Abstract
The relative safety of parenteral iron preparations is a controversial issue in the management of anemia in chronic kidney disease (CKD), as direct head-to-head comparative trials are lacking. In this study, patients of CKD were randomized to receive intravenous low molecular weight iron dextran (ID), sodium ferrigluconate complex (SFGC), and iron sucrose (IS) at doses and infusion rates recommended by the product manufacturer. One time test dose was used only for ID and SFGC. A total of 2,980 injections (n = 339) of i.v. iron was given, and 49 patients (14.45% per patient) and a total of 56 adverse events (1.88% per infusion) were noted. Odds ratios (OR) of serious adverse drug events (ADE; i.e., death, anaphylaxis, or suspected immuno-allergic events) per patient was not significant between ID vs. SFGC (3.566) and SFGC vs. IS (2.129), whereas that between ID vs. IS (7.594) was highly significant (p = 0.034). OR of serious ADE exposure was significantly higher in ID vs. SFGC (OR = 5.670, p = 0.0147) and ID vs. IS (OR = 7.799, p < 0.001). No significant difference was seen between the three groups in terms of non-serious ADEs. Drug discontinuation occurred significantly more often with ID. One patient who developed anaphylactoid reaction with SFGC and ID tolerated iron sucrose well.
INTRODUCTION
Parenteral iron plays a key role in the management of anemia of chronic kidney disease (CKD), as the use of erythropoiesis-stimulating agents and blood loss associated with hemodialysisCitation[1] is invariably associated with depletion of iron stores. The incidence of iron deficiency anemia is as high as 25–37.5%Citation[2] in hemodialysis patients, while CAPD patients fair marginally better. On average, a patient on maintenance hemodialysis loses as much as 1 to 1.5 g of elemental iron per year.Citation[2] During the correctional phase (i.e., the increase of hemoglobin from baseline to target levels), approximately 600 mg of iron will be incorporated into newly formed RBCs if hemoglobin rises by 4g/100mL within 2–3 months, and during the maintenance phase, 1–3 g/year may be required.Citation[2]
Both the NKF-DOQICitation[1] and the European Best Practice GuidelinesCitation[3] recommend the use of parenteral iron instead of oral iron for the treatment and prevention of iron deficiency anemia in CKD patients. Among the earliest parenteral iron formulations, iron dextran preparations were found to be effective in replenishing iron-stores but had a high incidence of life-threatening anaphylactic reactions.Citation[4] Low molecular weight (LMW) iron-dextran resulted in reduced incidence of fatal adverse drug events (ADEs) associated with anaphylaxis.Citation[5] Early adverse effects with parenteral iron can be due to toxic effects of free/labile iron as well as a hypersensitivity reaction,Citation[6] and there may be delayed toxic effects as well. The hypersensitivity reaction is probably due to antibodies to dextran molecules,Citation[4] which are large carbohydrate molecules of high antigenicity. Non-dextran formulations of parenteral iron, such as iron-sucrose and ferric-gluconate complex, have of late become available in clinical practice. Although the efficacy of these agents is comparable with conventional iron dextran preparation,Citation[7] controversy arises in the relative safety of these agents. Despite much retrospective analysis using data from large medical centers or from central agencies like the U.S. Federal Drug Agency pointing to the definite safety of these agents vis-à-vis iron dextran, no head-to-head comparative trial appears to be available to clarify this issue. Most data on comparative safety have relied on patients' self-report.Citation[4],Citation[5] The present study was therefore designed to recognize and compare various ADEs of three parenteral iron preparations: iron dextran/low molecular weight (ID), iron-sucrose (IS), and sodium ferric gluconate complex (SGFC) at the safe doses listed in the package insert.
