Abstract
Visfatin was recently defined as an adipocytokine; however, the pathophysiological role of visfatin is not completely understood. A few studies suggest that visfatin may be a new proinflammatory adipocytokine. The aim of the present study was to compare serum visfatin levels between hemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients and evaluate the relationship between visfatin levels to IL-6, TNF-α, and left ventricular hypertrophy. Serum visfatin, IL-6, and TNF-α levels were measured by using the ELISA method, and echocardiographic evaluations were performed in 31 hemodialysis patients, 30 CAPD patients, and 21 healthy volunteers. Serum visfatin levels were higher in the CAPD group (265.27 ± 387.86 ng/mL) than hemodialysis (97.68 ± 244.96 ng/mL,) and control (41.33 ± 48.87 ng/mL) groups (p = 0.04, p = 0.01, respectively). No significant difference was observed between the hemodialysis and control groups. In univariate analysis, visfatin levels were positively correlated with IL-6 (r = 0.24, p = 0.03), TNF-α (r = 0.34, p = 0.002), and BMI (r = 0.26, p = 0.03) and negatively correlated with some left ventricular diastolic parameters [Em and Em/Am (r = −0.305, p = 0.01), (r = −0.251, p = 0.03), respectively]. No relationship was found between visfatin and left ventricular mass index. In the linear regression analysis, visfatin levels independently related with TNF-( (β = 0.369, p = 0.001) and IL-6 (β = 0.284, p = 0.015). This study has found significantly higher levels of serum visfatin in CAPD patients when compared to healthy individuals. Increased visfatin levels seem to associate with proinflammatory cytokines such as IL-6 or TNF-α. As for the effects of on left ventricular structure and functions, visfatin might have negative effects on left ventricular diastolic function parameters but have no effects on left ventricular mass index.
INTRODUCTION
Visfatin was recently defined by Fukuhara et al. as a visceral adipose-derived secretory adipocytokine that mimics the properties of Pre-B Cell Colony-Enhancing Factor (PBEF), which stimulates IL-7 and B cell precursors.Citation[1]
In vitro studies with visfatin initially led to the assumption that it had insulin-mimetic effectsCitation[1],Citation[2] and increased glucose consumption in peripheral tissue, thus increasing insulin sensitivity. However, more recent in vivo and in vitro studies have suggested that visfatin may be related to insulin-resistance, endothelial dysfunction, and increased inflammation.Citation[3–6] Despite these findings, it should be noted that comprehensive results do not yet exist about the pathophysiological role of visfatin.Citation[4]
Dialysis patients are suggested to have increased several adipocytokines (adiponectin, leptin, interleukin 6 [IL-6], tumor necrosis factor-α [TNF-α]), particularly due to low renal clearance.Citation[7] This group of patients may be expected to have higher visfatin levels along with other adipocytokines. There seems to be only one other in the literature that addresses this topic, but it offers corroborating evidence.Citation[7] However, to the best of our knowledge, there is no study evaluating visfatin levels with respect to dialysis modalities.
IL-6 and TNF-α are adipocytokines with proinflammatory characteristics that are known to have an inflammatory role in dialysis patients.Citation[8] Recently, a relationship between visfatin and these adipocytokines have been suggested in healthy subjects.Citation[3],Citation[6] Increased levels of IL-6 and TNF-α have been demonstrated to be related with left ventricular hypertrophy (LVH) in dialysis patients.Citation[8–10] This suggests a relationship between visfatin, which is thought to be a proinflammatory cytokine, and LVH, though such a relationship has not yet been explored in the literature.
In the present study, we aimed to compare serum visfatin levels between hemodialysis patients and continuous ambulatory peritoneal dialysis (CAPD) patients and to investigate the relationship of visfatin levels to IL-6, TNF-α, and LVH.
