Abstract
Serum levels of lipids and lipoproteins were determined in 98 post-renal transplant fasting patients, and lipids and non-high density lipoprotein-cholesterol (non-HDL-C) and lipid ratios in the same post-renal transplant non-fasting patients were compared. The reference group was 87 healthy subjects. All patients were divided into two groups: patients with dyslipidemia (n = 69) and patients with normolipidemic (n = 29). The post-renal transplant patients (TX) with dyslipidemia had a significantly increased concentration of triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), non-HDL-C, apoB, and TRL and lipid ratios, and decreased HDL-C level and lipoprotein ratios. The lipids, lipoproteins, and lipoprotein ratios were significantly beneficial in TX patients with normolipidemic than in those with dyslipidemia. However, TRL concentration and lipid ratios were significantly increased and apoAI/apoCIII significantly decreased as compared to the reference group. The TX patients with dyslipidemia showed a significant correlation between TG and apoB:CIII (r = 0.562, p < 0.001) and apoCIII (r = 0.380, p < 0.004), but those with normolipidemic showed a significant correlation only between TG and apoCIII (r = 0.564, p < 0.008). Regression and Bland-Altman analyses showed excellent correlation between fasting and nonfasting non-HDL-C levels (r = 0.987, R2 + 0.987) in TX patients both with dyslipidemia and normolipidemic. We think the finding that nonfasting labs that are reliable for non-HDL-C as well as total cholesterol is important, as fasting labs are not always available. Disturbances of lipids, lipoproteins, and TRLs depend not only on the kind of treatment, but due to multiple factors can accelerate cardiovascular complications in post-renal transplant patients with dyslipidemia and also with normolipidemic. Further studies concerning this problem should be completed.
INTRODUCTION
The lipid abnormalities seen after renal transplantation are a complex mixture, attributable partly to drug treatment with steroids and cyclosporine, to impaired renal function, and to other factors such as persistent hyperparathyroidism.Citation[1] The typical pattern includes marked hypercholesterolemia and moderate hypertriglyceridemia (TG) with increased apolipoprotein B (ApoB).Citation[1] Recently, mounting evidence from a number of sources has supported an independent association between hypertriglyceridemia and coronary artery disease (CAD) risk.Citation[2] This relation is likely to stem from the atherogenicity of some species of TG-rich lipoproteins (TRL), particularly small very-low-density lipoprotein (VLDL) and intermediate-density lipoprotein particles (IDL).Citation[2],Citation[3] One of the leading causes of long-term failure of transplanted organ is chronic rejection (CR) or chronic allograft nephropathy (CAN). The pathogenesis of CAN involves a combination of immunologic and nonimmunologic factors.Citation[4–6] The alloreaction participates in the development of CAN mainly through activated monocytes and macrophages. These cells have the ability to synthesis and secrete cytokines, and, more importantly, growth factors, which act on smooth muscle cells and fibroblasts to stimulate proliferation and synthesis of connective tissue proteins.Citation[6] Several nonimmunologic risk factors have also been shown to exert an impact on the CAN process.Citation[5] These factors include ischemia-reperfusion damage, hypertension, hypercholesterolemia, and obesity.Citation[4],Citation[5] Lipoprotein abnormalities have been reported to be common among transplant patients, particularly during the use of cyclosporine-based immunosuppressive protocols.Citation[4–6] The Assessment of Lescol in Renal Transplantation (ALERT) trial is an investigator-initiated and investigator-led study designed to investigate the effects of fluvastatin on cardiac and renal endpoints in renal transplant recipients.Citation[7] Fluvastatin treatment significantly improves lipid values in renal transplant recipients but has no effect on graft loss or doubling of serum creatinine.Citation[4]
ApoB 100 concentrations are not affected by meals; however, these measurements are expensive and not readily available in most clinical centers.
Non-HDL cholesterol represents the sum of LDL, IDL, and VLDL cholesterol levels and correlates highly with ApoB level. TC (total cholesterol) and HDL cholesterol (HDL-C) results are similar whether measured in the fasting or nonfasting state, which makes non-HDL-C a reliable measure for general population.Citation[8] However, the applicability of these data to post-renal transplant patients has never been evaluated.
