Abstract
Rationale. Few studies have evaluated the epidemiology of acute kidney injury (AKI) in trauma. Objective. To evaluate the incidence, risk factors, and outcomes associated with early AKI (evident within 24 hours of admission) in critically ill trauma patients. Methods. A retrospective interrogation of prospectively collected data from the Australian New Zealand Intensive Care Society Adult Patient Database. A total of 9,449 trauma patients were admitted for ≥24 hours to 57 intensive care units across Australia from January 1st, 2000, to December 31st, 2005. Main Findings. The crude incidence of AKI was 18.1% (n = 1,711). Older age, female sex (OR 1.60, 95% CI, 1.43–1.78, p < 0.0001), and the presence of co-morbid illness (OR 2.70, 95% CI 2.3–3.2, p < 0.0001) were associated with higher odds of AKI. Those with trauma not associated with brain injury (OR 2.40, 95% CI, 2.1–2.7, p < 0.0001) and a higher illness severity (OR 1.12, 95% CI, 1.11–1.12, p < 0.001) also had higher likelihood of AKI. Overall, AKI was associated with a higher crude mortality (16.7% vs. 7.8%, OR 2.36, 95% CI, 2.0–2.7, p < 0.001). Each RIFLE category of AKI was independently associated with hospital mortality in multi-variable analysis (risk: OR 1.69; injury OR 1.88; failure 2.29). Conclusions. Trauma admissions to ICU are frequently complicated by early AKI. Those at high risk for AKI appear to be older, female, with co-morbid illnesses, and present with greater illness severity. Early AKI in trauma is also independently associated with higher mortality. These data indicate a higher burden of AKI than previously described.
INTRODUCTION
Acute kidney injury (AKI), defined as an abrupt reduction in kidney function, is a common clinical problem in critically ill patients and independently predicts poor outcome.Citation[1–4] Observational data indicate the incidence of AKI is rising.Citation[5] Two large multi-center cohort studies recently reported the occurrence of AKI in an estimated 36% of ICU patients.Citation[6],Citation[7] This large and increasing burden of AKI has been attributed in part to shifts in patient demographics (older, greater co-morbid illness), severity of illness (multi-organ dysfunction syndrome) and AKI associated with complex interventions (organ transplantation).Citation[8] Recently, the RIFLE classification (acronym for Risk, Injury, Failure, Loss, End-stage), a novel consensus definition for AKI, has been published.Citation[9] The RIFLE classification defines three grades of severity of AKI (i.e., Risk, Injury, and Failure) based on composite changes to serum creatinine and urine output. The RIFLE classification has now been evaluated in numerous clinical studies enrolling critically ill patients and found to be a valid and robust tool for the diagnosis and staging of AKI, along with having predictive ability for mortality.Citation[6],Citation[7],Citation[10–13]
Injury related to major trauma represents a leading cause of preventable morbidity and mortality.Citation[14–20] Major trauma comprises approximately 10% of all admissions to ICU.Citation[6] To date, only a few studies have described the epidemiology of AKI attributable to trauma.Citation[21–27] Morris et al. performed a retrospective study of 72,757 admissions to nine regional trauma centers over a five-year period and found only 78 patients (0.1%) developed AKI requiring renal replacement therapy (RRT).Citation[25] While these data might suggest AKI in trauma is rare, it did not focus on trauma requiring ICU admission. More recently, Brandt et al. conducted a single-center retrospective study of 1,033 ICU admissions for trauma and found AKI occurred in 23.8%.Citation[21] Of these, 10% required initiation of RRT. Moreover, this study found that AKI in trauma was associated with a longer duration of mechanical ventilation and stay in ICU.Citation[21] Emerging data suggest that the burden of AKI in trauma may be increasing; however, most studies to date are limited by small sample size,Citation[23],Citation[24],Citation[26] single-center design,Citation[21],Citation[26],Citation[28] focus on selected trauma populations,Citation[22] or use of variable definitions for AKI,Citation[21],Citation[25],Citation[27] or are potentially confounded by including late or delayed-onset of AKI that may not be attributable to trauma.Citation[21],Citation[25],Citation[27]
An evaluation of the epidemiology of AKI in trauma is important for health resource allocation, identification of high risk populations, development of preventative strategies, and for surveillance of long-term clinical consequences. Accordingly, we interrogated the Australian and New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) to obtain information on the epidemiology of early AKI, defined as AKI evident within 24 hours of ICU admission, for all ICU admissions related to trauma from 57 Australian ICUs over a five-year period. Our primary objectives were to describe the incidence and clinical characteristics of patients with early AKI in trauma, the severity of early AKI stratified by the RIFLE criteria, and the association of early AKI on clinical outcomes and prognosis.
