Abstract
Recently, mild AKI has been considered as a risk factor for mortality in different scenarios. We conducted a retrospective analysis of the risk factors for two distinct definitions of AKI after elective repair of aortic aneurysms. Logistic regression was carried out to identify independent risk factors for AKI (defined as $25% or $50% increase in baseline SCr within 48 h after surgery, AKI 25% and AKI 50%, respectively) and for mortality. Of 77 patients studied (mean age 68 ± 10, 83% male), 57% developed AKI 25% and 33.7% AKI 50%. There were no differences between AKI and control groups regarding comorbidities and diameter of aneurysms. However, AKI patients needed a supra-renal aortic cross-clamping more frequently and were more severely ill. Overall in-hospital mortality was 27.3%, which was markedly higher in those requiring a supra-renal aortic cross-clamping. The risk factors for AKI 25% were supra-renal aortic cross-clamping (odds ratio 5.51, 95% CI 1.05–36.12, p = 0.04) and duration of operation for AKI 25% (OR 6.67, 95% CI 2.23–19.9, p < 0.001). For AKI 50%, in addition to those factors, post-operative use of vasoactive drugs remained as an independent factor (OR 6.13, 95% CI 1.64–22.8, p = 0.005). The risk factors associated with mortality were need of supra-renal aortic cross-clamping (OR 9.6, 95% CI 1.37–67.88, p = 0.02), development of AKI 50% (OR 8.84, 95% CI 1.31–59.39, p = 0.02), baseline GFR lower than 49 mL/min (OR 17.07, 95% CI 2.00–145.23, p = 0.009), and serum glucose > 118 mg/dL in the post-operative period (OR 19.99, 95% CI 2.32–172.28, p = 0.006). An increase of at least 50% in baseline SCr is a common event after surgical repair of aortic aneurysms, particularly when a supra-renal aortic cross-clamping is needed. Along with baseline moderate chronic renal failure, AKI is an independent factor contributing to the high mortality found in this scenario.
INTRODUCTION
Acute kidney injury (AKI) imparts a dim prognosis to different clinical scenarios.Citation[1–4] Recently, it has been demonstrated that even mild increases in serum creatinine is associated with mortality.Citation[2],Citation[4],Citation[5] Therefore, the definition of AKI has recently changed toward more sensitive criteria.Citation[6],Citation[7] The surgical repair of aortic aneurysms (AA) is associated with a high incidence of renal dysfunction,Citation[8–10] and older studies suggest that there is a close relationship between the development of severe forms of AKI and mortality.Citation[11–14] Surgical trauma (e.g., bleeding, hypovolemia, inflammation), ischemia reperfusion to kidneys, and other viscera and limbs are potentially involved in the pathogenesis of AKI related to AA surgical repair. However, few data exist on the incidence, predictors, and outcomes of different definitions of mild AKI. We sought to investigate, using two different definitions, the baseline and peri-procedure factors involved in mild AKI that occurs early after elective operation for AA, as well as whether a mild AKI is associated with in-hospital mortality.
CHARACTERIZATION OF POPULATION AND METHODS
We retrospectively reviewed 102 patients submitted to repair of aortic aneurysms throughout the year 2000 in a tertiary, 900-bed university hospital. From these 102 patients, 15 were excluded due to exclusive endovascular procedure, and in 10 patients we could not retrieve sufficient data. Thus, we studied 77 patients who were submitted to elective open repair of aortic aneurysms, either thoraco-abdominal or abdominal. Surgeries were carried out by the same team of surgeons and anesthetists. Post-operative care was undertaken in a 20-bed surgical ICU. We retrospectively analyzed demographic and pre-,intra-, and post-operative clinical and laboratories variables.
The primary endpoint was AKI defined as a 50% increase in baseline serum creatinine (SCr) within 48h after operation. Secondary endpoints were a 25% decrease in glomerular filtration rate (GFR) from baseline, a 25% SCr increase from baseline (both also within 48h after operation), length of stay in ICU, and in-hospital mortality.