The present study investigates disturbed lipid and lipoprotein and triglyceride-rich lipoprotein concentration, and tests the hypothesis that nonfasting non-HDL-C level is as reliable as fasting non-HDL-C level in post-renal transplant patients with normo- and dyslipidemia.
PATIENTS AND METHODS
Serum levels of lipids and lipoproteins were determined in 98 post-renal transplant fasting patients, and lipids and non-HDL-C and lipid ratios compared in the same post-renal transplant non-fasting patients. The reference group were 87 subjects (45 males and 42 females, 25–54 years of age) chosen from among apparently normolipidemic individuals. The studied patients were without active inflammatory disease and malignancy. Moreover, 7 patients had diabetes, and 65 patients had hypertension. The cause of renal insufficiency in post-renal transplant patients with dyslipidemia was 58 glomerulonephritis, 5 interstitial nephritis, 10 polycystic disease, 3 hypertensive nephrosclerosis, 7 congenital defects, 25 unknown. The post-renal transplant patients received cyclosporine A + prednisone (n = 67), tacrolimus + prednisone (n = 28) and sirolimus + prednisone (n = 3), and lovastatin or simvastatin (n = 46) and fibrate (n = 2). Fifty patients were without anti-lipid drugs treatment because 5 of them with dyslipidemia had dyspepsia or myopathy disturbs, 2 did not regularly receive medicines, 26 had minimal lipids disturbs, and 17 with normolipidemic did not require anti‐lipid lowering therapy.
Hypertensive patients were using anti-hypertensive medications of either calcium channel blockers or angiotensin converting enzyme antagonists, blockers AT1, and alpha-blockers, but not diuretics. The patients who received beta-blockers were in groups with normo- and dyslipidemia.
All patients (n = 98) were divided into two groups: patients with dyslipidemia (n = 69) and with normolipidemic (n = 29). The difference between normolipidemic and dyslipidemia in post-renal transplant patients concern LDL-C, HDL-C, and non-HDL-C because the value of these parameters infringe the norm. The concentration of TG in normolipidemic patients is lower than 150mg/dL as a cut of patients. The clinical and laboratory parameters in post-renal transplant patients are presented in .
Table 1 Clinical and laboratory parameters is post–renal transplant patients
Lipid and lipoprotein profiles were obtained in serum after 14-hour overnight fasting, and lipid profiles in the same patients were obtained after a meal (non-fasting).
Routine laboratory parameters (the level of urea, uric acid, creatinine, total protein, albumin) were determined using Au 400 analyzer (Olimpus), and hemoglobin using ADVIA analyzer, Bayer. Lipids and lipoproteins were determined on Hitachi 902 analyzer. The total cholesterol (TC) was estimated by the enzymatic-colorimetric method, Biomaxima; and HDL-C by the direct method with immunoinhibition, AB-VACO, Biomaxima. HDL cholesterol, which is not bound with enzymes (cholesterol esterase and cholesterol oxidase), and chromogens producing a colored complex were determined. Triglycerides (TG) were determined using the standard enzymatic technique (Biomaxima). LDL-cholesterol (LDL-C) was calculated according to the Friedewald formula. Non-HDL-C was calculated as total cholesterol minus HDL-C. Apo AI, apoB were measured by Roche kit using the turbidimetric methods. TRL were separated as non-HDL lipoproteins from apoCIIInonB in the HDL fraction using anti-apoB antibodies. The method with applied anti-apoB antibody separating apoB-containing lipoproteins in VLDL+LDL (non-HDL) and non-apoB-containing lipoproteins in HDL can be used in the diagnosis and treatment of patients both with chronic allograft nephropathy and atherosclerosis in post-renal transplant patients. Lipoproteins (total apoCIII, apoCIIInonB and total apoE and apoEnonB) were measured by electroimmunodiffusion according to Laurell using a commercial kit Sebia, USA, as described previously.Citation[9]
Statistical Analysis
Statistical analysis was performed using one-way analysis of the ANOVA variance and multiple comparisons for assessment of the mean ± standard deviation (SD) in post-renal transplant patients and compared to the reference group, but to compare post-renal transplant fasting and nonfasting patients, the paired t-test was used. The correlation between variables was analyzed using Pearson's correlation coefficient. Linear regression between fasting and nonfasting lipids and lipid ratios in post-renal transplant patients were used. Bland-Altman analysis was to compare the two methods. The statistical significance of all variables was established at the level p < 0.05, and statistical analysis was performed using the STATISTICA program (Statsoft Polska, Krakow, Poland).