METHODS
This was a retrospective analysis of prospectively collected data. We interrogated the ANZICS APD for all adult (age ≥18 years) ICU admissions for a duration ≥24 hours associated with trauma from January 1st, 2000, to December 31st, 2005. In the event of multiple admissions, only the initial ICU admission was considered to avoid bias. Those patients re-admitted within 72 hours after initial discharge were considered as part of the initial index admission. The ANZICS APD is a high quality clinical database containing data from >600,000 individual adult admissions to 135 intensive care units (ICUs) from 1987 to the present.Citation[29] We selected only those ICUs that had continuously contributed data to the APD during this five-year period. This cohort included 57 ICUs (19 tertiary referral, 15 metropolitan, 12 regional/rural, and 11 private hospitals).
Identification of Cases
Trauma cases were identified by those admitted to ICU with a primary diagnosis of trauma. The trauma diagnosis was further stratified into two categories: trauma associated with brain injury (TBI) and trauma not associated with TBI.
Acute Kidney Injury
The presence of AKI was assessed for within the first 24 hours after ICU admission only. Therefore, we have referred to this as “early” AKI and classified it according to the RIFLE criteria.Citation[9] The RIFLE criteria (acronym indicating Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease) classifies AKI into three categories of severity (i.e., Risk, Injury, and Failure) and two categories of clinical outcome (i.e., Loss and End-stage kidney disease), and can be easily determined at the bedside (see ).Citation[9] For this study, the outcome RIFLE categories Loss and End-stage kidney disease have not been evaluated. Urine output was described in 94.8% of trauma patients; however, the ANZICS APD only captures the initial cumulative 24 hour output and does not record patient weights.Citation[6] Thus, we used a minor modification of the RIFLE urine output criteria, assuming an average patient weight of 70 kg and dividing patients into <35mL/hr (Risk), <21 mL/hr (Injury) or <4mL/hr (Failure). This modification has been performed previously and shown to be associated with clinical outcome.Citation[6] Baseline serum creatinine values were not available for the study cohort and were estimated by the Modification of Diet in Renal Disease (MDRD) equation, as recommended by the Acute Dialysis Quality Initiative (ADQI) Working Group (assuming a lower limit of normal baseline glomerular filtration rate [GFR] of 75 mL/min), in order to calculate each patient's worst RIFLE category. This has been performed similarly in previous studies.Citation[6],Citation[7],Citation[10],Citation[11],Citation[13] For analysis, patients were assigned to their worst RIFLE category according to either serum creatinine or urine output criteria. Those with end-stage kidney disease requiring chronic dialysis were excluded.
Table 1 The RIFLE definition and classification of AKI
Data Collection
Standard demographic, clinical, and physiologic data were retrieved. Demographic information included age, sex, and dates and source of admission. Clinical data encompassed trauma diagnosis, surgical status, presence of co-morbidities, and need for mechanical ventilation. Physiologic data included Glasgow Coma Scale (GCS), vital signs, PaO2/FiO2 ratio, serum pH, serum sodium, potassium, bilirubin, hematocrit, and white cell count. Data on kidney function included serum creatinine, urea, and urine output.Citation[29] Severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II, and APACHE III.Citation[30] Pre-existing co-morbidities were defined by use of the chronic health evaluation for APACHE II and APACHE III systems as outlined in the ANZICS APD data dictionary.Citation[29] The definitions for pre-existing co-morbidities categories are shown in the appendix.