The pre-operative variables analyzed were demographics, baseline renal function (mean SCr in the week before operation, and GFR calculated from Cockcroft-Gault formula), systemic arterial pressure, presence of comorbidities, and type and maximal diameter of aneurysms. Baseline chronic renal failure was defined as baseline SCr ≥ 1.5 mg/dL. Systemic hypertension and diabetes mellitus were defined based on history of use of medication. Coronary artery disease was defined as positive history of myocardium infarct, previous CABG or coronary intervention, or positive scintillography in pre-operative evaluation. Intra-operative variables studied were length of operation, duration of supra- and infra-renal aortic cross-clamping, mean arterial pressure, hypotension (defined as a mean arterial pressure less than 70 mmHg), need and volume of transfusion, need of vasoactive drugs, net fluid balance, and urinary output. We also analyzed the following variables 48 h post-operatively: mean arterial pressure, need of use of vasoactive drugs, creatinophosphokinase levels (CK), net fluid balance and urinary output, and total volume of daily infused fluids. Laboratory biochemical and hematological values were recorded during the period.
Statistical Analysis
Data are reported as mean ± SD for continuous variables and percentage for discrete variables. Nonparametrical data were shown as median and interquartile range (IQ). Categorical variables were analyzed with Fisher's exact and chi-square tests, as appropriated. Non-paired continuous variables were analyzed by Student's t-test or Mann-Whitney U-test, according to normality test. Some continuous variables were categorized through a ROC curve using the value with the best sensitivity and specificity. The resulting cutoff points were clinically relevant. Univariate analysis was performed using Pearson chi-square test. The candidate variables that achieved p < 0.10 on univariate analysis were included in a multivariate model (logistic regression) to identify independent predictors of AKI and in-hospital mortality. A two-tailed p < 0.05 was considered statistically significant. All analyses were done with assistance of SPSS 11.0 software.
RESULTS
Characterization of Overall Population
The demographic and clinical characteristics of the studied patients are shown in . Mean age was 68.4 ± 9.7 years. Eighty-three percent of patients were hypertensive and 10% had diabetes. Among the 77 patients, 32.5% had baseline preoperative SCr values ≥ 1.5 mg/dL. The mean glomerular filtration rate (GFR) was 58.6 ± 28.9 mL/min and mean baseline SCr was 1.47 ± 1.18 mg/dL. Eight out of 77 (10.4%) patients had thoraco-abdominal aortic aneurysms. Nonetheless, the intraoperative need for a supra-renal aortic cross-clamping occurred in 19/77 (24.6%) of the patients. The median duration of supra- and infra-renal aortic cross-clamping were 33 [IQ 20–42] and 55 [IQ 42–68] minutes, respectively (see ).
Table 1 Demographic and clinical characteristics of the overall population (n = 77)
Incidence of AKI
Forty-four out of 77 patients (57%) developed AKI defined as a 25% increase in baseline SCr, whereas 33.7% reached the definition of a 50% increase in baseline SCr. Only 10/77 patients (13%) needed renal replacement therapy (see ).
Table 2 Outcomes of the overall population (n = 77)
Analysis of Risk Factors for AKI 25%
The incidence of diabetes, hypertension, or baseline chronic renal failure was not different between AKI 25% group and control (data not shown). Moreover, baseline SCr (1.35 ± 0.53 vs. 1.63 ± 1.6, p = 0.30) and GFR (59.8 ± 29.9 vs. 56.9 ± 27.9 mL/min, p = 0.66) did not statistically differ between AKI 25% and control. The maximal aneurysm diameter was not different between AKI 25% and controls (6.46 ± 1.43 vs. 6.49 ± 1.28 cm, respectively, p = 0.66). Importantly, 38% of patients that developed AKI 25% had a supra-renal aortic cross-clamping, compared to only 6% in control (p = 0.001), although the duration of supra-renal clamping, when it occurred, did not statistically differ (32 [IQ 14–34] vs. 22 [IQ 20–26] minutes, p = 0.398).