RESULTS
The lipid and lipoprotein parameters of post-renal transplant patients and the reference group are shown in . The post-renal transplant patients with dyslipidemia have a significantly increased concentration of TG, LDL-C, non-HDL-C, apoB, apoCIII, apoCIIInonB, and TRL and lipid ratios (TC/HDL-C, LDL-C/HDL-C, TG/HDL-C), and decreased HDL-C level and lipoprotein ratios (apoAI/apoB, apoAI/apoCIII). These disturbances very clearly characterized dyslipidemia in post-renal transplant patients. The patients with normolipidemic had a concentration of TG < 150 mg/dL, but the concentration of TRL was significantly higher compared with the reference group. The lipid, lipoprotein, and lipoprotein ratios were significantly beneficial in post-renal transplant patients with normolipidemic than in those with dyslipidemia. However, TRL concentration and TC/HDL-C, LDL-C/HDL-C, TG/HDL-C were significantly increased and apoAI/apoCIII significantly decreased as compared to the reference group. The post-renal transplant patients with dyslipidemia showed a significant correlation between TG and apoB:CIII (r = 0.562, p < 0.001) and apoCIII (r = 0.380, p < 0.004), but with normolipidemic only between TG and apoCIII (r = 0.564, p < 0.008). Paired t-test analysis of fasting and nonfasting lipid profiles and lipid ratios presented a reduction of TC, LDL-C, HDL-C and LDL-C/HDL-C ratio, and elevated TG concentration and TG/HDL-C ratio post-renal transplant patients (see ). Regression and Bland-Altman analyses (see ) showed excellent correlation between fasting and nonfasting non-HDL-C levels (see and ; r = 0.994, R2 = 0.988) in post-renal transplant patients both with dyslipidemia and normolipidemic. Moreover, TG/HDL-C ratio was significantly increased in both studied groups of patients, but the correlation between TG/HDL-C and non-HDL-C was statistically significant only in fasting and nonfasting post-renal transplant patients with dyslipidemia.
Figure 1. Regression and Bland-Altman analysis for non-HDL cholesterol (Pearson's coeff. corr. R = 0.987, coeff.determ. R2 = 0.987).