Statistical Analysis
Analysis was performed using Intercooled Stata (Stata Corp, College Station, Texas, USA). In the event of missing data values, data were not replaced. Normally or near normally distributed variables are reported as means with standard deviations (SD) and compared by Student's t-test, analysis of variance, or simple linear regression. Non-normally distributed continuous data are reported as medians with inter-quartile ranges (IQR) and compared by Mann Whitney U test or Kruskal-Wallis test. Categorical data were reported as proportions and compared using Fisher's Exact Test. Incidence estimates for early AKI on admission to ICU were calculated as a proportion of all admissions to ICU with 95% confidence intervals (CI). Incidence estimates are presented as cumulative over five years. The estimated annual percent change in the incidence of AKI was assessed by fitting a straight line regression of the natural logarithm of the rates with calendar year used as an independent variable. The estimated annual percent changes was equal to [100 × (exp(b)-1)], where “b” represents the slope of the regression. If the estimated annual percent change is statistically greater than zero, then the incidence rate has an increasing trend over the study period.Citation[5] Multivariable logistic regression analysis was used to assess the association of each RIFLE category with hospital mortality. A priori selected variables included age, sex, co-morbidity, trauma diagnosis, need for mechanical ventilation, APACHE score, hospital site, and AKI. Model fit was assessed by the goodness-of-fit test, and discrimination was assessed by the area under the receiver operator characteristic (AuROC) curve. Data are presented as odds ratios (OR) with 95% confidence intervals (CI). A p value of <0.05 was considered statistically significant for all comparisons.
RESULTS
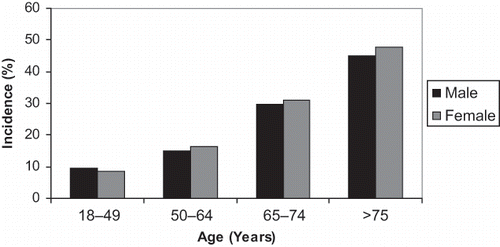
During the five-year study period, 124,088 patients were admitted to the 57 ICUs (see ). A total of 9,449 patients (7.95%) were admitted with a primary diagnosis of trauma. The majority of patients with a trauma diagnosis were admitted to tertiary (n = 6518, 69%), metropolitan (n = 1597, 16.9%), or regional (n = 935, 9.9%) hospitals, while 399 trauma patients (4.2%) were admitted to private hospitals during the study period. There was no significant change in the annual incidence rates of trauma over the study period (p = 0.94). Trauma patients were younger, more likely to be male, had a lower burden of co-morbid illness, and had lower overall severity of illness scores (see and ). Overall, trauma patients were more likely to have lower GCS scores and require mechanical ventilation. An estimated 42.2% of trauma cases were associated with TBI.
Table 2 Patient demographics at intensive care unit admission stratified by primary diagnosis of trauma
Figure 1. Age- and sex-stratified incidence of major trauma admitted to ICU from the ANZICS APD, 2000–2005.
Incidence of Early AKI
In the total cohort, AKI occurred in 36.1% within 24 hours of ICU admission, with a maximum RIFLE category Risk in 16.3%, Injury in 13.6%, and Failure in 6.3%. The crude incidence of early AKI in trauma was 18.1% (n = 1,711; see ). The incidence of early AKI was significantly lower in trauma compared with non-trauma admissions (18.1% for trauma vs. 37.7% non-trauma, odds ratio [OR] 0.37, 95% CI, 0.35–0.39, p < 0.0001). There was no significant change in the annual incidence of early AKI in trauma over the study period (p = 0.65). Older patient age and female sex (23.2% for female vs. 15.9% for male, OR 1.60, 95% CI, 1.43–1.78, p < 0.0001) were associated with a higher odds of AKI (see ). Similarly, the presence of co-morbid illness increased the odds of AKI (35.1% with co-morbidities vs. 16.7% with no co-morbidities, OR 2.70, 95% CI 2.3–3.2, p < 0.0001). Non-TBI-related trauma increased the odds of early AKI compared to those with TBI-related trauma (23.1% for non-TBI vs. 11.3% for TBI, OR 2.40, 95% CI 2.1–2.7, p < 0.0001). A higher severity of illness (per point increase in APACHE III score) within the first 24 hours was also associated with an incremental increase in the odds of early AKI (OR 1.03, 95% CI, 1.02–1.03, p < 0.001).
Table 3 Incidence of AKI stratified by RIFLE criteria for intensive care unit patients with an admission diagnosis of trauma
Hospital Mortality, Length of Stay, and Discharge Location
For trauma patients, early AKI was associated with a significantly higher crude mortality (16.7% for AKI vs. 7.8% for no AKI, OR 2.4, 95% CI, 2.0–2.7, p < 0.001). The presence of early AKI was also associated with increased odds of death after adjustment for co-variates (OR 1.8, 95% CI, 1.5–2.2, p < 0.001, AuROC 0.85, goodness-of-fit, p = 0.14; see ). Each RIFLE category was associated with elevated crude and adjusted odds ratios for death (see ). Early AKI in trauma was associated with a significantly longer duration of stay in hospital (see ). Similarly, early AKI was associated with a lower likelihood of discharge home from hospital and greater likelihood of transfer to another hospital or rehabilitation facility.
