Abstract
Background. Peritonitis is a common complication of end stage renal failure (ESRF) patients receiving continuous ambulatory peritoneal dialysis (CAPD). Peritoneal macrophage may participate in the activation of specific T cells and in the generation of local cell-mediated immunity to various pathogens. The purpose of this study is to investigate the possible role of macrophage in CAPD patients with peritonitis. Methods. We evaluated the expression of Fas receptor (CD95), ICAM-1 (CD54), CD25, and CD69 by two-color flow cytometry on extravasted macrophages from 16 ESRF patients on CAPD with peritonitis (peritonitis-positive) and compared them to 11 ESRF patients on CAPD without peritonitis (peritonitis-negative) and normal controls. Results. We found an increased expression of CD95, CD54, and CD25 on macrophage in peritonitis-positive group compared to controls (all p < 0.001). In the peritonitis-positive group, the CD95 expression was significantly higher than that of the peritonitis-negative group (p < 0.001). The expression of CD54, CD25, and CD69, however, was not significantly different between the peritonitis-positive and peritonitis-negative CAPD subgroups. Conclusion. We found an abnormally increased percentage of macrophage-expressing Fas receptor and ICAM-1, and the percentage of CD95+ macrophage, but not those of other markers, were increased among the subset of CAPD patients with peritonitis. The later finding suggests that this macrophage phenotype is associated with peritonitis occurring in CAPD.
INTRODUCTION
Macrophages are an important component of the peritoneal environment, as they may participate in the activation of specific T cells and in the generation of local cell-mediated immunity to various pathogens. The activation and clonal expansion of T helper cells are key events in the immune response. CD4+ T helper cells, in fact, provide help to specific B lymphocytes and cytotoxic cells that are responsible for effector functions of the immune response. Because T cell activation depends on the recognition of antigen displayed by antigen-presenting cells (APC) it is important to define the APC that are present in various anatomical sites and participate in local immune reactions. The peritoneum is an anatomical site in which immune responses are vigorous, and this site can be used for m vivo immunization. The immune responses that occur in the peritoneum are of special relevance in end stage renal failure (ESRF) patients treated with continuous ambulatory peritoneal dialysis (CAPD). These patients, in fact, carry a transcutaneous catheter inserted in the peritoneum, and peritonitis is a frequent complication. For these reasons, we investigated whether the cells that line the peritoneal cavity can participate in specific immune responses by exhibiting antigen-presenting function. Peritoneal macrophages are well known to be antigen-presenting cells. There are few studies that have examined CD95 expression on peritoneal macrophages and the function of CD95 signaling in peritoneal local defense. The purpose of this study was to investigate potential roles of CD95 that may be involved in the inflammation of the peritoneal membrane.
PATIENTS AND METHODS
Patients
Sixteen patients (nine men, seven women; mean age 57 years, range 42–71 years) on CAPD were enrolled in the study after giving their informed consent. The patients were treated with the standard double-bag system (Baxter Healthcare, Deerfield, Illinois, USA). A sample of dialysate from the night bag was collected from each patient during the noninfected state. When peritonitis was suspected, the patient brought the first cloudy dialysate bag to the ward for examination. Immediately after arrival, the effluent was drained, followed by two quick changes with dwell times of half an hour. Samples were obtained from these three bags and from night bags on day 3 and day 10. All dialysate samples were saved at −70°C until analyzed. Bacteria cultures were performed on days 1 and 3. The patients served as their own controls, as samples were obtained from them during the noninfected study state.
The control group consists of 11 patients who just started their CAPD treatment. Peritoneal effluents were obtained on the day of commencement of CAPD and at 1, 2, 4, and 8 weeks.
Bacteriological Cultures
Dialysate (100 ml) was cultured on aerobic and anaerobic blood agar plates and incubated for four days at 37°C. All dialysate were processed within two hours; 5 mL of the dialysate was inoculated into aerobic and anaerobic blood culture bottles (BacT/Alert, Organon Teknika, Durham, North Carolina, USA) and incubated for seven days.
