Abstract
A young female with essential hypertension developed progressive azotemia; renal biopsy showed hypertensive nephrosclerosis with considerable tubulointerstitial disease and cellular infiltration. The addition of mycophenolate mofetil (MMF) to her antihypertensive treatment resulted in a dramatic improvement of renal function during the following three months. When the patient discontinued MMF treatment, end-stage renal failure rapidly developed. This patient represents the first report of the beneficial use of MMF in non-immune chronic renal disease and demonstrates that significant functional improvement may be obtained with the addition of MMF to the treatment of hypertensive nephrosclerosis for patients in whom there is significant tubulointerstitial inflammatory infiltration.
INTRODUCTION
Recent evidence suggests that progressive renal failure of non-immune etiology may be slowed or improved with a combination of anti-inflammatory therapies; among them, the suppression of angiotensin II activity and the immunosuppressive drug mycophenolate mofetil (MMF) have been used alone or in combination Citation[1–5] to retard or revert the end-stage renal disease resulting in the remnant nephrons after extensive renal ablation. In humans, renal functional deterioration may be prevented and, in some cases, improved if aggressive treatment is started early in the course of renal disease.Citation[6] To date, there have been no reports with the combined use of angiotensin-converting enzyme inhibitors (ACEI) and MMF in chronic renal disease of non-immune etiology in man.
This study presents a case of hypertension-induced nephrosclerosis and progressive renal failure that was not improved after blood pressure control with multiple drug therapy, including the use of ACEI, but was dramatically ameliorated with the addition of MMF to the treatment.
CASE PRESENTATION
A 22-year-old patient with strong family history of essential hypertension was found to have elevated blood pressure during her second pregnancy and remained hypertensive since that time. She did not receive regular treatment. Serum creatinine and urinary sediment were normal after her last pregnancy. She had consulted for pelvic inflammatory disease (PID). Her past medical history was otherwise unremarkable; she denied episodes of hematuria or edema. Blood pressure was 157/110 mmHg. She had proteinuria (0.6 gr./day), and urinary sediment was unremarkable. Serum creatinine was 2.6 mg/dL, hemoglobin was 10.9 g/dL, total cholesterol was 197 mg/dL, and the rest of the routine hematologic and biochemical studies were within normal limits. Serum anti-DNA, anti-nuclear antibodies, ANCA, and anti-GBM antibodies were all negative, and serum C3 and C4 levels were normal. She was started in antibiotic treatment for her PID and referred for outpatient control, receiving hydralazine (200 mg/day), furosemide (60 mg/day) and captopril (150 mg /day) for her hypertension.
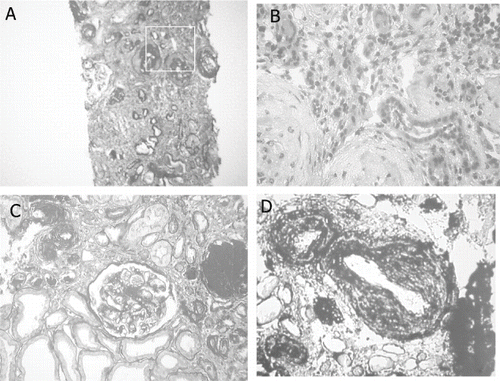
She failed to report to the outpatient clinic until four months later, when her blood pressure was 160/110 and her serum creatinine 5.2 mg/dL. She had taken medication irregularly because she was unemployed. She was admitted to the hospital, and her blood pressure controlled with hydralazine (300 mg/day), furosemide (240 mg/day), and captopril (100 mg/day). Renal artery stenosis was ruled out by angiography. Ultrasound evaluation revealed a reduced kidney size (right kidney: 7.9 cm, left kidney: 7.5 cm). Percutaneous renal biopsy was performed (see ): one glomerulus (out of 13) was normal, one showed mesangial expansion, and the rest showed variable degrees of sclerosis. Three glomeruli were completely obliterated with global sclerosis. Interstitial fibrosis and cellular infiltration were very prominent. The arterioles showed medial wall thickening and myointimal cell proliferation. Staining for IgG, IgM, IgA, and C3 were negative. She was discharged, and for the next three months, blood pressure was under control (varying between 140/90 to 130/80). However, serum creatinine rose to 6.3mg/dL; plasma clearance of 125I-thalamate (U*V/P) was 6.5 mL/min (mean of three determinations).
Figure 1 Renal biopsy findings. Panel 2A (PAS staining) shows the widening of interstitial areas with cellular infiltration and tubular dilatation. In 2B, two obsolescent glomeruli surrounded by intense interstitial infiltration are shown. 2C (PAS) shows a glomerulus with preserved structure and mesangial expansion, tubular dilatation, and atrophy, as well as areas of mesangial thickening; in the upper right-hand corner, two arterioles show appreciable wall thickening. 2D (silver methenamine staining) shows arterioles with myointimal proliferation.
