Abstract
Cardiac dysfunction is highly prevalent in dialysis patients in the developed world, and is a major cause of morbidity and mortality. The relative impact of pre-existing cardiac disease and dialysis/uremia on cardiovascular morbidity are not clear. We conducted a retrospective, cross-sectional analysis of cardiac function and mortality in 202 incident and prevalent dialysis patients over an 18-month period in a population with a low prevalence of cardiovascular disease at dialysis initiation. Systolic dysfunction was defined as an ejection fraction (EF) of <50%. Left ventricular hypertrophy (LVH) was determined by echocardiography or electrocardiogram. Clinical data was collected by chart review. Ninety-nine percent of patients were black, with a mean age of 41.7 ± 10.1 years, and median follow up 28 months (range 1–216 months). Echocardiograms were available in 132 patients. Seventy-seven patients received hemodialysis, and 55 received peritoneal dialysis. Mean EF was 63.2 ± 11.1. EF was not lower in patients with greater duration of dialysis, although LVH tended to increase (not statistically significant). In 39 patients who died during the study period, cardiac function was not different from survivors, and no patient died of ischemic heart disease or heart failure. In conclusion, in a population of patients with a low prevalence of cardiovascular disease at dialysis initiation, cardiac function appears preserved over time, and cardiac morbidity and mortality are low. This finding suggests that dialysis and uremia per se, in the absence of pre-existing cardiac disease, may not be major contributors to cardiovascular morbidity.
INTRODUCTION
Cardiac dysfunction is highly prevalent in dialysis patients in developed countries, and existing or de novo cardiac disease is a major cause of morbidity and mortality.Citation[9–15] Whether this is due to underlying cardiac disease that progresses during the course of chronic kidney disease (CKD), shares a common etiology to that of the renal dysfunction, or is a consequence of uremia and dialysis per se is not fully understood. Some studies suggest the greatest risk for cardiovascular mortality is pre-existing cardiac disease, either a known history of ischemic heart disease or reduced systolic function, or diagnosed on screening of asymptomatic patients at the start of dialysis.Citation[9–15] De novo cardiac dysfunction does develop in some patients, usually within the first year of dialysis, but it also appears to be determined in part by baseline cardiac status.Citation[16–18] Emerging data also support cardiovascular stress resulting from dialysis and/or ultrafiltration procedures as potential contributors to cumulative myocardial injury.Citation[19–25] In hemodialysis (HD) patients, subtle changes in diastolic relaxation or global changes in myocardial perfusion are detectable during HD treatments in patients without major cardiovascular disease.Citation[22] Interestingly, these myocardial changes persist for varying periods after dialysis, suggesting a degree of myocardial stunning. Long-term outcomes associated with these changes are still unknown, but it is conceivable that repetitive myocardial stunning may gradually result in loss of cardiac myocytes and contribute to eventual systolic dysfunction.Citation[26] Interestingly, however, cardiac outcomes tend to be worse in peritoneal dialysis (PD) patients with diabetes or known cardiovascular disease, suggesting that the hemodynamic changes associated with HD are not the only potential contributors to cardiac dysfunction.Citation[3],Citation[18],Citation[27–29] Lipid abnormalities are more prevalent in PD patients, but PD fluid exchanges themselves have been shown to result in changes in central venous pressure and vascular tone, which may lead to increased cardiac work.Citation[23],Citation[25],Citation[30]
In South Africa, dialysis is supported by the public health service, but because of resource limitations, patients accepted for chronic dialysis should be eligible for transplantation (i.e., have good functional status and minimal co-morbidities at the start of dialysis). The South African black population has traditionally had a low prevalence of cardiovascular disease.Citation[31] The major causes of end-stage renal disease (ESRD) in this population are hypertension and glomerulonephritis.Citation[32],Citation[33] Study of cardiac function in this population on dialysis (i.e., patients with relatively little co-morbidity at dialysis initiation and a low prevalence of background cardiovascular disease) may provide some insight into the relative impact of pre-existing cardiac disease and dialysis/uremia on cardiovascular outcomes.