MATERIALS AND METHODS
In this head-to–head, open label, prospective, randomized study, adult CKD patients who were on conservative management or on renal replacement therapy; followed up at the Nehru Hospital of PGIMER, Chandigarh, India, from January 2004 to May 2006; and were given i.v. iron therapy were enlisted. After providing written consent to be included in the study, patients were randomized into three groups on the basis of the type of parenteral iron preparation: group A, ID; group B, SFGC; and group C, IS. Oral iron use was discontinued during the study period. The study was approved by the Ethics Committee of our institute. No specific brand was used, and no help from any pharmaceutical company was sought.
The patients had overt or functional iron-deficiency anemia or were receiving parenteral iron preparations as maintenance therapy to prevent iron-deficiency anemia. Our study excluded patients with any of the following conditions:
iron overload, defined as serum ferritin > 800 ng/mL and/or TSAT > 50%;
known hypersensitivity to all three iron disaccharide-polysaccharide preparations;
non-iron deficiency anemia, like hemolytic anemia or macrocytic anemia;
history of asthma, eczema, or other atopic allergy;
decompensated liver disease or hepatitis;
acute or chronic infections;
associated inflammatory joint disease, like rheumatoid arthritis with evidence of active inflammation;
acute or chronic use of corticosteroids or immunosuppressives and/or anti-histaminics;
on hemodialysis with first time exposure to a new type of dialysis membrane; and
on hemodialysis requiring volume removal of >1 L/h for a 70 kg patient.
A detailed history of patients was taken regarding basic etiology of CKD disease; dialysis status; usage of EPO; drug history, including history of hypersensitivity to specific iron preparations or atopy and eczema, along with history of blood loss; drug prescriptions; and any history that would preclude iron use, such as liver disease. Baseline investigations included hemoglobin levels, total leucocyte count, serum transaminases, serum creatinine, iron studies, serum iron levels, total iron binding capacity (TIBC) TSAT, and serum ferritin.
Randomization into the three groups was done using computer-generated numbers. Patients with previous history of reaction to ID were randomized to either IS or SFGC, and the same principle was followed for patients with history of sensitization with SFGC or IS. Patients with prior history of imferon (still sold in India) or jectofer (iron sorbitol citrate ) sensitivity were also included in the study and randomized to receive any of the three parenteral irons.
The treatment protocol followed was as follows:
iron dextran: iron deficiency anemia (functional and overt), 100 mg of iron dextran twice a week for a total of 10 doses; maintenance therapy with EPO, 100 mg of iron dextran once a week for a total of 10 doses;
sodium ferric gluconate complex: iron deficiency anemia (functional and overt), 125 mg of SGFC once a week for a total of eight doses; maintenance therapy with EPO, 125 mg of SGFC once a week for a total of eight doses;
iron sucrose: iron deficiency anemia (functional and overt), 100 mg of iron sucrose twice a week for a total of 10 doses; maintenance therapy with EPO, 100 mg of iron sucrose once a week for a total of 10 doses.
The administration protocol followed was as follows:
iron dextran: 100 mg diluted in 100 mL of 0.9% NaCl to be given over 30 mins.
sodium ferric gluconate complex: 125 mg diluted in 100mL of 0.9% NaCl given over 60 mins.
iron sucrose: 100 mg diluted in 100 mL of 0.9% NaCl given over 15 mins.
A one-time test dose of 25 mg was given for iron dextran and SFGC only.
The primary outcome measure was the incidence of serious or non-serious ADE. We did not analyze the efficacy of the three agents on the hematological parameters. Adverse drug events were observed and recorded at time of test dose, prior to infusion, 5 mins into injection, and 5 and 20 minutes and 48 hours after parenteral infusion. Monitoring included symptoms and detailed general physical examination. No premedications like hydrocortisone, antihistaminics, or NSAIDs were given. Adverse effects were divided into serious ADEs and non-serious ADEs.