Table 4 Clinical outcomes in intensive care unit patients admitted with trauma stratified by RIFLE category
Table 5 Summary of crude and co-variate adjusted odds ratios for hospital mortality for trauma admissions to the intensive care unit
DISCUSSION
We conducted a five-year multi-center analysis of more than 9,000 ICU admissions for trauma to evaluate the incidence, risk factors, and outcome associated with early AKI (AKI occurring within 24 hours of ICU admission). We found that early AKI occurred in 18.1% of ICU patients admitted for trauma. We also found that these patients were more likely to be older and female, and have pre-existing co-morbid illness. Moreover, early AKI was more likely in non-TBI-related trauma and associated with a higher illness severity at the time of admission. Importantly, we found that early AKI in trauma was associated with both crude and co-variate-adjusted increases in hospital mortality. Similarly, for those with trauma, a greater severity of AKI, when defined by the RIFLE criteria, was associated with incremental increases in hospital mortality. Finally, we found that AKI was associated with a longer duration of hospitalization for survivors and a reduced likelihood of discharge home.
Our study has shown the incidence of early AKI in trauma to be higher than generally reported in prior investigations.Citation[25],Citation[27],Citation[28],Citation[31] There are a few plausible explanations for our findings. First, the occurrence of AKI in our cohort may in part reflect a higher overall severity of illness. These patients were all critically ill and supported in an ICU environment. Second, AKI in our cohort was defined by the RIFLE criteria. The RIFLE criteria incorporate both serum creatinine and urine output for defining AKI. These criteria have now been evaluated in more than 200,000 patients and found to be simple, robust, and valid as a tool for the diagnosis and classification of AKI.Citation[6],Citation[7],Citation[12],Citation[13] However, the RIFLE criteria are also a more sensitive indicator of AKI.Citation[12] Thus, the higher incidence of early AKI in our study may, in part, reflect the broader capture of previously undiagnosed AKI in this population. The true burden of AKI in prior studies was likely underestimated due to defining AKI by arbitrary biochemical thresholds or by the need for initiation of RRT only.Citation[25],Citation[27],Citation[28],Citation[31] For example, in a multi-center study from Finland, Ala-Kokko et al. described evidence of “renal dysfunction” in 10.8% and “renal failure” in 3.5% within 24 hours of ICU in a cohort of 1,044 patients with trauma.Citation[27] However, renal dysfunction and failure in this study were classified by SOFA score. Brandt et al. found that “renal failure” occurred in 23.8% of patients when defined by an absolute serum creatinine >133μmol/L or a relative changes of >50% or >44 μmol/L.Citation[21] While similar to our incidence estimate, this study was performed at a single level I trauma center and thus potentially prone to selection bias. Recently, the RIFLE criteria were applied in a single-center study of 304 critically ill burn patients (>10% total-body surface area).Citation[22] In this study, AKI occurred in 26.6% and was associated with longer duration of mechanical ventilation, longer stay in hospital, and higher mortality.Citation[25] Our findings suggest that the burden of early AKI in trauma may be greater than previously appreciated, and that this may have important health resource implications. Similarly, another interesting finding from our data was that an estimated 11.3% of TBI patients had evidence of early AKI. At present, there is a paucity of data on the incidence of AKI in TBI.Citation[32],Citation[33] However, AKI alone and certainly the need to provide RRT for AKI in those with TBI can be problematic due to risk for the potential aggravation of cerebral edema.Citation[34],Citation[35]
Several studies have attempted to characterize risk factors associated with the development of AKI in trauma. Similar to our findings, older ageCitation[23],Citation[28] and sexCitation[28] have been shown to be associated with increased risk of AKI in trauma. Pre-existing co-morbid illness and severity of injury have both consistently been shown to be associated with higher risk of AKI in major trauma.Citation[23],Citation[26],Citation[28] While our study was not able to capture data on specific solid organ injuries, direct injury to the kidney has been associated with an increased risk for both AKI and need for nephrectomy.Citation[28],Citation[36] Finally, complex abdominal and/or pelvic injury, concomitant sepsis, and multiple organ dysfunction have also shown to be important risk factors for AKI.Citation[23],Citation[26] Overall, however, many of these studies are limited due to describing the occurrence of AKI at any time point, and often late in the course (>7 days) after ICU admission. Rather, early AKI (present within 24 hours after ICU admission) may in fact differ from delayed-onset AKI (>48 hours after ICU admission). This distinction may be important as the temporal differences in the development of AKI after ICU admission may relate to differences in the underlying pathophysiology and thus have different prognostic implications. AKI occurring early is more likely to be directly attributable to trauma (e.g., shock, rhabdomyolysis, direct kidney blunt or penetrating trauma, exposure to radiocontrast media, early intra-abdominal hypertension),Citation[27] whereas delayed-onset AKI is more likely associated with complications such as sepsis and multiple organ dysfunction syndrome.Citation[24],Citation[26],Citation[37] We believe future investigations of AKI in trauma should attempt to define the impact of timing of development of AKI on clinical outcomes.