During surgery and in the perioperative period, there were no significant differences in the minimal MAP or the rates of hypotension between controls and AKI25%. The most important differences between AKI 25% and controls were related to the duration of operation (420 [IQ 258–420]) vs. 300 [IQ 208–337] minutes, p = 0.001), need of transfusion (77% vs. 51%, p = 0.018), and the overall volume of packed red cell (data not shown). Vasoactive drugs were more largely used in the immediate post-operative time in the AKI 25% group compared to controls (48% vs. 22%, p = 0.017), and the lactate levels were significantly higher in the AKI 25% group (27.8 ± 14.3 vs. 19.8 ± 8.2 mg/dL, p = 0.013). In the first post-operative day, the net fluid balance (1.05 ± 1.1 vs. 0.67 ± 1.4 mL/min, p = 0.025) and the glucose serum levels (165 ± 65 vs. 145 ± 52 mg/dL, p = 0.046) were higher in the AKI 25%, compared to controls. Clinical data regarding the second post-operative day did not differ between AKI 25% and controls (data not shown). Nonetheless, the highest CK level reached during post-operative period was significantly higher in AKI 25% than controls (1228 [IQ 437–2758] U/L vs. 326 [IQ 156–507] U/L, respectively, p = 0.01).
The candidate variables for the risk of developing AKI 25% that were included in the logistic regression model were supra-renal aortic cross-clamping, duration of operation longer than 339 minutes, need of blood transfusion, volume of packed red cells transfused more than 800 ml, total volume of infused fluids intraoperatively >15 mL/min, net fluid balance intraoperatively >12 mL/min, and the use of vasoactive drugs in perioperative period. After adjusting for these covariates, the remaining independent factors associated with development of AKI 25% were supra-renal aortic cross-clamping (OR 5.514, 95% CI 1.058–36.128, p = 0.042), and the duration of operation longer than 339 minutes (OR 6.678, 95% CI 2.238–19.928, p = 0.0007).
Analysis of Risk Factors for AKI 50%
Likewise, AKI 25%, there was no difference in demographics and clinical characteristics between those patients that developed AKI 50% and controls (see ). Regarding intra-operative variables, duration of operation, presence of supra-renal aortic cross-clamping, use of vasoactive drugs, and need and volume of blood transfusion were significantly higher in AKI 50% compared to controls. However, duration of either infra-renal or supra-renal aortic cross-clamping did not differ between groups (see ).
Table 3 Demographic and clinical characteristics of the patients that developed AKI 50% compared to controls
Table 4 Comparison between AKI 50% and controls regarding perioperative variables (intra-operative [IOP] and immediate post-operative [POI])
In the immediate post-operative period, MAP (68.9 ± 13.7 vs. 107.8 ± 20.5 mmHg, p = 0.012) and the need for vasoactive drugs (65.4% vs. 21.5%, p = 0.001) were significantly different between groups (AKI 50% and control, respectively). Net fluid balance was significantly higher in the AKI 50% group during this period (see ). The MAP values were not different beyond the immediate post-operative period, although AKI 50% patients needed vasoactive drugs for a longer time (see ). Serum glucose levels were significantly higher in first post-operative day in AKI 50% group compared to controls (184 ± 77 vs. 143 ± 44 mg/dL, p = 0.006). The highest CK levels were also different in the post-operative period when considering AKI 50% patients compared to controls (974 [IQ 237–2700] vs. 463 [IQ 188–1479] U/L, p = 0.001; see ).
Table 5 Clinical data of the first two post-operative days comparing AKI 50% patients to controls
Candidate variables that were included in the logistic regression model were duration of operation >383 minutes, need of supra-renal aortic cross-clamping, need of intraoperative blood transfusion, total volume of packed red cells transfused intra-operatively, need of vasoactive drug in the post-operative period, net fluid balance in the post-operative period, serum glucose levels in the post-operative period, and hemoglobin levels in post-operative period. After adjusting for these covariables, the independent risk factor for AKI 50% that remained in this model were need of supra-renal aortic cross-clamping (OR 7.318, 95% CI 1.667–32.117, p = 0.008), duration of operation >383 minutes (OR 7.162, 95% CI 1.691–30.326), and the need of vasoactive drugs in post-operative period (OR 6.137, 95% CI 1.648–22.854, p = 0.005; see ).
Table 6 Regression analysis of the risk factors for AKI 50%
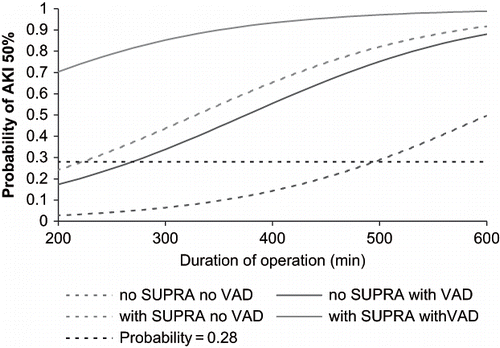
depicts the cumulative risk for developing AKI 50%, considering the level of aortic clamping, duration of operation, and need of vasoactive drugs in the post-operative period. The need of vasoactive drugs in the post-operative period was a strong determinant for AKI 50% after aneurysm open repair, whether the patient had either a supra- or infra-renal aortic cross-clamping.