Serious ADEs were defined as those that required immediate admission and/or resuscitation. These included the following:
death;
anaphylactoid reaction, definedCitation[5] as wheeze, dyspnea, hypotension, urticaria, or angioedema, without any prior history of sensitization occurring immediately at start or within four hours of infusion;
myocardial infarction;
pruritus;
coma;
seizures;
arrhythmias;
respiratory depression;
tachycardia, unexplained by other cause;
bradycardia,unexplained by other cause;
anaphylactic reaction, definedCitation[5] as wheeze, dyspnea, hypotension, urticaria, or angioedema, with prior history of sensitization occurring immediately at start or during infusion until four hours of infusion;
hypotension, unexplained by other cause;
hypertension;
cyanosis;
cardiac arrest;
sepsis secondary to iron infusion; and
exacerbation of inflammatory joint disease.
Non-serious ADEs included any symptoms and signs other than the above. Patient with both a serious and non-serious ADE reporting was done of only the serious ADE.
Patients who developed a serious ADE were given appropriate resuscitative measure, and the specific iron preparation was withdrawn. Patients with minor ADEs were given symptomatic treatment but were withdrawn from the specific preparation if patients found these symptoms intolerable and given an option to shift to other iron preparations.
ADE rate was calculated by dividing the number of overall or specific ADE by total number of doses dispensed. Adjusted ADEs rates per 100 mg of iron and per patient for iron dextran, SFGC, and iron sucrose were calculated. The odds ratio (OR) of ADEs associated with iron dextran vs. ferric gluconate and iron dextran vs. iron sucrose was calculated using 2 × 2 tables. The level of statistical significance was calculated using the chi-square test, and two-tailed p values <0.05 was taken as significant. Demographic characteristics and biochemical parameters were analyzed by univariate and logistic regression analysis to find predictive variables for reactions with parenteral iron. Analysis was done using SPSS version 13.0.
RESULTS
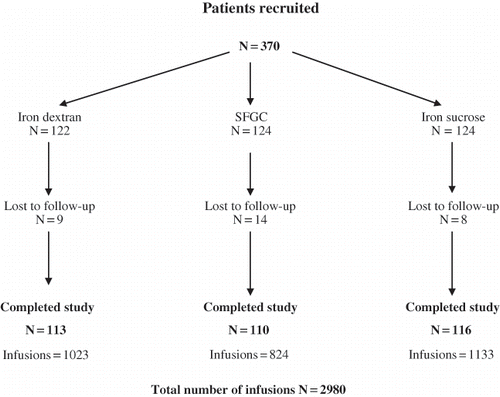
Three hundred and seventy CKD patients receiving i.v. iron from January 2005 to April 2006 were randomized into three groups based on the type of parenteral iron therapy, of whom 339 patients received i.v. iron as per protocol (see ) and were analyzed. Of the 31 patients not analyzed, four expired due to cause other than that attributed to iron related adverse events, and in 27 patients, no follow-up data were available. Clinical features and baseline characteristics of patients are depicted in . The main indication for starting i.v. iron was overt iron deficiency D (47%), functional iron deficiency (40%), and maintenance therapy (13%). Sixteen patients (4.72%) already had prior sensitivity to some form of parenteral iron before inclusion into the study, and three patients had sensitivity to both SFGC and iron dextran.
Table 1 Baseline characteristics of patients
Overall incidence of ADE with i.v. iron was 14.45 per 100 pts and 1.88 per 100 infusions (see ). In the ID group (n = 113), seven patients showed serious ADEs and 16 showed non-serious ADEs; in the SFGC group (n = 110), two showed serious ADEs while 15 showed non-serious ADEs; and in the IS group (n = 116), serious ADEs was seen in one patient while non-serious ADEs was seen in eight patients. Applying the chi-square test using the 2 × 2 contingency tables, the odds ratio (OR) of serious ADEs and non-serious ADEs of ID vs. SFGC, ID vs. IS, and IS vs. SFGC were calculated. Of these, only the OR of serious ADEs between IS and ID was significant. The number of discontinuations was significantly less in IS vs. the ID group, OR with 95% CI, was 5.765 (5.612–14.652), p = 0.003.
