Abstract
A 72-year-old woman with a previous diagnosis of non-tuberculous mycobacteria (NTM) pulmonary disease was admitted because of hemoptysis and acute renal failure. A chest x-ray showed interstitial infiltration over bilateral lung fields. A kidney biopsy showed immune complex-mediated crescentic glomerulonephritis and diffuse endocapillary hypercellularity with exudative neutrophils. Reactive NTM infection of the lungs was suspected when mycobacterial cultures of the sputum repeatedly yielded Mycobacterium avium. A lung biopsy revealed chronic inflammation without evidence of alveolar capillaritis. NTM pulmonary disease was further confirmed by tissue culture of the lung biopsy specimens. Anti-tuberculous drugs in combination with clarithromycin were given for the treatment of NTM infection. Because of the risk of aggravating underlying infectious disease, immunosuppressive therapy for crescentic glomerulonephritis was not carried out. Pulmonary symptoms promptly responded to treatment. Furthermore, renal function steadily improved after the initiation of anti-NTM therapy. To our knowledge, this is the first report of crescentic glomerulonephritis associated with NTM infection.
INTRODUCTION
Over the centuries, two well-known mycobacterial species—Mycobacterium tuberculosis and M. leprae—have been the known causes of much human suffering. Many other mycobacteria are ubiquitous in the environment with low virulence but can be opportunistic and at times deadly pathogens. They are referred to as atypical mycobacteria, mycobacteria other than tuberculosis, or non-tuberculous mycobacteria (NTM). Among the more than 90 known species of NTM, about one-third has been associated with human diseases. Today, the prevalence of NTM infection is increasing worldwide. It is partly explained by the increase in the number of immunocompromised individuals, such as those suffering from acquired immunodeficiency syndrome, and is also due to an increasing awareness of NTM as a human pathogen and improvements in methods of mycobacterial culture. Infection has been well documented to cause glomerulonephritis, with Streptococcus being the most common pathogen and predominantly affecting children. The pattern of the disease has changed over recent decades. Not only Streptococcus but also other bacterial, viral, fungal, and parasitic infections can trigger the disease in both children and adults. Although glomerulonephritis caused by tuberculosis infection has been reported in the literature,Citation[1–5] the association of NTM infection with glomerulonephritis has not been described before. Herein, we report a case of crescentic glomerulonephritis with the characteristics of infectious glomerulonephritis in which NTM was the most possible causal pathogen.
CASE REPORT
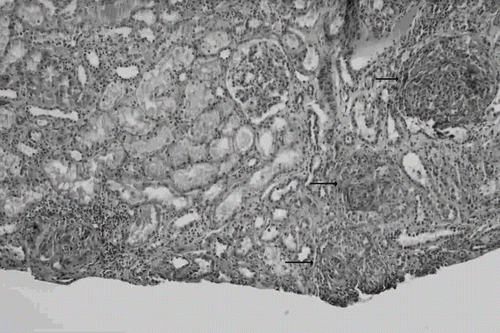
A 72-year-old woman was admitted with a two-week history of cough productive of yellowish, occasionally blood-streaked sputum. She also noticed profound malaise, decreased appetite, and a weight loss of 5 kg within this time period. She had received medications from a local medical doctor. There was no complaint of fever, epistaxis, or arthralgia. She had been diagnosed to have NTM pulmonary disease approximately five years ago and had received a 12-month course of anti-tuberculous therapy. Her past medical history was otherwise unremarkable. On admission, physical examination showed a body temperature of 36.5°C, regular pulse rate of 92/min, respiratory rate of 18/min, and blood pressure of 128/76 mmHg. Head and neck examinations were unremarkable. Auscultation of her chest revealed diffuse crackles over bilateral lung fields. Examinations of the heart and abdomen were normal. She had 1+ lower extremity pitting edema. No skin lesion was found. A chest x-ray demonstrated interstitial infiltration over bilateral lung fields and fibrous change of right upper lung. Laboratory investigations showed hemoglobin 9.5 g/dL, white blood cell count 15,400/μL, and platelet count 256,000/μL. A blood chemistry panel showed urea nitrogen 28 mg/dL, creatinine 2.2 mg/dL, sodium 136 mmol/L, potassium 3.8 mmol/L, calcium 8.1 mg/dL, glucose 93 mg/dL, uric acid 8.4 mg/dL, albumin 3.0 g/dL, and globulin 3.2 g/dL. Urinalysis revealed 4+ blood and 2+ protein, with numerous red blood cells and 35 to 40 white blood cells per high-power field in the urinary sediment. Daily urinary protein excretion was 1.9 g. A renal ultrasonography showed normal-sized kidneys with increased parenchymal echogenicity suggestive of acute renal parenchymal