Analysis of RIFLE and AKIN Criteria
When patients who developed AKI were evaluated according to the maximum RIFLE criteria reached within 48 h after operation based solely on serum creatinine,Citation[6] 26 out of 77 patients were classified as risk in 8/26 (31%), injury in 4/26 (15%), and failure in 14/26 (54%). The length of ICU stay (1.9 ± 2.1, 7.6 ± 3.3, and 10.6 ± 3.2 days; p < 0.05), need of dialysis (0, 25%, 35%; p < 0.05), and mortality (25%, 50%, and 70%; p < 0.05) were different when maximal risk class was compared to maximal injury and failure classes.
According to the AKIN classification, also based in the SCr criteria 48 h after operation, 47 (61%) had at least 0.3 mg/dL elevation above baseline SCr. Among these patients, 29/47 (61.7%) were classified in stage 1, 4/47 (8%) in stage 2, and 14/47 (29%) in stage 3. The length of ICU stay (4.8 ± 4.61, 7.6 ± 3.3, and 10.6 ± 3.2 days; p < 0.05), need of dialysis (0, 25%, 35%; p < 0.05), and mortality (20%, 50%, and 70%; p < 0.05) were different between stages 1, 2, and 3.
Risk Factors for Mortality
The overall in-hospital mortality was 27.3% (21/77). Fifty-six patients out of 77 (72.7%) were delivered from hospital. The comparison of survivors and non-survivors showed that there was no difference regarding demographics, comorbidities (diabetes, hypertension, and coronary artery disease), or size of aneurysm. However, the incidence of baseline CRF was higher in non-survivors (57% vs. 23.2%, respectively, p = 0.005). Baseline SCr was not significantly higher in non-survivors compared to survivors (1.61 ± 0.65 vs. 1.41 ± 1.32 mg/dL, respectively, p = 0.297), but baseline GFR was significantly different (45.86 ± 19.24 in non-survivors vs. 63.37 ± 30.57 mL/min in survivors, p = 0.009; see ).
Table 7 Demographic and clinical characteristics comparing survivors and non-survivors after aortic aneurysm open repair
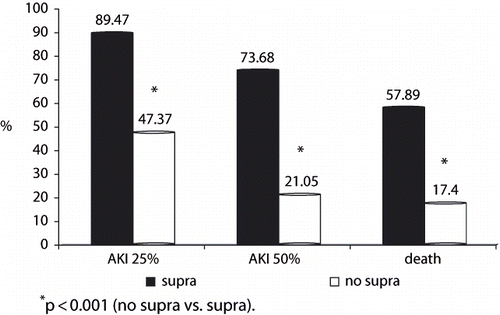
Among 21/77 patients who died, 11 (52.3%) had a supra-renal aortic cross-clamping. Moreover, the mortality of those patients who needed a supra-renal aortic cross-clamping was 57.8% (11/19), compared to 17.24% (10/58) in those who needed exclusively an infra-renal aortic cross-clamping. Nonetheless, the duration of supra-renal aortic cross-clamping was not different between non-survivors and survivors, for those who needed this maneuver (28 [IQ13–32] vs. 32 [IQ 14–35] min, respectively, p = 0.260; see and ).
Figure 2. Outcomes (AKI 25%, AKI 50%, and mortality rate) after aortic aneurysm open repair according to the level of aortic cross-clamping.
During the intraoperative period, there was a significant difference between non-survivors and survivors regarding the lower MAP (58.5 ± 10.1 vs. 66.1 ± 12.3 mmHg, respectively, p = 0.003), need of vasoactive drugs (71.4% vs. 9%, respectively, p = 0.001), duration of operation (420 [IQ 244–500] vs. 330 [IQ 207–380] min, respectively, p = 0.002), need of blood transfusion (95% vs. 55%, respectively, p = 0.001), and volume of packed red cells infused (1,964 ± 1,735 vs. 685 ± 1,032 mL, respectively, p = 0.001). Intraoperative urinary volume was also significantly higher in survivors (3.2 ± 1.5 vs. 2.2 ± 1.5 mL/min, respectively, p = 0.011; see ).