Table 2 Adverse drug effects noted in the three groups
The total number of adverse effects was analyzed as incidence rates per 100 patients, per 100 mg dose, and per 100 ampoules/exposure by the chi-square tests using the 2 × 2 contingency tables (see ). While a unit exposure was the same in the ID and IS groups as one single dose of 100 mg, in the case of SFGC, a single dose of 125 mg was equivalent to an exposure to two ampoules of 62.5 mg. Serious ADEs rates per exposure were significantly higher in the ID versus IS group and in the ID vs. SFGC groups, whereas rates per patients were significantly high in ID vs. IS only. The incidence rates in the non-serious ADEs were not significantly different in any of the three groups. Serious ADE was significantly higher in the dextran vs. non-dextran groups (OR = 4.908, 95% CI, 2.138–5.353, p = 0.018), whereas non-serious ADEs were not significant (OR = 1.455, 95% CI, 0.671–8.891, NS). The total number of discontinuations was higher in the dextran vs. non-dextran groups, which was highly significant (OR = 2.991, 95% CI, 1.672–3.128, p = 0.009). In the analysis of potential risk factors for developing ADE with parenteral iron (see and ), serious and non-serious ADEs of all the three groups were combined to increase the predictive power of the studied variables. Univariate analysis showed previous drug reaction, previous reaction to i.v. iron, and use of dextran preparation were significantly associated with serious ADEs while adverse effects were seen with iron dextran (see ).
Table 3 Prediction of adverse effects with parenteral iron
Table 4 Spectrum of adverse events with various parenteral iron preparations
Table 5 Univariate analysis of factors affecting adverse drug events
Serious ADE with ID included anaphylactoid reaction (n = 3), pruritus (n = 2), anaphylaxis (n = 1), and hypotension (n = 1). Prior history of drug sensitivity was present in four of these patients, which included sensitivity to imferon (n = 2) and other drugs (n = 2). Anaphylactic reaction in the form of urticaria and wheeze was seen in patient 11 within 5 mins of administering the test dose of 25 mg. This was considered to be a true allergic reaction, as the patient had a past history of sensitivity to imferon. Non-serious ADEs due to ID mostly occurred in the first dose itself and were mild in intensity. In patient 109, a case of diabetic nephropathy without any pre-existing history of autonomic neuropathy experienced nausea, vomiting, and one episode of melena 24 hours after his first dose of ID infusion, which resolved on expectant management for two days. There was no history of acid peptic disease or portal hypertension in the patient, and upper g.i. endoscopy had not shown any abnormality. Although further investigations to assess the cause of this melena were planned while the patient was on iron dextran, he refused any further injections of ID.
Adverse Effects Seen with Sodium Ferrigluconate Complex
Among the patients with serious ADEs, patient 219 developed generalized pruritus after 30 mins of starting the first infusion of SFGC (see ), which improved after stopping the infusion pump and administering inj. chlorpheniramine and hydrocortisone. There was a significant past history of fixed drug eruption to some traditional medicine. Patient 183 had postural dizziness due to hypotension 15 mins after completion of the first infusion of SFGC. This patient also had a history of pruritus to amoxicillin. None of these patients had shown any reaction to the test dose. Most of the non-serious ADEs were mild in nature and occurred in the first dose itself. A majority of the non-serious effects were related to the gastrointestinal system, although upper respiratory tract symptoms, thrombophlebitis, and joint complaints were also noted. Patient 167, an old case of rheumatoid arthritis, also had nonspecific pain in bilateral ankle and knee joint after the eighth dose, which resolved with paracetamol.
Adverse Effects Seen with Iron Sucrose
No anaphylaxis or anaphylactoid reaction was noted with IS, although hypotension presumably due to IS was the only serious ADE observed in one patient (patient 289) in whom infusion was given during dialysis, and we could not ascribe any other obvious dialysis related cause for the same. Blood pressure improved on stopping IS. This patient had a previous history of anaphylactoid reaction with ID. Increasing the infusion time of IS to 30 mins in subsequent dialysis sittings did not produce hypotension. However, as per our protocol, subsequent recording of infusions were not included in the final analysis. Non-serious ADEs with IS were seen in eight patients, with a majority of symptoms pertaining to the gastrointestinal system (see ) occurring in the initial infusions and were mild. However, in patient 225, nausea, vomiting, and muscle cramps were severe and appeared 24 hours after infusion to the extent that patient became oliguric; he was admitted in the Emergency Room, where he received anti-emetics along with Frusemide challenge, after which urine output improved. This patient had a past history of reaction with both ID and SFGC. Subsequent i.v. iron was not given.
