Abstract
Background. Sirolimus has been considered to be a non-nephrotoxic agent. It may delay graft function due to a potential hindrance of the recovery from acute tubular necrosis. It remains controversial as to whether the concomitant administration of sirolimus (SRL) with calcineurin inhibitors delays graft function in Asian patients. Method. This study enrolled 61 patients who received primary renal transplantation. Twenty-one patients aged 38.9 ± 11 years received early treatment with 6 mg/day sirolimus, 8 mg/kg/day cyclosporine (CsA) and prednisolone (SRL group). Forty patients with a mean age of 36.7 ± 9 years in the control group were treated with the standard immunosuppressive therapy (8 mg/kg/day CsA, mycophonolate mofetil and prednisolone). Results. The creatinine level at one week following transplantation in the SRL group was not significantly different from that in the control group (4.7 ± 1.0 versus 2.7 ± 0.4 mg/dL, p = 0.17). Similarly, there was no significant difference in the creatinine level at week 2 (3.4 ± 0.7 versus 2.7 ± 0.3 mg/dL, p = 0.36), week 3 (2.0 ± 0.3 versus 2.1 ± 0.2 mg/dL, p = 0.76), and week 4 (1.8 ± 0.2 versus 1.8±0.1 mg/dL, p = 0.92) between SRL and control groups, respectively. Similarly, the number of the patients with renal function impairment in both groups was not significantly different. The one-year patient and graft survival was 100% and 96%, respectively, in the SRL group and 100% and 98%, respectively, in the control group. Conclusion. The combination of sirolimus and cyclosporine did not prolong the recovery from transplantation-associated ischemic injury in kidney graft recipient. Our study demonstrated the safety of early usage of the concomitant administration of sirolimus and cyclosporine in kidney transplantation.
INTRODUCTION
Because one-year renal graft survival rates now exceed 90%,Citation[1] the clinical challenge is to develop immunosuppressive regimens that reduce the risk of long-term graft loss while preserving the current low rates of acute rejection. In view of the known nephrotoxic potential of calcineurin inhibitors (CNIs), CNI-sparing regimens represent an interesting therapeutic alternative. Proliferation signal inhibitors are potent immunosuppressants that appear to be non-nephrotoxic.Citation[2] Therefore, they may permit CNI dose reduction without loss of immunosuppressive potency or increased renal toxicity.
Sirolimus (SRL) inhibits protein kinase activity, termed mammalian target of rapamycin (mTOR), which is necessary to continue the cell cycle. The inhibition of mTOR affects the activity of the 40S ribosomal protein S6 kinase (p70s6k), which plays a key role in cell proliferation and apoptosis pathways.Citation[3] Recent data have shown that despite the absence of specific nephrotoxicity, sirolimus might increase the prevalence, length, and severity of delayed graft function (DGF) when used during the initial phase after transplantation.Citation[4–6]
Delayed graft function (DGF), defined as the need for at least one hemodialysis session during the first week after transplantation, has been associated with both long- and short-term decrease of graft survival. DGF is secondary to ischemia-reperfusion injury and is associated with acute tubular necrosis.Citation[7] The rate of recovery of tubular cell function is thought to depend on a balance between tubular cell death and the ability of injured cells to enter the cell cycle and proliferate.Citation[8],Citation[9] A possible mechanism of rapamycin for delaying graft function recovery may be secondary to its antiproliferative action. Furthermore, the co-administration of cyclosporine and sirolimus may amplify the nephrotoxic effects of the former drug.Citation[10]
In our review, there is scant literature regarding DGF using the combination of sirolimus and cyclosporine in Asia. The aim of this study was to evaluate the influence of the combination of sirolimus and cyclosporine in the DGF setting and the impact of the one-year, two-year, graft function, and acute rejection rate for patients treated with the combination of sirolimus and cyclosporine in Asia.
MATERIALS AND METHODS
Study Population
All adults older than 16 years who had undergone a primary renal transplantation from July 2000 through August 2005 in Chang Gung Memorial Hospital were retrospectively reviewed. No patient who received a second transplant or whose graft failed within 24 hours of the transplantation operation was included in the analysis. All patients were on a conventional hemodialysis schedule before surgery. Our final study group included 61 recipients, who were divided into two nonrandomized groups according to the immunosuppressive regimen:
treated early with sirolimus 6mg per day, cyclosporine (CsA) 8mg/kg/day, and steroids (SRL group, n = 21); or
standard immunosuppressive therapy CsA 8mg/kg/day, mycophenolate mofetil (MMF) 2 gm/day, and steroids (control group, n = 40).
