in activated macrophages that arginase can compete with NOS for its common substrate L-arginine and thus inhibit NO production and NO-mediated vasodilatatory function. In addition, in microvascular endothelial cells, arginase has been shown to counteract nitric oxide-mediated vasodilatation, and inflammatory molecules turned on the arginase pathway while, at the same time, turned down the eNOS pathway.Citation[23]
In many studies, it has been reported that melatonin has a scavenging effect on NO and has an inhibiting NOS enzyme activity.Citation[4],Citation[5],Citation[9],Citation[24–26] Melatonin can regulate the production of NO through its interaction with the enzymes that synthesize it. It has been shown that melatonin inhibits nNOS, iNOS, and mtNOS activity in various models.Citation[9] In addition, in the study of Aerseth et al.,Citation[27] it has been reported that melatonin has increased the vasoconstrictive effect of noradrenaline on the kidney arteries of seals and pigs, and that the selective melatonin receptor antagonist luzindole has reduced this potentiation 80%. Sokkary et al.Citation[28] have shown that melatonin significantly decreased kidney NO levels in mice infected with Schistosoma mansoni. These decreasing effects of melatonin on NO that were shown in the previous studies were parallel to the current findings. The finding of the inhibitory effect of melatonin on kidney NO level in this study may be due to inhibiting NOS activity, which has been shown previously.Citation[25],Citation[26] Moreover, the stimulatory effect of melatonin on the arginase enzyme activity may be seen as a result of NOS inhibition. In other words, the inhibitory effect of melatonin on the NOS activity may lead to an increased arginine concentration, which is the common substance for both the arginase and NOS enzymes. Therefore, an increased arginine concentration may result in an increment of the arginase enzyme activity. The present finding of low-dose melatonin has a higher increase on the arginase enzyme activity than the higher dose, and this effect may be a result of further ischemic conditions formed by a higher dose of melatonin in this model.
Previously, the current authors demonstrated that there was an increase in plasma urea and creatinine levels, which is an indicator of impaired glomerular function; an increase in MDA levels, which reflects lipid peroxidation; a significant decrease in SOD, CAT, and GPx enzyme activities; and severe renal morphological impairment in the glycerol treatment group. In addition, NO, which is an important mediator in the pathophysiology of this model, was also shown to be decreased in the same study. In this model, no beneficial effects of melatonin in different doses was shown. Moreover, melatonin significantly reduced plasma NO levels in both of the doses.Citation[29] The depletion effect of melatonin on NO may be responsible for worsening the effects of melatonin in the glycerol-induced ARF model. The decreasing effect of melatonin on NO may lead to intensify the renal ischemia and also increase the tubular damage. As a result of this mechanism, no protective effects of melatonin were found. The nitric oxide system has been studied in renal impairment associated with heme protein. One of these studies showed that the administration of arginine protected against renal dysfunction, whereas the administration of an inhibitor of nitric oxide synthase worsened the renal function in the glycerol model of ARF.Citation[30] In the present authors' previous study, no beneficial effects of melatonin in different doses was found.Citation[29] The decreasing effect of melatonin on NO may lead to an intensification of the renal ischemia, and therefore, no protective role of melatonin in glycerol-induced ARF was found. On the other hand, the protective role of melatonin in the myoglobinuric ARF was reported by Ferraz et al.Citation[31] The contradictory results between Ferraz et al.Citation[31] and this study may be due to different doses of given glycerol to obtain ARF, different times for the second injection of melatonin, or the investigated parameters taken from the glycerol-induced ARF model in the different times.
In conclusion, in this study, the authors have shown that while melatonin increased arginase activity and ornithine levels, it decreased NO levels. It is suggested that besides the direct scavenging of NO, the stimulatory effect of melatonin on arginase activity may result in an inhibition of NOS activity and decrease the kidney NO level. Moreover, it is possible that one of the protective mechanisms of melatonin might be through the stimulation on ornithine levels. Possible relation(s) and mechanism(s) between arginase enzyme and melatonin need to be further investigation.