Adverse Drug Effect Profile in Patients with Prior History of Reactivity to Different Forms of Parenteral Iron
Patients who experienced some form of ADEs with parenteral iron prior to recruitment in the study tended to show ADEs in the majority of cases. Among patients with a prior history of imferon sensitivity (n = 9), three showed similar features with ID. However none of the imferon-sensitive patients developed any reaction with IS. Of the seven patients with prior history of sensitization to ID, two receiving SFGC experienced the same gastrointestinal side effects as with ID in the first infusion itself, while patient 136, who had already received a total dose infusion of 1.0 g of ID two months previously, developed muscle cramps after the seventh dose of SFGC, which was mild and did not persist in subsequent doses. Among ID-sensitive patients allotted to IS, patient 289, who had previously developed an anaphylactoid reaction with 100 mg of ID, showed an episode of intradialytic hypotension for which no dialysis-related cause was apparent. The other three ID-sensitive patients tolerated IS well. Amongst patients with prior sensitivity to SFGC randomized to the ID group, patient 112 with a prior history of pruritus with SFGC and fixed drug eruption with traditional medicines developed frank features of anaphylactoid reaction after first dose of i.v. ID. In the double sensitive (SFGC and ID) patients 225 and 332, vomiting, diarrhea, and melena occurred after 24 hours of the first dose of IS as with SFGC and ID. However, patient 339 who had a history of anaphylactoid reaction with SFGC and ID in the very first dose did not show any reaction with IS.
DISCUSSION
Our study is probably the first open label randomized prospective trial testing the relative safety of three commonly used parenteral iron preparations in CKD patients. As with all prospective studies,Citation[8] the present study also suffers from a lack of sufficient predictive power due to small sample size, but gives useful information on the pattern of adverse events and patient characteristics. Another limitation of our study is that it is an open labeled trial, which, however, is ethical because it is necessary to avoid repeated exposure to a specific parenteral iron formulation to which the patient had been sensitized previously. We included patients who had previously received imferon, a low molecular weight iron dextran preparation (banned by the FDA in the United States but available in India), as no data are available on the cross-sensitivity between imferon and newer i.v. iron preparations.
Studies on comparative safety of parenteral iron suffer from a number of biases.Citation[7] Newly introduced drugs will show a higher incidence of ADE because they receive more scrutiny and report activity within a few years following their introduction. Hence, iron sucrose, which has been only recently introduced into the Indian Market in the last two years, will have a slightly greater incidence of adverse effects, and iron dextran slightly less incidence. Other biases include protopathic bias (i.e., premonitory symptoms that evolve over days into a serious diagnosable event) and publicity bias among the patient and the treating physician. Another reason for variability is the absence of standardized definitions of serious and non-serious adverse effectsCitation[8] in literature . We have divided ADE into two groups wherein suspected immune-allergic and anaphylactoid events were grouped as serious ADEs and non-allergic or free iron-related ADEs were categorized as non-serious ADEs. Standard definitions of anaphylaxis was used,Citation[11] and although the distinction of anaphylaxis from anaphylactoid reaction is impossible clinically, we have used the definitions of HamstraCitation[6] for anaphylactoid reaction. In our study, hypotension has been placed in the serious ADEs group to allow heightened surveillance, as it can have a toxic effect as well as an isolated manifestation of anaphylaxis.Citation[10] Pruritus, which is an allergic event to i.v. iron,Citation[12] can also be an isolated feature of anaphylactoid/anaphylaxis reactionsCitation[11] and hence was considered as a serious ADE.