Table 8 Comparison of peri-operative (within 48 h after operation) variables between survivors and non-survivors
Regarding the variables in the immediate post-operative, lowest MAP (65.3 ± 13.1 vs. 77.5 ± 13.2 mmHg, p = 0.001), need of vasoactive drugs (71.2% vs. 21.5%, p = 0.001), and net fluid balance (1.53 ± 1.68 vs. 0.43 ± 1.30 mL/min, p = 0.003) wgpere significantly different between non-survivors and survivors, respectively. Among the laboratory values, the platelet count was lower in non-survivors during post-operative period, in accordance with an increased need of blood transfusion intra-operatively. The increased need of vasoactive drugs persisted throughout the first and second post-operative period (data not shown), and during the first post-operative day, serum lactate levels were higher in non-survivors (30.8 ± 10.5 vs. 20.5 ± 8.5 mg/dL, p = 0.006). The level of serum albumin during the post-operative period was significantly lower in the non-survivor group (2.0 ± 0.3 vs. 2.4 ± 0.4 g/dL, p = 0.001). Serum glucose levels was higher in non-survivor group throughout the post-operative period, reaching significance in the second post-operative period (188 ± 138 vs. 127 ± 73 mg/dL, p = 0.036; see ).
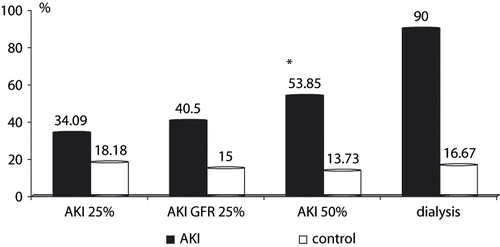
Regarding the development of AKI, there was a trend, although not statistically different, toward higher mortality in those who developed AKI 25% (34% vs. 18% in control, p = 0.12). Likewise, the length of ICU stay did not differ for AKI 25% compared to controls (5.7 ± 5.3 vs. 4.7 ± 6.7 days, p = 0.13). When a 25% decrease in GFR was taken into account, this event was associated with higher mortality rates than control (see ). Accordingly, when patients who developed AKI 50% were compared to controls, there was a striking difference in mortality (53% vs. 13.7%, respectively, p = 0.001) and length of ICU stay (7.0 ± 6.0 vs. 4.4 ± 5.7 days, respectively, p = 0.005). Importantly, among patients who needed renal replacement therapy, mortality reached 90% (see ).
Figure 3. Mortality after aortic aneurysm open repair according to AKI definitions, compared to controls. Legend: AKI 25% vs. control, p = 0.121; AKI GFR 25% vs. control, p = 0.012; AKI 50% vs. control, p = 0.001; dialysis vs. control, p = 0.0001.
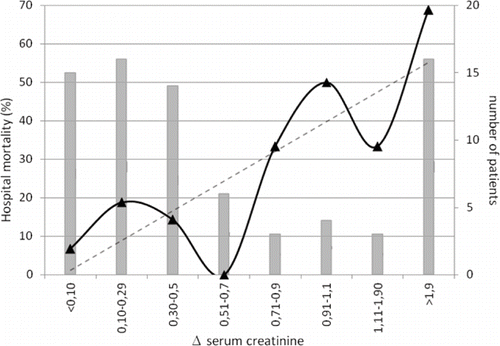
Fifteen percent of all patients presented a decrease or an elevation lower than 0.10mg/dL in SCr, these patients having a hospital mortality of 6.67 percent. Otherwise, patients with creatinine elevation presented a progressively increasing in hospital mortality with the degree of increase (see ).
Figure 4. Hospital mortality and change in serum creatinine within 48 h after aortic surgery. Legend: Distribution of Δ serum creatinine and mortality rates calculated for serum creatinine intervals. Bars represent the number of patients in each interval. Broken line represents tendency of death with Δ serum creatinine elevation.