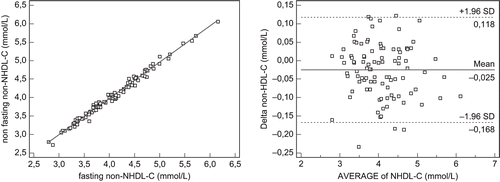
Table 2 Fasting lipid and lipoprotein profiles and lipid and lipoprotein ratios
Table 3 Paired t-test analysis of fasting and nonfasting lipid profiles
Table 4 Linear regression between fasting and nonfasting lipids and lipid
Table 5 Pearson's correlation
DISCUSSION
Renal transplant recipients are at increased risk of premature cardiovascular disease.Citation[7] After renal transplantation, various types of metabolic dysfunctions are associated with chronic renal failure reverse, but lipid abnormalities appear to progress in a large fraction of patients.Citation[1]
Previously, we showed that the CRF patients had disturbed lipoprotein composition, but dyslipidemia was secondary to the disturbed lipoproteins, which can be modified in dependence of the advanced renal failure and type of dialysis or CRF treatment. The variability of TG and HDL-C concentration depends on the variability of TRLs and cholesterol-rich lipoprotein concentrations, but TG decrease and HDL-C concentration increase are caused by apoAI concentration variability.Citation[10] The post-renal transplant patients in one long-term study showed that they had disturbed lipoprotein composition, and its consequence of hyperlipidemia was probably in part due to the increased use of immunosuppressant and steroids.Citation[9] In the present study, we have shown for the first time the results of lipids, lipoproteins, and TRLs in a large group of post-renal transplant patients with normo- and dyslipidemia, and of fasting and non-fasting state of non HDL-C. The post-renal transplant patients were treated with cyclosporine A and prednisone, as well as prednisone and tacrolimus which were used as the main medicines. The post-renal transplant patients presented high TRL levels (VLDL+LDL) not only in dyslipidemia but in normolipidemic, too. The patients with normolipidemic showed significant differences between the concentrations of apoCIII, apoCIIInonB, apoB:CIII and that of apoE, apoEnonB, apoB:E, and significant differences in lipid and lipoprotein ratios as compared to the reference group. The post-renal transplant patients had a significant correlation between the concentrations of non-HDL-C and TG and apoCIII and apoB:CIII, which suggests impaired metabolism of TRL (small dense LDL). However, the disturbed the concentration of apoCIII and apoE can cause a decrease in cell uptake of apoB:CIII lipoproteins.Citation[10] A high apoCIII level, which is an endogenous lipoprotein lipase inhibitor, may be involved in the occurrence of small LDL. Interestingly, it has been reported that immunosuppressive drugs enhanced apoCIII levels in kidney transplantation.Citation[11] ApoCIII/apoAI ratio could be of particular interest when TG levels (≥4.5 mmol/L) interfere with HDL-C measurement.Citation[11] Our post-renal transplant patients with dyslipidemia had a higher TG, non-HDL-C, apoCIII, TRL, and BMI, and lower HDL-C than those with normolipidemic. Several cross-sectional studies and prospective studies have demonstrated that atherogenic risk is higher in hypertriglyceridemic patients with elevated apoB than in hypertriglyceridemic patients with normal apoB.Citation[12],Citation[13] However, the risk is not related directly to TG levels but to the number of small dense LDL particles.Citation[11–13] We showed that post-renal transplant patients with dyslipidemia had elevated both the mass of cholesterol (non-HDL-C) and apoB (the number of atherogenic particles) in apoB-containing lipoproteins (TRL). Moreover, post-renal transplant patients with normolipidemic had a normal mass of cholesterol (non-HDL-C) and apoB, but they had elevated TRL (apoB:CIII) and decreased apoAI/CIII ratio. These results suggest disturbed lipoprotein metabolism in normolipidemic post-renal transplant patients (small dense LDL and small dense HDL). Our study clearly shows that TRL (apoCIII, apoB:CIII) and lipid (TC/HDL-C, LDL-C/HDL, TG/HDL) and lipoprotein (apoAI/apoCIII) ratios are very sensitive markers of early dyslipoproteinemia in post-renal transplant patients. Chan et al.Citation[14] demonstrated that in overweight-obese individual's plasma concentrations of apoB-48, remnant-like particle (RLP)-cholesterol and non-HDL-C were partly dependent on the catabolism of apoB-100-containing lipoproteins, and this may be the consequence of increased competition for hepatic uptake between chylomicron and VLDL-remnants. Further studies should examine the effect of weight reduction, fibrate treatment, or insulin sensitizers on the association between these surrogate TRL markers and apoB-100 kinetics in obese and/or diabetic post-renal transplant patients.
We showed that 70% of our post-renal transplant patients had dyslipidemia and dyslipoproteinemia, and only 30% post-renal transplant patients had normolipidemic.