We found significantly higher incidence of total ADE per patient and exposure in the ID compared to IS group, but no difference between IS and SFGC. Although the sample size is less for definitive conclusion, similar results were noted by Chertow et al.Citation[7] in a retrospective analysis of a large cohort of patients receiving all three preparations. Serious drug events per exposure in this study was significantly higher in ID compared to SFGC or IS or between dextran vs. non-dextran but was not different between IS and SFGC. Interestingly, we found no anaphylactoid reactions with SFGC and IS. Our study suggests that serious ADEs occur mostly with iron dextran followed by SFGC and iron sucrose. Bailie et al.Citation[13] had previously shown that the incidence of type I adverse effects (anaphylaxis, anaphylactoid reaction, angioedema, and urticaria) were in the order of ID > SFGC > IS.
Contrary to our study, Chertow et al.Citation[5] showed that risk of non-life-threatening ADEs was 4–14 times more in the SFGC group than in the LMW iron dextran group. However, many of the so-called non-life-threatening events in this study included urticaria, pruritus, and facial edema, many of which have an underlying allergic basis and hence should have been included in the serious ADEs group. Michael et al.Citation[9] found the incidence of drug intolerance (due to both life-threatening and non-serious ADEs ) to be significantly less with SFGC as compared to ID (0.44% vs. 2.47%, p < 0.0001 ). In a recent update of their comparative data, Chertow et al.Citation[7] have, however, shown that the incidence of SFGC-related life-threatening and non-serious ADEs have decreased with respect to iron dextran in the recent years, attributing the initial high incidence to a “new agent vigilance” bias and the progressive under-reporting of non life- threatening ADEs, given their relative mild nature.
The incidence rates of all adverse effects with iron dextran in the present study is higher than previous studies,Citation[5–7] a majority of which were retrospective, and serious ADEs in these studies referred to life-threatening anaphylactoid reactions. Another reason could be the absence of premedication in our study, as Barton et al.Citation[14] demonstrated no serious ADEs even with total dose dextran infusion in premedicated patients. Our incidence rates of anaphylactic reaction with ID are similar to those of Auerbach et al.,Citation[15] who mention an incidence of 3.5% for anaphylactoid reactions. One of the two patients with anaphylactoid reaction and the sole patient with anaphylaxis had shown sensitivity to imferon in the past, thereby suggesting a high cross-reactivity between dextran compounds of different molecular weight. Non-serious ADEs rates with ID were 15.92% per patient and 1.76% per exposure, which is similar to the studies of McCarthy et al.Citation[16] (12.2% per patient) and BartonCitation[14] (13% per patient). We have included patients developing respiratory tract infection in view of the suspected toxic effects of parenteral iron on the host-defense mechanisms.Citation[17],Citation[18]
The incidence of serious ADEs with SFGC in the present study are lower than 3.4%, as noted in previous studies,Citation[20],Citation[21] but higher than that of Micheal et al.Citation[9] (0.6%), who interestingly observed the same ADE rate as in the placebo group. It is possible that the etiology of ADE with SFGC is toxic rather than immuno-allergic, as a compilation of all safety profile studies on SFGCCitation[8] shows a proportionate increase in serious ADEs with increasing dose of infusion. The incidence rates of non-serious ADEs with SFGC compares well with Nissenson's rateCitation[21] of 10.2% per patient, whereas Michael et al.Citation[9] report an incidence of 3.4% in single exposure and 0.6% on repeated exposure.Citation[22]
Iron sucrose may be the safest parenteral iron, as no serious ADEs have been documented in previous prospective trialsCitation[23–26] and an FDA databaseCitation[7] gives an incidence of life-threatening ADEs as 0.6 per million doses. In our analysis of the safety profile of the IS group, we observed hypotension as a possible serious ADE that decreased on reducing the rate of sucrose infusion, although it could still be due to intra-dialytic factors. The incidence of non-serious ADEs with IS in the present study was 10.34% per patient, which is similar to Wyck et al.,Citation[26] who observe rates of 9% when iron sucrose was given as a 100 mg push over 5 mins. Using lower infusion rates of 4.2 mg/min to give 250 mg of iron sucrose, Kosch et al.Citation[24] did not observe any serious or non-serious ADEs. It is possible that many of the so-called non-serious ADEs may well represent intradialytic events, which may at times be very difficult to segregate from true drug events and therefore need observer discretion for reporting.