Phenotypic Analysis
Macrophage was separated using Ficoll-Hypaque method, washed in cold phosphate-buffered saline with 2% fetal calf serum and 0.1% sodium azide, and then stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibodies for two-color immunophenotyping stated below. The FITC/PE paired antibodies used were anti-CD3/CD95 (Fas), anti-CD3/CD54 (ICAM-1), anti-CD3/CD25 (interleukin-2 receptor), and anti-CD3/CD69. All antibodies were purchased from Becton-Dickinson (Worldwide Inc, Taiwan). CD3 is derived from hybridization of mouse myeloma cells with spleen cells from BALB/c mice immunized with human thymocyte. PE conjugates are supplied as 25 μg in 2.0 mL (12.5 μg/mL) of phosphate-buffered saline. Twenty microliters of monoclonal antibody or PE-conjugated antibody stains 1,000,000 peripheral blood mononuclear cells. The sample tubes were incubated for 30 minutes in an ice bath.
Samples from patients and controls were analyzed on a FACS Caliber flow cytometer (Becton-Dickinson). Cells were analyzed by selective gating based on forward and side scatter parameters. The cell gate was checked for its purity by using CD14 (monocyte marker)/CD45 (nucleated cells) double-staining, and was regarded as appropriate if the gate included at least 95% of all cells. The percentage of cells staining with each monoclonal antibody was determined by comparing each histogram with one from control cells stained with FITC- or PE-labeled isotype-control monoclonal antibodies.
For immunofluorescence analysis, the cells were seeded on slide flasks (Nunc. Roskilde, Denmark) and incubated for 24 h. The adherent layer was washed with PBS and treated with mouse MoAb specific for cytokeratin (1:10; Amersham, Aylesbury, UK) and with rabbit anti-Factor VIM serum (1:10; Dako, Glostrup. Denmark). After 30 min, the slides were washed and treated with the corresponding fiuoresceinated anti-immunoglobulin antibody (1:40; Kontron, Milan. Italy) for 45 min, washed and mounted with 10/il buffered glycerol. MC stained positive for cytokeratin but negative for Factor VIII. Cytometric examination showed a homogeneous population with respect to size and light scatter.
Statistics
Data were presented as median+/−SD, and were analyzed using a non-parametric unpaired Mann-Whitney test. A p value less than 0.05 was considered statistically significant.
RESULTS
Bacterial growth was found in all dialysates. Staphylococcus epidermidis was found in six episodes. Staphylococcus aureus was found in two episodes, and Klebsiella pneumonia in one episode. All patients were treated with cephazolin and vancomycin intraperitoneally. If necessary, therapy was changed according to the susceptibility pattern.
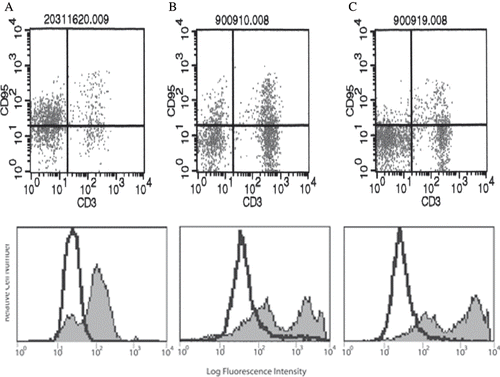
showed that the macrophages were identified and gated using forward scatter versus side scatter dot plot profiles. showed the representative FACS histogram for the expression of CD95 on macrophage from healthy controls, peritonitis-negative CAPD patients, and peritonitis-positive CAPD patients.
Figure 1. Macrophages were identified and gated using forward scatter versus side scatter dot plot profiles.
Figure 2. Representative FACS histogram for the expression of CD95 on macrophages from (A) healthy control, (B) CAPD patient with peritonitis, and (C) CAPD patient without peritonitis.
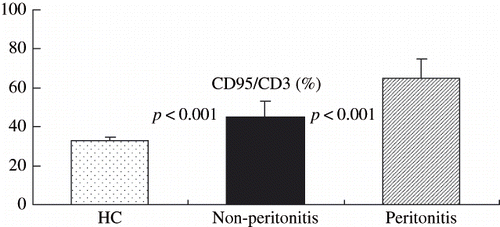
Expression percentage of CD95, CD54, CD25, and CD69 on macrophage were shown in , which showed that the expression of CD95, CD54, and CD25 in peritonitis-positive CAPD patients was significantly higher than those of control (all p < 0.001).