The possibility of using MMF for a short (one-month) therapeutic trial was presented to the patient, who gave her informed consent, and MMF (2 g/day, reduced to 1.5 g/day after nausea and diarrhea) was begun. In the following weeks, creatinine decreased progressively. MMF was continued indefinitely. Creatinine reached 3.6 mg/dL, and the anti-hypertensives could be reduced (hydralazine: 100 mg/day, furosemide: 80 mg/day, enalapril: 40 mg/day). However, after three months with this combination therapy, compliance became irregular again. She had severe socioeconomic problems and was lost from control. Afterwards, she was re-admitted with uncontrolled hypertension (180/110 mmHg), and her serum creatinine was 8.2 mg/dL. Hemodialysis was started.
DISCUSSION
This patient illustrates how the addition of MMF to antihypertensive treatment may result in the improvement in renal function in patients with hypertension-induced nephrosclerosis. This diagnosis was suspected clinically in this patient, with her strong family history of hypertension, moderate proteinuria, and no evidence of systemic disease, and confirmed by renal biopsy, as clinical observations may be misleading.Citation[7–10] The possibility that the patient had a primary renal disease that could conceivably benefit from MMF was considered, but the demonstration of a prominent interstitial infiltrate suggested that a trial with MMF may be useful, especially as antihypertensive treatment had not altered the progressive increment in serum creatinine.
The renal infiltration of immunocompetent cells is a common feature of progressive renal damage,Citation[11] and MMF may offer protection in experimentally induced atherosclerosis.Citation[12] Furthermore, experimental studies have already shown the benefit of using MMF and ACEI in the treatment of established renal damage of non-immune origin.Citation[4],Citation[5] This patient offers clinical validation to these experimental studies. Despite blood pressure control, her serum creatinine rose to 6.3 mg/dL, and only when MMF was started did creatinine diminish over the following months. It is not possible to speculate how much longer the improvement would have lasted had the patient not stopped treatment, but the cessation of MMF treatment was rapidly associated with deterioration of renal function.
REFERENCES
- Romero F, Rodríguez-Iturbe B, Parra G, González L, Herrera-Acosta J, Tapia E. Mycophenolate mofetil prevents the progression of renal failure induced by 5/6 renal ablation in rats. Kidney Int 1990; 55: 945–955, [CSA]
- Tapia E, Franco M, Santamaría J, Zafra I, Quiroz Y, Rodríguez-Iturbe B, Herrera-Acosta J. Mycophenolate mofetil prevents arteriolopathy and renal injury in subtotal ablation despite persistent systemic hipertensión. Kidney Int 2003; 63: 994–1002, [INFOTRIEVE], [CROSSREF], [CSA]
- Fujihara CK, Malheiros DM, Zatz R, Noronha ID. Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney Int 1998; 54: 1510–1519, [INFOTRIEVE], [CROSSREF], [CSA]
- Fujihara CK, Noronha IL, Malheiros I, Antunes GR, de Oliveira IB, Zatz R. Combined mycophenolate mofetil and losartan therapy arrests established injury in the remnant kidney. J Am Soc Nephrol 2000; 11: 283–290, [INFOTRIEVE], [CSA]
- Remuzzi G, Zoka G, Gagliardini E, Corna D, Abbate M, Benigni A. Combining an antiproteinuric approach with mycophenolate mofetil fully suppresses progressive nephropathy of experimental animals. J Am Soc Nephrol 1999; 10: 1542–1549, [INFOTRIEVE], [CSA]
- Ruggenenti P, Schieppatu A, Remuzzi G. Progression, remission, regression of chronic renal disease. Lancet 2001; 357: 1601–1608, [INFOTRIEVE], [CROSSREF], [CSA]
- Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study Kidney Disease (AASK) Trial. Kidney Int 1997; 51: 244–252, [INFOTRIEVE], [CSA]
- Mazzuchi N, Schwedt E, Fernandez JM, Cusumano AM, Ancao MS, Poblete H, Saldana-Arevalo M, Espinosa NR, Centurion C, Castillo H, Gonzalez F, Milanes CL, Infante M, Ariza M. Latin American registry of dialysis and renal transplantation: 1993 Annual Dialysis Data Registry. Nephrol Dial Transplant 1997; 12: 2521–2527, [INFOTRIEVE], [CROSSREF], [CSA]
- Zucchelli P, Zuccalla A. Recent data on hypertension and progressive renal disease. J Human Hypertens 1996; 10: 679–682, [CSA]
- Caetano ER, Zatz R, Saldanha LB, Praxedes JN. Hypertensive nephrosclerosis as a relevant cause of chronic renal failure. Hypertension 2001; 38: 171–176, [INFOTRIEVE], [CSA]
- Rodríguez-Iturbe B, Pons H, Herrera-Acosta J, Jonson RJ. The role of immunocompetent cells in non-immune renal diseases. Kidney Int 2001; 59: 1626–1640, [CROSSREF], [CSA]
- Romero F, Rodríguez-Iturbe B, Pons H, Parra G, Quiroz Y, Rincón J, González L. Mycophenolate mofetil reduces cholesterol-induced atherosclerosis in the rabbit. Atherosclerosis 2000; 152: 127–133, [INFOTRIEVE], [CROSSREF], [CSA]