In the present study, dextran-intolerant patients who received SFGC showed non-serious ADEs, but none showed any serious allergic symptoms. Coyne et al.Citation[10] noted a seven times greater risk of intolerance to SFGC in dextran-sensitive patients, but the risk ratio was comparable with that of the placebo and did not appeared to be immune-mediated. The authors therefore proposed that these reactions may be a pseudo-allergic response. As previously demonstrated,Citation[29] double sensitive patients (ID and SFGC) did not show any anaphylactoid reaction with iron sucrose. Gastrointestinal ADE of parenteral iron can occur with all three forms, as these are free iron mediated,Citation[25] and free iron transiently increased during infusion time.Citation[28]
Given the rarity of true allergic events, the utility of a test dose especially for non-dextran molecules like the SFGC is debatable.Citation[3],Citation[30] In the present study, a one-time test dose was given to patients receiving ID or SFGC as per the latest EBPG guidelines.Citation[3] In the present study, a history of prior drug allergy, iron dextran use, and prior reaction to i.v. iron predicted the risk for serious ADE, while previous reaction to i.v. iron predicted non-serious ADE. Previously Hamstra et al.Citation[6] showed that female sex, pregnancy, dose of iron given, and history of connective tissue disease were significantly associated with higher risk of reaction to iron dextran. Fishbane et al.,Citation[19] however, found only a positive history of drug allergy (OR = 2.4, p = 0.03) to be a significant predictor of drug adverse events. As did previous studies,Citation[9] we did not find any added risk of ADEs in patients on ACE inhibitors. Rolla et al.,Citation[31] however, showed a statistically higher incidence of hypotension and anaphylactoid reaction in patients on ACE inhibitors, which was postulated to be due to higher bradykinin levels in patients on ACE inhibitors.
To conclude, this is the first prospective randomized study analyzing the relative safety of three commonly available parenteral iron preparation. Despite a small sample size and the observer bias inherent in an open label study, it was observed that the total number of adverse effects, especially serious ADEs, is significantly less in the non-dextran formulations like SFGC and iron sucrose. Iron sucrose as of now may be considered to be the safest form of parenteral iron.
REFERENCES
- NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: Update 2000. Am J Kidney Dis. 2001; 37(Suppl. 1)S182–S238
- Nissenson AR, Strobos J. Iron deficiency in patients with renal failure. Kidney Int. 1999; 69(Suppl. 1)S18–S21
- Locatell F, Aljama P, Barany P, et al. Revised European best practice guidelines for management of anemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19(Suppl. 2)i22–i25
- Fletes R, Lazarus JM, Gage J, Chertow GM. Suspected iron dextran-related adverse drug events in hemodialysis patients. Am J Kidney Dis. 2001; 37: 743–749
- Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004; 19: 1571–1575
- Hamstra RD, Block MH, Schocket AL. Intravenous iron dextran in clinical medicine. JAMA. 1980; 243: 1726–1731
- Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006; 21: 378–382
- Arnoff GR. Safety of intravenous iron in clinical practice: Implications for anemia management protocols. J Am Soc Nephrol. 2004; 15: 99–106
- Michael B, Coyne DW, Fishbane S, et al. Sodium ferric gluconate complex in hemodialysis patients: Adverse reactions compared to placebo and iron dextran. Kidney Int. 2002; 61: 1830–1839