Table 1 Expression percentage of CD95, CD54, CD25, and CD69 on macrophages in patients with CAPD peritonitis and normal control (NC)
showed the expression percentage of CD95, CD54, CD25, and CD69 on macrophage in the peritonitis-positive CAPD patients, compared to the peritonitis-negative CAPD patients. The CD95 expression of peritonitis-positive CAPD patients was significantly higher than that of the peritonitis-negative CAPD patients (p < 0.001). The expression of CD54, CD25, and CD69, however, was not significantly different between the two CAPD subgroups.
Table 2 The expression percentage of CD95, CD54, CD25, and CD69 on macrophages in seven CAPD peritonitis patients, compared to nine CAPD patients without peritonitis
showed the expression percentage of CD95 on macrophage of control and those of peritonitis-positive and peritonitis-negative CAPD patients. There was a significant difference between each of them (all p < 0.001).
DISCUSSION
Bacterial peritonitis remains a major problem in patients undergoing CAPD. It is well recognized that all three cell systems present in the peritoneum—peritoneal macrophages, mesothelial cells, and lymphocytes—are involved in the initial immune response and in the recruitment of leukocytes from the peripheral blood.Citation[1] Peritoneal host defense is regulated by different cytokines derived mainly from macrophages but also from leukocytes and mesothelial cells.Citation[2] Peritoneal macrophages are the predominant cells in dialysate and act as first line of cellular defense against bacterial invasion.Citation[3] Besides, CAPD peritoneal cells have features of both immaturity and activation compared to control cells.Citation[4] Peritoneal macrophages have been reported to have characteristics such as increased respiratory burst activity, and the killing of Candida albicans suggest they are functionally activated. Monocyte and macrophages are likely to play an active part during the peritoneum infection.Citation[5],Citation[6]
The expression of surface molecules/receptors that are important in the host response to infection was examined using peritoneal macrophages isolated from patients on CAPD with peritonitis. The macrophage phenotypic profile was compared with that of normal peripheral blood monocytes. Increased expression by macrophages of CD14, ICAM-1 (CD54), Fc gamma RI (CD64), Fc gamma RII (CDw32), Fc gamma RIII (CD16), and transferring receptors (CD71) was previously reported.Citation[7] CD40 ligand (CD154) is functionally expressed on peritoneal macrophages during continuous ambulatory peritoneal dialysis peritonitis. The expression of CD40 mRNA and ICAM-1 were upregulated markedly following stimulation with INF-gamma.Citation[8] The activity of inflammatory cytokines can account for the phenotypic profile of these peritoneal macrophages.
Fas (CD95) is a 36-KD surface protein that belongs to a member of receptor super-families for tumor necrosis factor, and Fas triggered the apoptosis through the activation of a cascade of caspase. Our results of increased Fas receptors in macrophages of CAPD peritonitis further supported the pathogenetic role of Fas in these patients. In this study, we have demonstrated an increased expression of CD95, CD54, and CD25 on macrophages in CAPD patients, and the CD95 expression was especially higher in CAPD peritonitis. Our results indicated an abnormal increase of Fas and ICAM-1 expression in the activated macrophages of CAPD patients. Moreover, Fas expression in macrophages can be associated with local defense in peritoneal inflammation. Most of the previous studies on the pathogenesis of CAPD peritonitis measured circulating inflammatory mediators such as the cytokines with their receptors and the soluble adhesion molecules, and showed marked heterogeneity, which may due to the short half lives of the cytokines they measured.Citation[9–11]
We have also demonstrated an increased ICAM-1 expression in macrophages of patients with CAPD peritonitis. This finding is important because the pathogenesis of CAPD peritonitis may be critically dependent on a series of cell-adhesion molecule interactions between peritoneal mesothelium and leukocytes.Citation[12] There could be several explanations for our finding of increased expression of CD25, but not CD69 in macrophages of patients with CAPD peritonitis. First, the high circulating levels of cytokines such as interleukin-2 in CAPD peritonitis may contribute to the persistent instead of early activation of macrophages.Citation[13] Second, macrophages may be activated via the effect of the up-regulation of MHC cryptic antigens on damaged mesothelial cells, which is common in CAPD peritonitis.Citation[14] Third, the subunit of bacteria toxin may be a source of critical T-helper epitopes, which help to activate CD25.Citation[15]
In conclusion, we found an abnormally increased percentage of macrophages expressing Fas receptor and ICAM-1, and the percentage of CD95+ macrophages but not those of other markers were increased among patients with CAPD peritonitis. The later finding suggests that this macrophages phenotype is associated with CAPD peritonitis. Further study is needed to determine the role of macrophage in the inflammation process of the peritoneum.