The variables that were included in the regression analysis model for mortality were AKI defined as 25% decrease in baseline GFR; AKI 50%, baseline GFR lower than 49 mL/min; maximal increase in SCr >0.5 mg/dL; need for a supra-renal aortic cross-clamping; need of intraoperative blood transfusion; volume of packed red cells infused >850 ml; use of vasoactive drugs in post-operative period; lowest MAP in the post-operative period <72 mmHg; net fluid balance in the post-operative period >0.85 mL/min; duration of operation >358 minutes; serum glucose levels higher than 118 mg/dL in the post-operative period; lactate levels in the post-operative period >23 mg/dL; HCO3− levels in the post-operative period <21 mEq/L; and lowest serum albumin levels in the post-operative period <2.2 g/dL.
After adjusting for the covariates above, the remaining independent risk factors for in-hospital mortality after aortic aneurysm open repair were need for a supra-renal aortic cross-clamping (OR 9.675, 95% CI 1.379–67.883, p = 0.022); development of AKI 50% (OR 8.846, 95% CI 1.318–59.390, p = 0.025); baseline GFR lower than 49 mL/min (OR 17.076, 95% CI 2.008–145.233, p = 0.009); and serum glucose >118 mg/dL in the post-operative period (OR 19.995, 95% CI 2.320–172.288, p = 0.006; see ).
Table 9 Regression analysis for risk of mortality after aortic aneurysm open repair
DISCUSSION
It appears that even elective open surgical repair of aortic aneurisms is a condition that predisposes to AKI development. Several authors describe an incidence that varies from 5–8% in isolated abdominal,Citation[13] to ∼10–25% in thoraco-abdominal aortic aneurysms.Citation[9],Citation[12] In the present cohort, we found an incidence of 33.7% for AKI, considered a 50% increase in baseline SCr, which is higher than the described in the literature. There are different definitions of AKI reported in other series, mostly regarding more severe forms of AKI. Recently, there is a trend toward more sensitive criteria for AKI, which were already validated in different settings.Citation[6],Citation[7],Citation[15–17] The reason we found a higher incidence is probably due to the definitions we employed. Both definitions, an increase of at least 25% or 50% in baseline SCr (or a 25% decrease in GFR for the last one) within 48 h after operation, encompass a mild form of AKI. A 25% increase in baseline SCr is less than proposed for the RIFLE criteria, whereas ≥50% increase in SCr (or a 25% decrease in GFR), the definition of risk for kidney injury, appears to confer a high risk for death, as was recently demonstrated.Citation[15–17] More recently, the Acute Kidney Injury Network groupCitation[18] proposed a classification that is even more extensive, comprising patients with elevations higher than 0.3 mg/dL in baseline SCr. By this classification, 61% of patients were classified with acute kidney injury. The majority of these patients met stage 1 (61.7%) and presented a significantly lower mortality than patients in stage 2 and 3, corresponding to RIFLE injury and failure. The causes for AKI were most probably due to ischemia-reperfusion and surgical trauma, as they occurred soon after operation and were largely transient, mild, and reversible in those who survived. Although we cannot exclude them, it is very unlikely that other causes such as cholesterol atheroembolism and renal artery thrombosis occurred in this series.
Our results demonstrate that the most important factors associated with AKI are the need for a supra-renal aortic clamping and the length of surgical trauma. The first determines renal ischemia-reperfusion injury and its inherent pathogenetic effects. The second is probably related to the level of surgical difficulty and probably determines a more severe inflammatory systemic response. Other studies have demonstrated a correlation of the duration of supra-renal aortic clamping with the risk of AKI and severity of renal injury.Citation[10],Citation[18],Citation[19] However, we were not able to demonstrate this relationship. It is likely that for a mild AKI, such as a 25% SCr increase, the average aortic cross-clamping duration of 30 minutes is more than enough to trigger a renal insult. For a more severe AKI (50% SCr increase), these two risk factors were accompanied by the need of vasoactive drugs in the post-operative period, which is certainly a marker of a more severe surgical trauma and probably reflects a difficulty in intra-operative volemic resuscitation.
Even though our patients had a mild to moderate depression in baseline renal function, surprisingly it was not a risk factor for AKI, as demonstrated by others.Citation[9],Citation[20] Also interestingly, we did not find very high levels of CK in the AKI group. Therefore, we did not analyze CK levels as an independent variable for AKI. It appears, as demonstrated by other studies,Citation[21],Citation[22] that isolated post-operative rhabdomyolysis, at least in the range of values we found, is not a strong risk factor for AKI as long as the patients receive adequate fluid therapy and maintain high urine outputs, as occurred in our cohort.