Corticosteroids appear to interact with cyclosporin A to worsen lipid profiles, and hyperlipidemia has been related to corticosteroid dose.Citation[15] Corticosteroids may raise HDL-C levels by increasing apoA-I production by the liver or/and via a mechanism decreasing CETP activity. Significantly reduced HDL-C levels and higher plasma CETP activity were observed after prednisone therapy.Citation[16–18] It was observed that the levels of TC, LDL-C, TG, and apoB in renal transplant recipients treated with cyclosporin A and prednisone were significantly higher than in patients treated with azathioprine plus prednisone. Cyclosporin A may cause alterations in lipids and lipoproteins by binding to the LDL receptor and altering its activity or by inhibiting bile acid synthesis.Citation[15],Citation[19] Post-renal transplant diabetes mellitus has been recognized as one of the most serious complications related to the use of corticosteroids and calcineurin-inhibitors.Citation[20] The maintenance of immunosuppression with cyclosporine (CSA) is associated with nephrotoxicity, hyperlipidemia, and hypertension.Citation[21] Significant improvements in creatinine and BUN were observed following conversion from cyclosporine to tacrolimus.Citation[22],Citation[23] A triple-therapy regimen of MMF, steroids, and low-dose CSA has been reported to be successful in allowing for improved renal function and good prevention of acute rejection episodes.Citation[21] CETP facilitates the transfer of cholesteryl esters (CE) from HDL to apoB-containing lipoproteins (VLDL and LDL) with a reciprocal transfer of TG.Citation[24] CETP, along with PLTP (phospholipid transfer protein), plays an important role in the metabolism and the remodeling of plasma lipoprotein. The CETP activity has been reduced in response to corticosteroids and lipopolysaccharides. It was suggested that CETP-mediated transfer of triglycerides, but not phospholipids, regulates the transfer of CSA between lipoproteins. The distribution of CSA among lipoproteins is partially influenced by CETP.Citation[24] It is necessary to aggressively control post-renal transplant hyperlipidemia and important to reduce or withdraw steroids in selected, low-risk recipients as early as possible from the viewpoint of preventing post-renal transplant hyperlipidemia.Citation[1]
Unfortunately, little is known about non-HDL-C, the metabolic basis for lipoprotein disturbances, and non-HDL-C in fasting or nonfasting post-renal transplant patients. However, lipoprotein measurements are expensive and not readily available in most clinical centers. Taking into account the above information, we have determined fasting and nonfasting lipid profiles and calculated non-HDL-C concentration and lipid ratios in the same post-renal transplant patients. We have shown an excellent correlation between the fasting and nonfasting non-HDL-C levels in post-renal transplant patients both with dyslipidemia and normolipidemic. Non-HDL-C is the only lipid measurement that has correlated positively with cardiovascular mortality in a hemodialysis population.Citation[8] Desmeules et al.Citation[8] in their study tested the hypothesis that nonfasting non-HDL-C level is as reliable as fasting non-HDL-C level for the management of dyslipidemia in hemodialysis patients. We think the finding that nonfasting labs are reliable for non-HDL-C as well as total cholesterol is important, as fasting labs are not always available.
We conclude that disturbances of lipids, lipoproteins, and TRLs not only depend on the kind of treatment, but due to multiple factors can accelerate cardiovascular complications in post-renal transplant patients with dyslipidemia and also with normolipidemic. Further studies concerning this problem need to be performed.