Steroid was administered to all recipients beginning in the immediate post-operative period: methylprednisolone (solumedrol) 500 mg the fist day followed by 20 mg per day for 2 more days, intravenously) and medrol orally, subsequently. Sirolimus, CsA, and MMF were given immediately before surgery. The target trough blood level for sirolimus was 8–10 ng/mL and target C2 levels between were 1200–1400 ng/mL.
Laboratory Study
We obtained complete laboratory and dialysis-related data in these patients. Laboratory data included serum hemoglobin, albumin, calcium, blood urea nitrogen (BUN), and creatinine. In addition, Kt/V (Daugirdas), which represented urea clearance, was analyzed for the adequacy of dialysis. Pre-dialysis and post-dialysis blood samples were collected from a single dialysis session according to recommended procedures for dialysis by NKF-K/DOQI guidelines.Citation[11] Hemoglobin, albumin, calcium, BUN, and creatinine concentrations were determined by using standard autoanalyzers.
Transplant and Post-Transplant Parameters
HLA mismatches calculated the total number of mismatches at the HLA-A, -B, and –DR loci. Glomerular filtration rate (GFR) was estimated by Cockcroft-Gault formula. Delayed graft function (DGF) was defined as the requirement for hemodialysis during the first week after transplantation. Acute rejections were proved with renal biopsy.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. Two-tailed Student's unpaired t-test was employed to evaluate the difference between means. Differences between groups of categorical variables were analyzed by chi-square test. Actuarial graft survival was calculated from the date of renal transplantation to graft failure or patient death. p < 0.05 was considered statistically significant. The statistical analyses were done by the software packages of SPSS 12.0 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
shows the demographic data of the entire group of donors and recipients. The patients in the two groups did not significantly differ for the number of HLA mismatches, but donor age was significant younger in the SRL group than in the control group. After two years post-transplantation, we were unable to find differences between the two immunosuppressive regimens both in terms of graft and patient survival and in terms of kidney graft function (GFR, 53.1 ± 19.1 versus 51.6 ± 24.8 mL/min, p = 0.812; SRL group versus control group).
Table 1 Clinical features of donors and renal transplant recipients divided according to the immunosuppressive regimen adopted
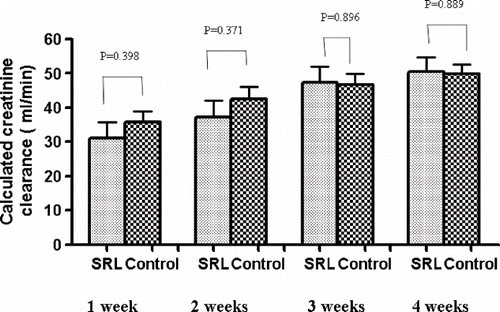
Also, in the early post-transplantation period, the two groups of patients showed a similar behavior. The one-, two-, three-, and four-week GFR in the two groups was not significantly different (see ). Delayed graft function occurred at a similar rate in the two groups of patients: 28% in SRL group and 25% in control group (p = 0.763; see ). Furthermore, the duration of post-transplant hemodialysis time was similar (SRL group versus control group: 3.7 ± 6.5 versus 3.3 ± 6.3 days, p = 0.843).
Figure 1. Kidney graft function in the two groups of patients examined at one, two, three, and four weeks after transplantation.
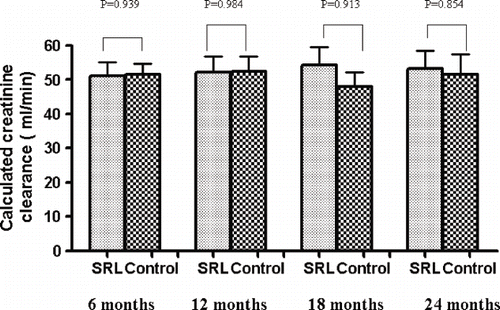
Regarding long-term graft function, the clearance of creatinine was similar between the two groups in 6, 12, 18, and 24 months (see ). Finally, both immunosuppressive regimens were associated with a low acute rejection rate (SRL group versus control group: 23% versus 20%, p = 0.730; see ).