Numerous investigations have shown that AKI in ICU patients overall exerts an independent effect on mortality.Citation[2],Citation[3],Citation[7],Citation[38–40] However, few have focused on describing the mortality associated with AKI in trauma.Citation[21],Citation[25],Citation[27] Our data further strengthen the findings of prior investigations by showing that early AKI is associated with higher crude and adjusted mortality in ICU patients with trauma.Citation[21],Citation[25],Citation[27] In the only other study to evaluate early AKI in trauma, Ala-Kokko et al. showed that “renal failure” defined by SOFA score >2 had an adjusted OR of 8.2 for hospital mortality.Citation[27] In addition, our study further highlights how higher severity of early AKI, defined by worse RIFLE category, is associated with a significant and incremental increase in mortality, even after adjusting for co-variates. These data indicate early AKI in ICU patients with trauma may exert an important and independent impact of survival.
There are notable limitations to our study. First, we were only able to collect data on AKI occurring within the initial 24 hours of ICU admission and were not able to obtain data on whether AKI improved or worsened over subsequent days. Thus, some trauma patients may have been classified as AKI; however, following prompt resuscitation may have demonstrated rapid improvement in kidney function. We recognize this may contribute to some misclassification.Citation[27] This may also lead to the suggestion that in these cases the etiology was simply “pre-renal azotemia” rather than true AKI; however, our contention is that acute declines in kidney function and/or genuine injury were still apparent within the initial 24 hours of ICU admission, and this correlated significantly with outcome. In addition, we recognize that the pathophysiology of trauma is complex, frequently confounded by other factors, and generally associated with intense systemic inflammation that may contribute to both early and downstream AKI.Citation[3],Citation[41] Prior clinical studies have suggested the majority of AKI in trauma develops late and generally as a complication of septic shock and/or progressive multiple organ dysfunction syndrome.Citation[23–25],Citation[42] Alternatively, our study is strengthened by being the largest multi-center study of AKI in trauma performed to date. We contend our data provides a crude descriptive analysis of the occurrence of early AKI, defined by the RIFLE criteria, that is more likely to be attributable to trauma rather than to delayed or additional confounding factors (i.e. operative interventions, sepsis).
Second, we were unable to accurately estimate the prevalence of chronic kidney disease (CKD) (except for those requiring chronic dialysis) in our cohort. This may also contribute to some misclassification and over-estimation of the incidence of AKI. Population-based investigations have suggested an estimated 20% of ICU patients have pre-morbid CKD.Citation[1] In a sensitivity analysis, if we assumed 20% of critically ill trauma patients in our cohort had pre-morbid CKD and were re-classified, the resulting incidence of early AKI would be 14.8%. This is not a significant change, and this crude estimate would still indicate a higher burden of illness due to early AKI that previously reported.
Third, the ANZICS APD does not capture sufficient data to calculate Injury Severity Scores (ISS) or Revised Trauma Scores.Citation[43] We acknowledge this as an important limitation, and we recognize that although not an ideal surrogate of injury severity due to the absence of an anatomic diagnosis, we have used the APACHE II and APACHE III scores as an index of overall severity of illness.Citation[44–46] The APACHE scores described in our cohort were comparable to those reported in previous large epidemiologic studies of trauma in which accompanying ISS were greater than 16.Citation[27],Citation[47]
Fourth, we were not able to describe risk of early AKI by mechanism of trauma (blunt, penetrating, burn). We recognize that the risk of early AKI and overall prognosis may be influenced by these factors.
Finally, we were unable to describe the proportion of patients ultimately requiring RRT.