- Coyne WD, Adkinson F, Jr, Nissenson Allen R, et al. SFGC in hemodialysis patients, II: Adverse reactions in iron-dextran sensitive and dextran tolerant patients. Kidney Int. 2003; 63: 217–224
- Sampson HA, Munoz-Furlong A, Bock SA, et al. Symposium on the definition and management of anaphylaxis: Summary report. J Allergy Clin Immunol. 2005; 115: 584–591
- Novey HS, Pahl M, Haydik I, et al. Immunologic studies of anaphylaxis to iron dextran in patients on renal dialysis. Ann Allergy. 1994; 72: 224–228
- Bailie GR, Clark JA. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005; 20: 1443–1449
- Barton JC, Barton EH, Bertoli LF, Gothard CH, Sherrer JS. Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med. 2000; 109: 27–32
- Auerbach M, Witt D, Toler W, et al. Clinical use of total dose intravenous iron dextran infusion. J Lab Clin Med. 1988; 111: 566–570
- McCarthy JT, Regnier CE, Loebertmann CL, Bergstralh EJ. Adverse events in chronic hemodialysis patients receiving intravenous iron dextran—a comparison of two products. Am J Nephrol. 2000; 20: 455–462
- Burns DL, Pomposelli JJ. Toxicity of parenteral iron dextran therapy. Kidney Int. 1999; 55(Suppl. 69)S119–S124
- Kletzmayr J, Ferrara I. Impairment of transendothelial leukocyte migration by iron complexes. J Am Soc Nephrol. 2002; 14: 2639–2644
- Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996; 28: 529–534
- Panesar A, Agarwal R. Safety and efficacy of sodium ferric gluconate complex in patients with chronic kidney disease. Am J Kidney Dis. 2002; 40: 924–931
- Nissenson AR, Lindsay RM, Swan S, Seligman P, Strobos J. Sodium ferric gluconate complex in sucrose is safe and effective in hemodialysis patients: North American clinical trial. Am J Kidney Dis. 1999; 33: 471–482
- Michael B, Coyne DW, Folkert VW, Daahl NV, Warnock DG. Ferrlecit Publication Committee. Sodium ferric gluconate complex in hemodialysis patients: A prospective evaluation of long-term safety. Nephrol Dial Transplant. 2004; 19: 1576–1580
- Charytan C, Levin N, Al-Saloum M, et al. Efficacy and safety of iron sucrose in iron deficiency in patients of dialysis-associated anemia: North America Clinical Trial. Am J Kidney Dis. 2001; 37(2)300–307
- Kosch M, Bahner U, Bettger H, Matzkies F, Teschner M, Schaefer RM. A randomized, controlled parallel-group trial on efficacy and safety of iron sucrose (Venofer) vs. iron gluconate (Ferrlecit) in hemodialysis patients treated with rHuEpo. Nephrol Dial Transplant. 2001; 16: 1239–1244
- Geisser P, Baer M, Schaub E. Structure/histotoxicity relationship of parenteral iron. Arzneimittel-forschung. 1992; 2(12)1439–1452
- Van Wyck DB, Cavallo G, Spinowitz BS, et al. Safety and efficacy of iron sucrose in patients sensitive to iron dextran: North American clinical trial. Am J Kidney Dis. 2000; 36: 88–97
- Aronoff GR, Bennett WM, Blumenthal S, et al. Iron sucrose in hemodialysis patients: Safety of replacement and maintenance regimens. Kidney Int. 2004; 66: 1193–1198
- Van Wyck DB, Danielson BG, Aronoff GR. Making sense: A scientific approach to intravenous iron therapy. J Am Soc Nephrol. 2004; 15: 91–92
- Charytan C. Safety of iron sucrose in hemodialysis patients intolerant to other parenteral iron products. Nephron Clin Pract. 2004; 96(2)63–66
- Fishbane S, Kowaliski E. The comparative safety of intravenous iron dextran, sacchharate, and sodium ferric gluconate. Semin Dialysis. 2000; 13(6)381–384
- Rolla G, Bucca C, Brussino L. Systemic reactions to intravenous iron therapy in patients receiving ACE inhibitors. J Allergy Clin Immun. 1994; 93: 1074–1075