DISCUSSION
In this study, we report that the administration of SRL, in combination with a calcineurin inhibitor (cyclosporine), to kidney transplantation recipients is associated with a similar incidence of DGF and a similar outcome compared to standard immunosuppressive therapy with cyclosporine, mycophenolate mofetil, and steroids.
DGF was considered to be critical for the prognosis of graft survival. In previous journal reports, they found that combination of SRL and CNI may increase the rate of DGF. We review their patient's condition to compare with our data and found that the recipient's age may be the critical factor accounting for the different results.
There were relatively older donors in previous report journals with a mean age around 45–50 years.Citation[5] However, in our study, both groups of patients were relatively younger, with a mean age around 35–40 years. Recently, Gabriel et al.Citation[12] compared the donor characteristics, level of HLA matching, cold ischemic time, and transplant outcome according to the recipient's age at transplantation. He found that older recipients had a high incidence of DGF and younger recipients possibly had a higher incidence of acute rejection.
The first explanation for our finding is that the older patients who were predisposed for DGF may develop DGF when they undergo early exposure to the combination of sirolimus and a calcineurin inhibitor. However, the same regimen may not increase DGF risk in younger patients who are resistant to DGF occurrence.
It is well known that in Taiwan, the rate of kidney allograft transplantation is lower than in western nations, so that kidney allografts are given for selected people who had lower mean ages with a high HLA matching level. Therefore, the mean recipient age in our country may be lower than in western nations.
The influence of race on renal allograft survival cannot be ignored. Renal allograft survival in black recipient is significantly lower than in clinically comparable white recipients.Citation[13] For black cadaveric recipients, there was no significant improvement in allograft survival with the use of cyclosporine or with matching for HLA. Therefore, patients of different races may have different responses to immunosuppressive regimens.
In our study, we enrolled only patients of the Asian race, which was different from previous reports that were primarily comprised of Caucasians. Therefore, the risk of DGF occurrence may be different between Asian and Caucasian peoples.
The other detrimental factor for DGF in the present study was donor age. In our study, SRL group patients had a younger donor age than the control group. Apparently, age may influence DGF occurrence as an intervenient factor for our result. However, because donors and recipients are paired by HLA matching, which is not easily controlled for similar donor age, further large studies are required to remove donor age as an intervenient factor.
Data for cold ischemia time, a classically recognized risk factor, was not available in our report. This factor may also determine the DGF occurrence. In our report, patients in the SRL group had a similar rate of acute rejection with the control group. Furthermore, graft and patient survival rates were also similar and without significant difference.
In summary, we report that the combination regimen with sirolimus, calcineurin inhibitor, and corticosteroid has a similar DGF occurrence compared with a standard regimen of calcineurin inhibitor, MMF, and corticosteroid. Therefore, we believe that sirolimus is safe and effective for early usage in younger patients after renal transplantation.
REFERENCES
- Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000; 342: 605–612
- Kahan BD, Chang JY, Sehgal SN. Preclinical evaluation of a new potent immunosuppressive agent, rapamycin. Transplantation 1991; 52: 185–191
- Lieberthal W, Fuhro R, Andry CC, et al. Rapamycin impairs recovery from acute renal failure: Role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001; 281: F693–F706
- Smith KD, Wrenshall LE, Nicosia RF, et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J Am Soc Nephrol 2003; 14: 1037–1045
- McTaggart RA, Gottlieb D, Brooks J, et al. Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant 2003; 3: 416–423
- Davis C. Sirolimus delays renal allograft recovery. Am J Transplant. 2003; 3: 363–365
- Geddes CC, Woo YM, Jardine AG. The impact of delayed graft function on the long-term outcome of renal transplantation. J Nephrol. 2002; 15: 17–21
- Humes HD, MacKay SM, Funke AJ, Buffington DA. Acute renal failure: Growth factors, cell therapy, and gene therapy. Proc Assoc Am Physicians. 1997; 109: 547–557
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994; 93: 2175–2188
- Johnson RW, Kreis H, Oberbauer R, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001; 72: 777–786
- I. NKF-K/DOQI Clinical Practice Guidelines for Hemodialysis Adequacy: update 2000. Am J Kidney Dis. 2001; 37: S7–S64
- Oniscu GC, Brown H, Forsythe JL. How old is old for transplantation?. Am J Transplant. 2004; 4: 2067–2074
- Barger BO, Hudson SL, Shroyer TW, et al. Influence of race on renal allograft survival in the pre- and post- cyclosporine era. Clin Transpl. 1987; 217–233