disease. Owing to acute renal failure and urinalysis findings suggestive of glomerulonephritis, rapidly progressive glomerulonephritis (RPGN) was highly suspected. Serological studies showed negative findings for antinuclear antibody, antibasement membrane antibody, antineutrophil cytoplasmic antibody, hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antibody. Antistreptolysin O titer was < 200 IU/mL. Serum complement levels were normal. Rheumatoid factor was negative. A kidney biopsy showed diffuse proliferative glomerulonephritis, with cellular crescents affecting 10 of the 25 examined glomeruli (see ). The glomeruli displayed endocapillary hypercellularity with capillaries full of neutrophils. Mild interstitial infiltrate of inflammatory cells was noted. Interstitial fibrosis and tubular atrophy were present only focally and minimally. No glomerular tuft necrosis or vasculitis was seen. Immunofluorescent studies showed granular staining for IgG (2+), IgM (1+), C3 (3+), and C1q (1+) along the mesangium and capillary loops. By electron microscopy, subendothelial and mesangial electron-dense deposits were noted. No subepithelial hump-like deposit was found, and there was no evidence of cryoglobulin deposit. Because of histologically resembling infectious glomerulonephritis, a comprehensive workup for infection was performed. Echocardiography showed no evidence of infective endocarditis. Sputum, blood, and urine bacterial cultures were negative. However, sputum smears for acid-fast stain were positive. Furthermore, mycobacterial cultures of the sputum specimens collected by either spontaneous expectoration or bronchoscopic wash repeatedly yielded M. avium. To clarify the lung lesions, a thoracoscopic lung biopsy was performed and demonstrated dilated alveoli full of mucus, macrophages, and leukocytes. The alveolar septum was widened by lymphoid aggregation, fibrosis, and smooth muscle cells proliferation. No alveolar capillaritis or granuloma was found. NTM pulmonary disease was further confirmed when the mycobacterial culture of the lung biopsy specimens also grew M. avium. Anti-tuberculous agents comprising rifampicin, isoniazid, and ethambutol in combination with clarithromycin were administrated for the treatment of NTM infection. Because of the risk of aggravating underlying infectious disease, immunosuppressive therapy for crescentic glomerulonephritis was not given. Pulmonary symptoms promptly responded to the treatments. Repeated sputum smears for acid-fast stain and mycobacterial cultures became negative one month later. Follow-up chest x-ray also showed resolution of the pulmonary infiltrates. Furthermore, the renal function steadily improved, with serum creatinine level falling to 1.1 mg/dL three months after initiation of anti-NTM therapy.
DISCUSSION
The combination of acute glomerulonephritis, as manifested by hematuria and renal insufficiency, and pulmonary hemorrhage, as manifested by hemoptysis and pulmonary infiltrates, is characteristic of pulmonary-renal syndrome. However, these manifestations can be seen in acute glomerulonephritis, which is complicated by other pulmonary disorders such as pulmonary edema, pulmonary embolism, or pulmonary infection. There is no doubt that it is important to exclude any pulmonary infectious process before starting immunosuppressive therapy when pulmonary renal syndrome is suspected. However, definite diagnosis of pulmonary NTM infection remains to be difficult. First, NTM requires special media and growth conditions. Second, the diagnosis of pulmonary NTM infection is complicated by the variability in clinical and radiological manifestations and the frequent presence of significant prior pulmonary disease. Finally, in contrast to M. tuberculosis, NTM are ubiquitous in the environment. Isolation of NTM from the sputum may represent harmless colonization of the respiratory tract and does not constitute proof of the existence of pulmonary NTM infection. In an attempt to address these difficulties, the American Thoracic Society has established specific criteria that emphasize compatible clinical symptoms/signs, reasonable exclusion of other disease, and characteristic imaging findings such as persistent (more than two months) or progressive pulmonary infiltrates. Bacteriologic criteria include positive cultures of at least three sputum/bronchial wash samples within one year or any growth bronchopulmonary tissue biopsy.Citation[6] In our patient, the clinical, radiographic, and bacteriologic criteria all were satisfied and indicated NTM pulmonary disease.