MATERIAL AND METHODS
Along with 21 healthy volunteers, the study included 31 hemodialysis and 30 CAPD patients who had been receiving dialysis for at least the last six months in our department. Dialysis patients with diabetes mellitus; cardiovascular diseases, such as valve disease, atherosclerotic heart disease, or arrhythmia; uncontrolled blood pressure; hypervolemia; or a disease causing active inflammation were omitted from the study. Four patients were excluded from study because a loss of data. Of the participating patients, 4 had chronic renal failure due to amyloid, 4 due to polycystic kidney disease, 15 due to glomerulonephritis, and 6 due to nephrolithiasis. Chronic renal failure etiology of 32 patients was not known. Seventeen of 31 hemodialysis patients (67%) and 17 of 30 CAPD patients (70%) had hypertension. Our patients were performing their CAPD treatment using lactate containing standard dialysis solution for four or five exchanges. Hemodialysis patients were receiving bicarbonate dialysis for four hours, three times a week. Prior to the study, all patients were informed, and their signed consent was taken. The study was approved by the Ethical Committee on Studies Involving Human Beings at Gazi University, Faculty of Medicine. The systolic and diastolic blood pressure of all patients was measured, and their body mass index (BMI) was calculated by the formula (BMI = kg/m2). Serum glucose, albumin, high sensitive C-reactive protein (hsCRP), blood urea nitrogen (BUN), creatinine, calcium (Ca), phosphate (P04), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, hemoglobin, and intact parathormone (iPTH) levels were measured from blood samples drawn after a 12-hour fast in the routine biochemistry laboratory. Serum visfatin, IL-6, and TNF-α levels were identified using the ELISA method. The dialysis efficacy of all patients was assessed using the Kt/V method:
Echocardiography
All subjects in the study were evaluated with 2D, M-Mode, pulse wave Doppler, and tissue Doppler echocardiography by using a GE Vivid 7 dimension echocardiography machine. Echocardiographic evaluations were performed at the left lateral decubitus position with standard techniques. 2D long-axis views were used to obtain linear measurements of left ventricular cavity. Left ventricular mass (LVM) was estimated using the anatomically validated formula of Devereux et al.Citation[11]:
where IVST = interventricular septal thickness, LVID = left ventricular internal dimension, and LPWT = left posterior wall thickness. The left ventricular mass index (LVMI) was calculated by the formula, LVM/(height) Citation[2],Citation[7].Citation[11] Mitral inflow velocities were obtained by pulse wave Doppler in the apical four-chamber view with the sample volume placed at the tips of the mitral valve leaflets. The ratio of early diastolic to late diastolic mitral inflow velocities was measured. Tissue Doppler imaging was performed from the apical four-chamber view, and the images were digitized. Myocardial velocity profiles of the lateral mitral annulus were obtained by placing a 6 mm sample volume at the junction of the mitral annulus and lateral myocardial wall. Early and late diastolic velocities, systolic velocities, and isovolumetric relaxation time (IVRT) were measured from two consecutive cardiac cycles and averaged. The ratio of early to late diastolic mitral annular velocities was calculated.
Statistical Analysis
Data are shown as mean ± SD or as percentages. Comparisons between groups were performed by student's t test, chi-square test, or one-way analysis of variance (ANOVA), as appropriate. Univariate correlation was established by Pearson's correlation coefficient. All statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS version 13). Significance was defined as a p value less than 0.05.
RESULTS
A comparison of the anthropometric measurements and demographics and biochemical values of the CAPD, hemodialysis, and control groups is given in . No significant difference was present between the groups with respect to age and sex distribution. When visfatin, IL-6, and TNF-α levels were compared between the groups, visfatin levels were significantly higher in the CAPD group (265.27 ± 387.86 ng/mL) than the hemodialysis (97.68 ± 244.96 ng/mL) and control (41.33 ± 48.87 ng/mL) groups. On the other hand, no significant difference was observed between the hemodialysis and control groups (see ). IL-6 was significantly higher in the CAPD group compared to the control group (12.91 ± 14.63 pg/mL; 4.51 ± 7.52 pg/mL). However, there was no significant difference between other groups (see ). TNF-α was significantly higher in both hemodialysis (32.43 ± 8.57 pg/mL) and CAPD patients (36.03 ± 9.68 pg/mL) than the control group (9.25 ± 8.81 pg/mL). However, no significant difference was observed between the hemodialysis and peritoneal dialysis groups (see ). The results of the univariate correlation analysis yielded a positive correlation between serum visfatin levels and IL-6 (r = 0.24, p = 0.03), TNF-α (r = 0.34, p = 0.002), and BMI (r = 0.26, p = 0.03). The linear regression analysis took visfatin as the dependent variable and IL-6, TNF-α, and BMI as the independent variables, and found statistical significance between visfatin and TNF-α (β = 0.369, p = 0.001) and IL-6 (β = 0.284, p = 0.015).