REFERENCES
- Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nephrol 2000; 11: 1553–1561, [INFOTRIEVE], [CSA]
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996; 49: 314–326, [INFOTRIEVE], [CSA]
- Baliga R, Zhang Z, Baliga M, et al. Evidence for cytochrome p-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 1996; 49(2)362–369, [INFOTRIEVE], [CSA]
- Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev 2002; 123: 1007–1019, [INFOTRIEVE], [CSA], [CROSSREF]
- Tan DX, Manchester LC, Reiter RJ, et al. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept 2000; 9: 137–159, [INFOTRIEVE], [CSA], [CROSSREF]
- Morris SM. Enzymes of arginine metabolism. J Nutr 2004; 134: 2743S–2747S, [INFOTRIEVE], [CSA]
- Boucher JL, Moali C, Tenu JP. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci 1999; 55: 1015–1028, [INFOTRIEVE], [CSA], [CROSSREF]
- Cherla G, Jaimes EA. Role of L-arginine in the pathogenesis and treatment of renal disease. J Nutr 2004; 134: 2801S–2806S, [INFOTRIEVE], [CSA]
- Jeon J, Acuna-Castroviejo D, Sainz RM, et al. Melatonin nd mitochondrial function. Life Sci 2004; 75: 765–790, [CSA], [CROSSREF]
- Ash DE. Structure and function of arginases. J Nutr 2004; 134: 2760S–2764S, [INFOTRIEVE], [CSA]
- Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide 2000; 4: 441–458, [INFOTRIEVE], [CSA], [CROSSREF]
- Geyer JW, Dabich D. Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem 1971; 39: 412–417, [INFOTRIEVE], [CSA], [CROSSREF]
- Chinard FP. Photometric estimation of proline and ornithine. J Biol Chem 1952; 199: 91–95, [INFOTRIEVE], [CSA]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275, [INFOTRIEVE], [CSA]
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 1990; 36: 1440–1443, [INFOTRIEVE], [CSA]
- Que LG, Kantrow SP, Jenkinson CP, et al. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol 1998; 275: 96–102, [CSA]
- Li H, Meininger CJ, Hawker JR, et al. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 2001; 280: 75–82, [CSA]
- Ishii N, Ikenaga H, Carmines PK, et al. High glucose augments arginase activity and nitric oxide production in the renal cortex. Metabolism 2004; 53(7)868–874, [INFOTRIEVE], [CSA], [CROSSREF]
- Jansen A, Lewin S, Cattell V, et al. Arginase is a major pathway of L-arginine metabolism in nephritic glomeruli. Kidney Int 1992; 42: 1107–1112, [INFOTRIEVE], [CSA]
- Durak I, Ozturk HS, Elgun S, et al. Erythrocyte nitric oxide metabolism in patients with chronic renal failure. Clin Nephrol 2001; 55: 460–464, [INFOTRIEVE], [CSA]
- Erbas H, Aydogdu N, Kaymak K. Effects of N-acetylcysteine on arginase, ornithine and nitric oxide in renal ischemia-reperfusion injury. Pharmacol Res 2004; 50: 523–527, [INFOTRIEVE], [CSA], [CROSSREF]
- Cook HT, Jansen A, Lewis S, et al. Arginine metabolism in experimental glomerulonephritis: interaction between nitric oxide synthase and arginase. Am J Physiol. Renal Physiol 1994; 267: F646–F653, [CSA]
- Zhang C, Hein TW, Wanng W, et al. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB 2001; 15: 1264–1266, [CSA]
- Turjanski AG, Leonik F, Estrin DA, et al. Scavenging of nitric oxide by melatonin. J Am Chem Soc 2000; 122: 10468–10469, [CSA], [CROSSREF]
- Rao VSN, Santos FA, Silva RM, et al. Effect of nitric oxide synthase inhibitors and melatonin on the hyperglycemic response to streptozotocin in rats. Vascul Pharmacol 2002; 38: 127–130, [INFOTRIEVE], [CSA], [CROSSREF]
- Zhang S, Li W, Gao Q, et al. Effect of melatonin on the generation of nitric oxide in murine macrophages. Eur J Pharmacol 2004; 501: 25–30, [INFOTRIEVE], [CSA], [CROSSREF]
- Aarseth JJ, Nordoy ES, Stokkan KA. Melatonin potentiates the vasoconstrictive effect of noradrenaline in renal artery from newborn hooded seals (Cystophora cristata) and harp seals (Phoca groenlandica). J Comp Physiol [B] 2001; 171: 491–496, [CSA]
- Sokkary GHE, Omar HM, Hassanein AFMM, et al. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Rad Biol Med 2002; 32(4)319–332, [INFOTRIEVE], [CSA], [CROSSREF]
- Aydogdu N, Atmaca G, Yalcin O, et al. Effects of exogenous melatonin on myoglobinuric acute renal failure in the rats. Ren Fail 2004; 26(5)479–486, [INFOTRIEVE], [CSA], [CROSSREF]
- Maree A, Peer G, Schwartz DA, et al. Role of nitric oxide in glycerol-induced acute renal failure in rats. Nephrol Dial Transplant 1994; 9(Suppl.)78–81, [INFOTRIEVE], [CSA]
- Ferraz FF, Kos AG, Janino P, Homsi E. Effects of melatonin administration to rats with glycerol-induced acute renal failure. Ren Fail 2002; 24(6)735–746, [INFOTRIEVE], [CSA], [CROSSREF]