REFERENCES
- Marubayashi S, Ohdan H, Tashiro H, Tokita D, Onoe T, Hayamizu K, Asahara T, Doi S, et al. Studies on post-renal dyslipidemia in kidney transplant patients. Hiroshima J Med Sci. 2005; 54: 39–45
- Maki KC, Galant R, Davidson MH. Non-high-density lipoprotein cholesterol: The forgotten therapeutic target. Am J Cardiol. 2005; 96(Suppl.)59K–64K
- Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Junger I, Waddilius G. Apolipoproteins versus lipids as indicators of coronary risk and as targets for statin therapy. Lancet 2003; 361: 777–780
- Fellström B, Holdaas H, Jardine AG, Holme I, Nyberg G, et al. Effect of fluvastatin on renal and points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int. 2004; 66: 1549–1555
- Fellström B. Nonimmune risk factors for chronic renal allograft disfunction. Transplantation 2001; 71: SS10–SS16
- Paul LC. Chronic allograft nephropathy: An update. Kidney Int. 1999; 56: 783–793
- Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomized, placebo-controlled trial. Lancet 2003; 361: 2024–2031
- Desmeules S, Arcand-Bosse J-F, Bergeron J, Douville P, Agharazii M. Nonfasting non-high-density lipoprotein cholesterol is adequate for lipid management in hemodialysis patients. Am J Kidney Dis. 2005; 45: 1067–1072
- Kimak E, Solski J, Baranowicz-Gąszczyk I, Książek A. A long-term study of dyslipidemia and dyslipoproteinemia in stable post-renal transplant patients. Ren Fail. 2006; 28: 483–486
- Kimak E, Książek A, Solski J. Disturbed lipoprotein composition in non-dialyzed, hemodialysis, continuous ambulatory peritoneal dialysis and post-transplant patients with chronic renal failure. Clin Chem Lab Med. 2006; 44: 64–69
- Badiou S, Garrigue V, Dupuy AM, Chong G, Cristol JP, Mourad G. Small dense low-density lipoprotein in renal transplant recipients: A potential target for prevention of cardiovascular complications?. Transplant Proc. 2006; 38: 2314–2316
- Sattar N, Williams K, Sniderman AD, Agostino RD, Haffner SM. Comparison of the association of apolipoprotein B and non-high-density lipoprotein cholesterol with other cardiovascular risk factors in patients with the metabolic syndrome in the insulin resistance atherosclerosis study. Circulation 2004; 110: 2687–2693
- Lee S-J, Moye LA, Campos H, Williams GH, Sacks FM. Hypertriglyceridemia but not diabetes status is associated with coronary heart disease. Atherosclerosis 2003; 167: 293–302
- Chan DC, Watts GF, Ng TWK, Uchida Y, Sakai N, Yamashita S, Barrett PHR. Apolipoprotein B-100 kinetics and static plasma indices of triglyceride-rich lipoprotein metabolism in overweight men. Clin Biochem. 2005; 38: 806–812
- Fellström B. Risk factors for and management of post-transplantation cardiovascular disease. BioDrugs. 2001; 15: 261–278
- Lemieux I, Houde I, Pascot A, Lachance J-G, Noël R, Radeau T, Despres J-P, Bergeron J. Effects of prednisone withdrawal on the new metabolic triad in cyclosporine-treated kidney transplant patients. Kidney Int. 2002; 62: 1839–1847
- Tall AR. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990; 68: 379–384
- Hilbrands LB, Demacker PN, Hoitsma AJ, et al. The effects of cyclosporine and prednisone on serum lipid and (apo)lipoprotein levels in renal transplant recipients. J Am Soc Nephrol. 1995; 5: 2073–2081
- Donahoo WT, Kosmiski LA, Eckel RH. Drugs causing dyslipoproteinemia. Endocrinol. Metab. Clin. North. Am. 1998; 27: 677–697
- Walczak DA, Calvert D, Jarzembowski TM, Testa G, Sankary HN, Thielke J, et al. Increased risk of post-transplant diabetes mellitus despite early steroid discontinuation in Hispanic kidney transplant recipients. Clin Transplant. 2005; 19: 527–531
- Abramowicz D, del Carmen Rial M, Vitko S, del Castillo D, Manas D, Lao M, et al. Cyclosporine withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen: Results of a five-year, prospective, randomized study. J Am Soc Nephrol. 2005; 16: 2234–2240
- Woodside KJ, Nichols SD, Hunter GC, Gugliuzza KK, Daller JA. Conversion from cyclosporine to tacrolimus results in improved creatinine but does not alter lipid profile. Transpl Proc. 2005; 37: 1877–1879
- Włodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, Kokot F, et al. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus-based regiments. Transplant Int. 2005; 18: 157–162
- Kwong M, Wasan KM. Cholesteryl ester transfer protein facilitates the movement of water-insoluble drugs between lipoproteins: a novel biological function for a well-characterized lipid transfer protein. Biochem Parmacol. 2002; 64: 1669–1675