We found a high mortality rate in this study (27%), compared to others in the literature (4–19%).Citation[11–14] This mortality was more elevated in patients submitted to supra-renal aortic cross-clamping (42.8%) and with thoraco-abdominal aneurisms (61%). The intra-operative mortality among patients with thoraco-abdominal aneurisms was 30%.
We and others have demonstrated that mild AKI is associated with mortality in various scenarios.Citation[2],Citation[4],Citation[5] In this study, we demonstrated that, although largely used in clinical trials of AKI prevention, such as those in the prevention of contrast nephropathy,Citation[5] the use of a definition of AKI as a 25% increase in baseline SCr did not have a significant impact on mortality and length of ICU stay. Nevertheless, a ≥ 50% increase in baseline SCr, even transiently, clearly correlated with mortality after surgical open repair of aortic aneurysms. This finding has important consequences, as this subset of insult determining AKI, in contrast to the majority of cases of AKI in ICU, is scheduled in advance, allowing for potential preventive measures. Indeed, some efforts have already been made to prevent AKI associated with aortic aneurysms repair, mostly with conflicting and sometimes disappointing results.Citation[23–27]
Nevertheless, we found other risk factors for mortality that are also potentially suitable for intervention. We confirmed in this study that serum glucose levels higher than 118 mg/dL, even transiently within 48 h after operation, are strongly associated with increases in mortality. It is noteworthy that the patients included in this study had their ICU stay before the results from van den Berghe et al. and the current practice of post-operative strict glucose control in ICU were widespread.Citation[28],Citation[29] Supra-renal aortic cross-clamping, besides its role in determining AKI, is also a factor associated with high mortality. Supra-renal clamping increases splanchnic ischemia and probably contributes to systemic inflammation and multiple organ dysfunctions.Citation[30] Not surprisingly, most of the patients who developed AKI were those that had supra-renal cross-clamping, as well as longer use of vasoactive drugs, higher lactate, and need of larger volumes of packed red cell. It is conceivable that modern techniques involving distal aortic perfusion with pressure-controlled blood perfusion of the kidneys might decrease AKI and mortality associated with aortic cross-clamping, although this is controversial, and further controlled studies are waited to confirm this hypothesis.Citation[31],Citation[32] Lastly, baseline decrease in glomerular filtration rate to less than 49 mL/min (i.e., moderate CRF) was also a strong independent risk factor to death in this cohort, as demonstrated by Huynh et al.Citation[20] It is likely that CRF patients, independent of further decreases in renal function, are prone to an exuberant inflammatory and oxidative response, which can contribute to other organ failures, as demonstrated in CRF patients undergoing acute myocardium infarct and after myocardium revascularization.Citation[33],Citation[34] It is possible that this subset of patients should be preferentially candidates for endovascular procedures, as suggested by some, although recent data have argued against that.Citation[35],Citation[36] As aortic aneurysm open repair is still a standard treatment for most of the patients, CRF patients should at least be viewed as candidates for differentiated protocols of intra- and post-operative support.
In conclusion, mild AKI is highly prevalent among patients undergoing elective aortic aneurysms open repair, particularly in those submitted to supra-renal aortic cross-clamping. It has been demonstrated that this early and even transient renal dysfunction, along with post-operative hyperglycemia and baseline moderate chronic renal failure, has a serious impact on outcome.