In summary, we found trauma ICU admissions are frequently complicated by early AKI. Those at high risk for early AKI appear to be older, female, have pre-existing co-morbid illness, and generally present with higher acuity of illness. Early AKI in critically ill trauma patients also appears to be independently associated with higher mortality and prolonged duration of hospitalization. While our study has limitations, this descriptive analysis of a large cohort broadly suggest a higher burden of early AKI in critically ill trauma patients than previously described, and that early AKI may exert an important impact on prognosis. Based on these findings, we contend additional prospective evaluations of AKI in ICU admissions for trauma are needed that explore not only early AKI, but also the association of early dynamic changes in kidney function and clinical outcomes.
This study was authored on behalf of the ANZICS Database Management Committee. It was supported in part by the Austin Hospital Anaesthesia and Intensive Care Trust Fund.
REFERENCES
- Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit Care. 2005; 9: R700–R709
- Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004; 66: 1613–1621
- Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002; 30: 2051–2058
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005; 294: 813–818
- Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007; 11: R68
- Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008; 23: 1203–1210
- Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007; 35: 1837–1843; 1852
- Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care. 2006; 12: 557–560
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy, and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8: R204–R212
- Guitard J, Cointault O, Kamar N, Muscari F, Lavayssiere L, Suc B, Ribes D, Esposito L, Barange K, Durand D, Rostaing L. Acute renal failure following liver transplantation with induction therapy. Clin Nephrol. 2006; 65: 103–112
- Heringlake M, Knappe M, Vargas Hein O, Lufft H, Kindgen-Milles D, Bottiger BW, Weigand MR, Klaus S, Schirmer U. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006; 72: 645–654
- Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006; 10: R73
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006; 34: 1913–1917
- Airey CM, Chell SM, Rigby AS, Tennant A, Connelly JB. The epidemiology of disability and occupation handicap resulting from major traumatic injury. Disabil Rehabil. 2001; 23: 509–515
- Connelly J, Chell S, Tennant A, Rigby AS, Airey CM. Modelling five-year functional outcome in a major traumatic injury survivor cohort. Disabil Rehabil. 2006; 28: 629–636
- Demetriades D, Murray J, Sinz B, Myles D, Chan L, Sathyaragiswaran L, Noguchi T, Bongard FS, Cryer GH, Gaspard DJ. Epidemiology of major trauma and trauma deaths in Los Angeles County. J Am Coll Surg. 1998; 187: 373–383
- Laupland KB, Kortbeek JB, Findlay C, Hameed SM. A population-based assessment of major trauma in a large Canadian region. Am J Surg. 2005; 189: 571–576
- McNicholl B, Cooke RS. The epidemiology of major trauma in Northern Ireland. Ulster Med J. 1995; 64: 142–146
- Niemcryk SJ, Hines R, Brawley M, Yount SI. Intentional and unintentional injury in the State of Nevada: 1989–1992. Am J Prev Med. 1998; 14: 43–53
- Pickett W, Hartling L, Brison RJ. A population-based study of hospitalized injuries in Kingston, Ontario, identified via the Canadian Hospitals Injury Reporting and Prevention Program. Chronic Dis Can. 1997; 18: 61–69
- Brandt MM, Falvo AJ, Rubinfeld IS, Blyden D, Durrani NK, Horst HM. Renal dysfunction in trauma: Even a little costs a lot. J Trauma. 2007; 62: 1362–1364
- Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR. Contribution of acute kidney injury toward morbidity and mortality in burns: A contemporary analysis. Am J Kidney Dis. 2007; 49: 517–523
- Guly UM, Turney JH. Post-traumatic acute renal failure, 1956–1988. Clin Nephrol. 1990; 34: 79–83
- Matas AJ, Payne WD, Simmons RL, Buselmeier TJ, Kjellstrand CM. Acute renal failure following blunt civilian trauma. Ann Surg. 1977; 185: 301–306
- Morris JA, Jr., Mucha P, Jr., Ross SE, Moore BF, Hoyt DB, Gentilello L, Landercasper J, Feliciano DV, Shackford SR. Acute posttraumatic renal failure: A multicenter perspective. J Trauma. 1991; 31: 1584–1590