Although the potential virulence of NTM has been increasingly recognized in humans, NTM infection-related glomerulonephritis has not been described yet. Nguyen et al. reported a patient with RPGN who developed severe lower gastrointestinal bleeding two weeks after the initiation of aggressive immunosuppressive treatments and was diagnosed to have ulcerative colitis due to infection with M. avium.Citation[7] The intestinal bleeding stopped promptly after the administration of anti-NTM therapy. However, renal function did not recover, and the patient was maintained on hemodialysis. The report emphasized an aggravation of gastrointestinal NTM infection after immunosuppressive therapy but did not address the association of NTM infection with RPGN.
In our presenting case, arguments may arise as to whether the crescentic glomerulonephritis was an independent disease or was causally associated with NTM infection. In light of several observations, we strongly considered that NTM infection was responsible for the development of crescentic glomerulonephritis. First, the kidney biopsy revealed diffuse endocapillary proliferation with neutrophil exudate as the characteristics of infectious glomerulonephritis. Although subepithelial hump-like deposits were not seen in our case, they are often but not always found in cases of infectious glomerulonephritis. Secondly, immunofluorescence studies showed a granular glomerular labeling pattern indicative of immune complex-mediated glomerulonephritis. In the absence of other discernible causes such as a variety of primary glomerulonephritis, systemic lupus erythematosus, Henoch-Schönlein purpura, and mixed cryoglobulinemia, infectious glomerulonephritis was the most possible clinicopathological diagnosis. Most importantly, the recovery of renal function after treatment of NTM infection further suggested a casual relationship between NTM infection and crescentic glomerulonephritis.
In summary, crescentic glomerulonephritis could be associated with NTM infection. Although the prognosis of crescentic glomerulonephritis is generally believed to be poor, our case showed a favorable prognosis when anti-NTM therapy was promptly administrated. The natural history of infection-related glomerulonephritis perhaps contributes to the excellent outcome.
REFERENCES
- Teruel JL, Matesanz R, Mampaso F, Lamas S, Herrero JA, Ortuno J. Pulmonary tuberculosis, cryoglobulinemia and immune complex glomerulonephritis. Nephron. 1987; 27: 48–49
- Meyrier A, Valensi P, Sebaoum J. Mesangio-capillary glomerulonephritis and the nephrotic syndrome in the course of disseminated tuberculosis. Nephron. 1988; 49: 341–342
- Sopeña B, Sobrado J, Pérez AJ, Oliver J, Courel M, Palomares L, González L. Rapidly progressive glomerulonephritis and pulmonary tuberculosis. Nephron. 1991; 57: 251–252
- Pecchini F, Bufano G, Ghiringhelli P. Membranoproliferative glomerulonephritis secondary to tuberculosis. Clin Nephrol. 1997; 47: 63–64
- Matsuzawa N, Nakabayashi K, Nagasawa T, Nakamoto Y. Nephrotic IgA nephropathy associated with disseminated tuberculosis. Clin Nephrol. 2002; 57: 63–68
- Wallace RJ, Jr, O'Brein R, Glassroth J, Raleigh J, Dutta A. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990;1; 42: 940–953
- Nguyen HN, Frank D, Handt S, Rieband HC, Maurin N, Sieberth HG, Matern S. Severe gastrointestinal hemorrhage due to Mycobacterium avium complex in a patient receiving immunosuppressive therapy. Am J Gastroenterol. 1999; 94: 232–235