Table 1 Comparison of selected characteristics between CAPD and hemodialysis patients and healthy subjects
Figure 1. Comparison of visfatin serum levels between CAPD and hemodialysis patients and healthy subjects.
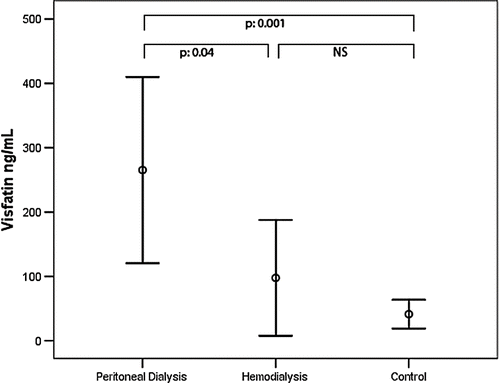
Figure 2. Comparison of IL-6 serum levels between CAPD and hemodialysis patients and healthy subjects.
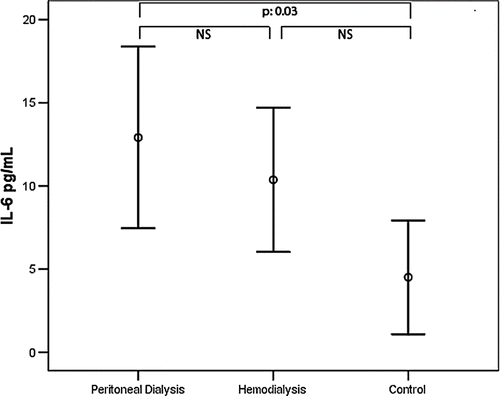
Figure 3. Comparison of TNF-α serum levels between CAPD and hemodialysis patients and healthy subjects.
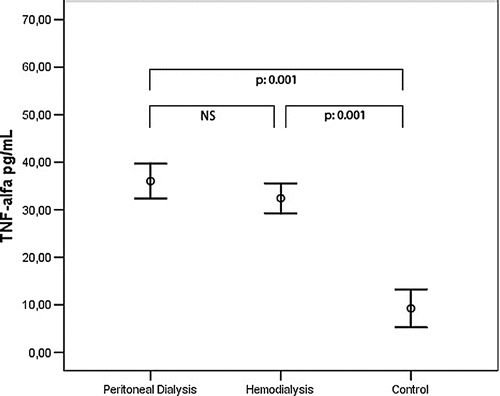
Echocardiographic Findings
shows a comparison of the tissue Doppler echocardiography parameters of the CAPD, hemodialysis, and control groups. LVMI was significantly higher in the CAPD patients than the control group. There was no significant difference between the other groups (see ). According to the results of the univariate correlation analysis, a negative relationship was observed between serum visfatin levels and Em (mitral annuler early velocity) (r = −0.305, p = 0.01), Em/Am (mitral annuler late velocity) (r = −0.251, p = .03). No relationship was found between visfatin and LVMI (r = .028, p = .81).
Table 2 Comparison of echocardiographic parameters between CAPD and hemodialysis patients and healthy subjects
DISCUSSION
Our study found higher visfatin levels in end stage renal disease (ESRD) patients than healthy subjects. When visfatin levels were compared with respect to dialysis modalities, it was found that peritoneal dialysis patients had higher values than hemodialysis patients.
In the only study that exists in the literature about visfatin levels in ESRD patients, Axelsson et al. reported increased visfatin levels in ESRD patients, which is consistent with our results.Citation[7] The mechanism behind increased visfatin levels in ESRD patients may be due to the reduction in the renal clearance of visfatin as other adipocytokines.
Another result of our study has been the identification of higher visfatin levels in peritoneal dialysis patients than in hemodialysis patients when dialysis treatment options were compared. To our knowledge, there is no such a comparison in the literature. However, both in vitro and in vivo studies suggested that increased serum level of glucose stimulates to the secretion of visfatin.Citation[12] Our study found no significant difference between the fasting blood glucose levels of peritoneal and hemodialysis groups. However, the increased glucose load of the dialysis fluid in peritoneal dialysis patients may lead to a significant increase in visfatin levels. Additionally, we have demonstrated a relationship between visfatin level and BMI. Similar to our study, there are several studies demonstrating a positive relationship between visfatin and BMI,Citation[13],Citation[14] suggesting that the increase in BMI may cause an increase in visfatin levels. Although no statistical significance was found in our study, the BMI of peritoneal dialysis patients was seen to be higher than that of hemodialysis patients. This difference might have contributed to the increased visfatin levels in peritoneal dialysis patients.