- Tran DD, Cuesta MA, Oe PL. Acute renal failure in patients with severe civilian trauma. Nephrol Dial Transplant. 1994; 9(Suppl. 4)121–125
- Ala-Kokko T, Ohtonen P, Laurila J, Martikainen M, Kaukoranta P. Development of renal failure during the initial 24 h of intensive care unit stay correlates with hospital mortality in trauma patients. Acta Anaesthesiol Scand. 2006; 50: 828–832
- Plurad D, Brown C, Chan L, Demetriades D, Rhee P. Emergency department hypotension is not an independent risk factor for post-traumatic acute renal dysfunction. J Trauma. 2006; 61: 1120–1128
- Stow PJ, Hart GK, Higlett T, George C, Herkes R, McWilliam D, Bellomo R. Development and implementation of a high-quality clinical database: The Australian and New Zealand Intensive Care Society Adult Patient Database. J Crit Care. 2006; 21: 133–141
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985; 13: 818–829
- Nadvi SS, Mokoena T, Gouws E, Haffejee AA. Prognosis in posttraumatic acute renal failure is adversely influenced by hypotension and hyperkalaemia. Eur J Surg. 1996; 162: 121–124
- Zygun DA, Doig CJ, Gupta AK, Whiting G, Nicholas C, Shepherd E, Conway-Smith C, Menon DK. Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003; 18: 238–244
- Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005; 33: 654–660
- Sipkins JH, Kjellstrand CM. Severe head trauma and acute renal failure. Nephron. 1981; 28: 36–41
- Davenport A. Renal replacement therapy in the patient with acute brain injury. Am J Kidney Dis. 2001; 37: 457–466
- Davis KA, Reed RL, II, Santaniello J, Abodeely A, Esposito TJ, Poulakidas SJ, Luchette FA. Predictors of the need for nephrectomy after renal trauma. J Trauma. 2006; 60: 164–170
- Ostric V, Radovic M, Stanojevic P, Djukanovic L. Acute renal failure in polytraumatized patients: Prediction of outcome. Ren Fail. 1996; 18: 607–613
- Bagshaw SM, Mortis G, Doig CJ, Godinez-Luna T, Fick GH, Laupland KB. One-year mortality in critically ill patients by severity of kidney dysfunction: A population-based assessment. Am J Kidney Dis. 2006; 48: 402–409
- Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995; 155: 1505–1511
- Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001; 29: 1910–1915
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005; 36: 691–709
- Regel G, Grotz M, Weltner T, Sturm JA, Tscherne H. Pattern of organ failure following severe trauma. World J Surg. 1996; 20: 422–429
- Baker SP, B O'Neill, Haddon W, Jr, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974; 14: 187–196
- McAnena OJ, Moore FA, Moore EE, Mattox KL, Marx JA, Pepe P. Invalidation of the APACHE II scoring system for patients with acute trauma. J Trauma. 1992; 33: 504–507
- Vassar MJ, Wilkerson CL, Duran PJ, Perry CA, Holcroft JW. Comparison of APACHE II, TRISS, and a proposed 24-hour ICU point system for prediction of outcome in ICU trauma patients. J Trauma. 1992; 32: 490–500
- Vassar MJ, Lewis FR, Jr, Chambers JA, Mullins RJ, PE O'Brien, Weigelt JA, Hoang MT, Holcroft JW. Prediction of outcome in intensive care unit trauma patients: A multicenter study of Acute Physiology and Chronic Health Evaluation (APACHE), Trauma and Injury Severity Score (TRISS), and a 24-hour intensive care unit (ICU) point system. J Trauma. 1999; 47: 324–329
- Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003; 55: 608–616
APPENDIX
Pre-existing co-morbidities were defined by use of the chronic health evaluation for APACHE II, APACHE III, and SAPS II systems, as outlined in the ANZICS APD data dictionary.
Cardiovascular disease was defined by the presence of New York Heart Association (NYHA) class IV symptoms.
Respiratory disease was defined by the presence of chronic restrictive, obstructive, or vascular respiratory disease causing severe exercise limitations or documented chronic hypoxemia, hypercapnia, secondary polycythemia, or pulmonary hypertension.
Liver disease was defined by the presence of biopsy-proven cirrhosis or documented evidence of portal hypertension (i.e., variceal bleeding, encephalopathy).
Metastatic cancer was defined by the presence of any documented metastatic solid organ tumor.
Leukemia or lymphoma was defined by prior documented presence of any form of hematologic malignancy.
Immunocompromised was defined by the presence of advanced disease sufficient to suppress resistance to infection (i.e., malignancy, AIDS) or therapy that suppresses resistance to infection (i.e., chemotherapy, steroids).