REFERENCES
- Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997; 95: 878–884
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol. 2004; 15: 1597–1605
- Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002; 30: 2051–2058
- Vieira JM, Jr, Castro I, Curvello-Neto A, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007; 35: 184–191
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105: 2259–2264
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative Workgroup: Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8: R204–R212
- Mehta RL, Chertow GM. Acute renal failure definitions and classification: Time for change?. J Am Soc Nephrol. 2003; 14: 2178–2187
- Tallgren M, Niemi T, Poyhia R, et al. Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur J Vasc Endovasc Surg. 2007; 33: 550–555
- Kashyap VS, Cambria RP, Davison JK, L'Italien GJ. Renal failure after thoracoabdominal aortic surgery. J Vasc Surg. 1997; 26: 949–955
- Back MR, Bandyk M, Bradner M, et al. Critical analysis of outcome determinants affecting repair of intact aneurysms involving the visceral aorta. Ann Vasc Surg. 2005; 19: 648–656
- Cambria RP, Davison JK, Zannetti S, L'Italien G, Atamian S. Thoracoabdominal aneurysm repair: Perspectives over a decade with the clamp-and-sew technique. Ann Surg. 1997; 226: 294–303
- Godet G, Fleron MH, Vicaut E, et al. Risk factors for acute postoperative renal failure in thoracic or thoracoabdominal aortic surgery: A prospective study. Anesth Analg. 1997; 85: 1227–1232
- Sayers RD, Thompson MM, Nasim A, Healey P, Tabú N, Bell PRF. Surgical management of 671 abdominal aortic aneurysms: A 13 year review from a single centre. Eur J Vasc Endovasc Surg. 1997; 13: 322–327
- LeMaire SA, Miller III CC, Conklin LD, Schmittling ZC, Koksoy C, Coselli JS. A new predictive model for adverse outcomes after elective thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2001; 71: 1233–1238
- Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006; 10: R73
- Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg. 2006; 81: 542–546
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network (AKIN): Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11: R31
- Ellenberger C, Schweizer A, Diaper J, et al. Incidence, risk factors and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med. 2006; 32: 1808–1816
- Kudo FA, Nishibe T, Miyazaki K, et al. Postoperative renal function after elective abdominal aortic aneurysm repair requiring suprarenal aortic cross-clamping. Surg Today. 2004; 34: 1010–1013
- Huynh TTT, Statius van Eps RG, Miller III CC, et al. Glomerular filtration rate is superior to serum creatinine for prediction of mortality after thoracoabdominal aortic surgery. J Vasc Surg. 2005; 42: 206–212
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference?. J Trauma. 2004; 56: 1191–1196
- de Meijer AR, Fikkers BG, de Keijzer MH, van Engelen BGM, Drenth JPH. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: A five-year intensive care survey. Intensive Care Med. 2003; 29: 1121–1125
- Wijnen MH, Vader HL, Van Den Wall Bake AW, Roumen RM. Can renal dysfunction after infra-renal aortic aneurysm repair be modified by multi-antioxidant supplementation?. J Cardiovasc Surg. 2002; 43: 483–488
- Franklin SC, Moulton M, Sicard GA, Hammerman MR, Miller SB. Insulin-like growth factor I preserves renal function postoperatively. Am J Physiol. 1997; 272: F257–F259
- Gilbert TB, Hasnain JU, Flinn WR, Lilly MP, Benjamin ME. Fenoldopam infusion associated with preserving renal function after aortic cross-clamping for aneurysm repair. J Cardiovasc Pharmacol Ther. 2001; 6: 31–36
- Macedo E, Abdulkader R, Castro I, Sobrinho AC, Yu L, Vieira JM, Jr. Lack of protection of N-acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair—a randomized controlled trial. Nephrol Dial Transplant. 2006; 21: 1863–1869
- Baldwin L, Henderson A, Hickman P. Effect of postoperative low-dose dopamine on renal function after elective major vascular surgery. Ann Intern Med. 1994; 120: 744–747
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001; 345: 1359–1367
- Clayton SB, Mazur JE, Condren S, Hermayer KL, Strange C. Evaluation of an intensive insulin protocol for septic patients in a medical intensive care unit. Crit Care Med. 2006; 34: 2974–2978
- Adembri C, Kastamoniti E, Bertolozzi I, et al. Pulmonary injury follows systemic inflammatory reaction in infrarenal aortic surgery. Crit Care Med. 2004; 32: 1170–1177
- Jacobs MJ, Statius van Eps RG, de Jong DS, Schurink GWH, Mochtar B. Prevention of renal failure in patients undergoing thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2004; 40: 1067–1073
- Safi HJ, Harlin SA, Miller CC, et al. Predictive factors for acute renal failure in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg. 1996; 24: 338–345
- Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004; 351: 1285–1295
- Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002; 137: 555–562
- Prinssen M, Verhoeven ELG, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004; 351: 1607–1618
- Parmer SS, Fairman SM, Karmacharya J, Carpenter JP, Velazquez OC, Woo EY. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg. 2006; 44: 706–711