More recent studies have focused on the effects of visfatin on the glucose metabolism. Although such studies initially concluded that it may have positive effects on insulin sensitivity, they have yielded contradictory results.Citation[1],Citation[4],Citation[5] Additionally, a few studies have suggested that visfatin may have proinflammatory characteristics.Citation[3],Citation[4],Citation[6] There are two studies in the literature demonstrating a positive relationship between serum level of IL-6 and visfatin.Citation[3],Citation[6] Likewise, our study has also found an independent and significant positive relationship between visfatin and two of the important inflammatory cytokines, TNF-α and IL-6. These results might confirm that visfatin might be a proinflammatory adipocytokine.
IL-6 and TNF-α are known to be closely related to cardiovascular events and increased mortality in ESRD patients.Citation[8] A possible relationship with these cytokines may imply that visfatin has a role in the development of cardiovascular events. In addition, two recent studies have concluded that there may be a relationship between visfatin and endothelial dysfunction in patients with Type 2 diabetes mellitus and ESRD patients.Citation[4],Citation[7]
It is well known that inflammatory state and endothelial dysfunction in dialysis patients have negative effects on left ventricular structure and function. In particular, it has been claimed that these factors have a role of the development of LVH in dialysis patients.Citation[9],Citation[15] LVH is an important indicator of increased cardiovascular risk in ESRD patientsCitation[10]; therefore, it is very important to define the factors leading to development of LVH. Visfatin, which is suggested to be related to inflammation and endothelial dysfunction, may be responsible of the development of LVH due to these factors.Citation[3],Citation[4],Citation[6],Citation[7] Although our study found no relationship between visfatin and LVMI, we concluded that visfatin might have negative effects on left ventricular diastolic function parameters.
In conclusion, this study has demonstrated significantly higher levels of serum visfatin in dialysis patients when compared to healthy individuals. This increase was particularly higher in peritoneal dialysis patients than in the hemodialysis group. This may have been caused by the increased glucose load due to dialysis fluid in peritoneal dialysis patients. Our study has also demonstrated a significant positive relationship between visfatin and proinflammatory cytokines such as IL-6 or TNF-α. This implies that visfatin might be a proinflammatory adipocytokine. As for the effects on left ventricular structure and functions, visfatin might have negative effects on left ventricular diastolic function parameters, but have no effects on left ventricular mass index. Larger-scale studies are needed into this topic.
REFERENCES
- Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005; 307(5708)426–430
- Hug C, Lodish HF. Visfatin: A new adipokine. Science. 2005; 307(5708)366–367
- Oki K, Yamane K, Kamei N, Nojima H, Kohno N. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol (Oxf). 2007; 67(5)796–800
- Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism. 2007; 56(4)451–458
- Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with Type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006; 91(1)295–299
- Seo JA, Jang ES, Kim BG, et al. Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women. Diabetes Res Clin Pract. 2008; 79(1)108–111
- Axelsson J, Witasp A, Carrero JJ, et al. Circulating levels of visfatin/pre-B-cell colony-enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am J Kidney Dis. 2007; 49(2)237–244
- Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005; 67: 216–233
- Erten Y, Tulmac M, Derici U, Pasaoglu H, et al. An association between inflammatory state and left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2005; 27(5)581–589
- Cottone S, Nardi E, Mule G, et al. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol. 2007; 67(4)209–216
- de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. Journal of the American College of Cardiology. 1992; 20: 1251–1260
- Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006; 49(8)1909–1914
- Berndt J, Kloting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005; 54(10)2911–2916
- Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006; 91(4)1578–1581
- Pannier B, Guerin AP, Marchais SJ, Metivier F, Safar ME, London GM. Postischemic vasodilation, endothelial activation, and cardiovascular remodeling in end-stage renal disease. Kidney Int. 2000; 57(3)1091–1099