REFERENCES
- Rapoport J, Hausmann MJ, Chausmann MJ, Chaimovitz C. The peritoneal immune system and continuous ambulatory peritoneal dialysis. Nephron. 1999; 81: 373–380
- Topley N. The cytokine network controlling peritoneal inflammation. Perit Dial Int. 1995; 15(Suppl. 3)S35–S39; S39–S40, discussion
- Goldstein CS, Bomalaski JSA, Zurier RB, Neilson EG, Douglas SD. Analysis of peritoneal macrophages in continuous ambulatory peritoneal dialysis patients. Kidney Int. 1984; 26: 733–740
- Betjes MG, Tuk CW, Struijk DG, Krediet RT, Arisz L, Hoefsmit EC, et al. Immuno-effector characteristics of peritoneal cells during CAPD treatment: A longitudinal study. Kidney Int. 1993; 43: 641–648
- Hart PH, Jones CA, Finlay-Jones JJ. Peritoneal macrophages during peritonitis. Phenotypic studies. Clin Exp Immunol. 1992; 88(3)484–491
- Peterson PK, Gaziano E, Suh HJ, Devalon M, Peterson L, Keane WF. Antimicrobial activities of dialysate-elicited and resident human peritoneal macrophages. Infect Immun. 1985; 49: 212–218
- Bos HJ, van Bronswijk H, Helmerhorst TJ, Oe PL, Hoefsmit EC, Beelen RH. Distinct subpopulations of elicited human macrophages in peritoneal dialysis patients and women undergoing laparoscopy: A study on peroxidatic activity. J Leukoc Biol. 1988; 43: 172–178
- Yang X, Ye R, Kong Q, Yang Q, Dong X, Yu X. CD40 is expressed on rat peritoneal mesothelial cells and upregulates ICAM-1 production. Nephrol Dial Transplant. 2004; 19(6)1378–1384
- Tzehoval E, De Baetselier P, Feldman M, Segal S. The peritoneal antigen-presenting macrophage: Control and immunogenic properties of distinct subpopulations. Eur J Immunol. 1981; 11(4)323–328
- Harvey DM, Sheppard KJ, Morgan AG, Fletcher J. Neutrophil function in patients on continuous ambulatory peritoneal dialysis. Br J Hematol. 1988; 68: 27–28
- Davies SJ, Yewdall VM, Ogg CS, Cameron JS. Peritoneal defence mechanisms and Staphylococcus aureus in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. 1990; 10: 135–140
- Brauner A, Lu Y, Hallden G, Hylander B, Lundahl J. Difference in the blood monocyte phenotype between uremic patients and healthy control: Its relation to monocyte differentiation into macrophages in the peritoneal cavity. Inflammation 1998; 22: 55–66
- Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The ligand ICAM-l provides an important costimulatory signal for T cell receptor mediated activation of resting T cells. J Immunol. 1990; 144: 4579–4586
- McGregor SJ, Topley N, Jorres A, Speekenbrink AB, Gordon A, Gahl GM, et al. Longitudinal evaluation of peritoneal macrophage function and activation during CAPD: Maturity, cytokine synthesis and arachidonic acid metabolism. Kidney Int. 1996; 49: 525–533
- Betics MGH, Bos HJ, Krediet RT, Arisz L. The mesothelial cells in CAPD effluent and their relation to peritonitis incidence. Perit Dial Int. 1991; 11: 22–26