MATERIALS AND METHODS
This study was conducted with approval from the Committee for Research on Human Subjects (Medical) of the University of the Witwatersrand, Johannesburg, South Africa. Retrospective chart review was conducted in 202 consecutive patients who received dialysis at a single center in the Chris Hani Baragwanath Hospital in Soweto, South Africa, from November 2000 to April 2002. Data including cause of ESRD, dialysis outcome, medications, echocardiograms, laboratory tests within two months of echocardiogram, and blood pressures taken on the day of echocardiogram were recorded. The echocardiograms were performed for clinical care. Data were analyzed by t-test and ANOVA for continuous variables that were approximately normally distributed, chi squared test for categorical variables and the Mann-Whitney test for those variables not approximately normally distributed (Kirkman, T.W., 1996, Statistics to use, available at http://www. physics.csbsju.edu/stats/). Systolic dysfunction was defined as an ejection fraction (EF) < 50% determined using the fractional shortening method. Echocardiograms were all performed in a single cardiology unit by clinical cardiologists. Left ventricular hypertrophy (LVH) was defined either on echocardiography described in the report, or by electrocardiogram with R in V1 + V5 or V6 > 35 mm. Diastolic dysfunction was defined as an E:A ratio ≥ 1. In patients who had repeat echocardiograms, the most recent echocardiogram was included for analysis.
RESULTS
Two hundred and two prevalent and incident patients received chronic dialysis during the study period, of whom 91 (45%) were on PD and 111 (55%) were on HD. Ninety-nine percent of the patients were of African origin, 2 patients were Caucasian. Of the 202 patients, 132 (65%) patients had echocardiograms performed at some time since initiation of dialysis. Patients on PD were prescribed four 2 L twin bag exchanges per day, although prescriptions were altered as needed to achieve adequate clearance. All patients on HD were dialyzed for 4 hours three times a week, with the exception of two who were dialyzed for 5 hours twice a week because of transportation difficulties. Because of cost constraints, all patients were dialyzed on low flux dialyzers. Dialysis was conducted using acetate until 2001, when a transition was made to bicarbonate dialysate. The target urea reduction ratio was 65%. Calcium carbonate was the only phosphate binder available. Causes of ESRD are outlined in . The majority of patients had hypertension as a cause of ESRD, although this may be over-represented, as renal biopsies were not routinely performed. Only 4% had diabetic ESRD, reflecting the fact that many patients with diabetes are not accepted for the chronic dialysis program in South Africa because of multiple co-morbidities. These data therefore do not reflect the true spectrum of ESRD in the general South African CKD population. No patients had a known history of coronary artery disease.
Table 1 Causes of end stage renal disease (ESRD), n = 202
Patient Outcomes
Mean patient age was 41.7 ± 10.1 years. Median follow-up was 28 months (range 1–216), 30 months (range 1–216) for HD and 28 (range 9–156) for PD. During the 18-month study period, 39 (19%) patients died, 15 (16.5%) on PD and 24 (21.6%) on HD. Median time to death from initiation of dialysis was 18 months (range 1–170 months). Causes of death are outlined in . Four patients died of cardiovascular events, due to severely elevated blood pressure, hemorrhagic stroke, and volume overload after missing dialysis. No patient is known to have died of an ischemic cardiac event. One patient who died of sepsis had an EF of 30% documented two years prior, upon returning to dialysis after losing an allograft after 10 years. Causes of death among patients who had echocardiograms compared to those who did not were not different. Nineteen patients (9.4%) were transplanted during the study period at a median of 27 months (range 5–152, average waiting time in South Africa is 2–3 years). Nine (4.5%) patients regained enough renal function to discontinue dialysis after a median of seven months (range 2–72), with diagnoses of malignant hypertension, scleroderma, and obstructive uropathy.
Table 2 Causes of death in all dialysis patients and those with echocardiograms
Clinical Characteristics of the Dialysis Population
At least one echocardiogram available was available in 132 patients. Demographic and clinical parameters are described in . Hemoglobins are low in the whole population, as erythropoietin was available routinely only from 2001, after which the target hemoglobin was 9–10 g/dL. As some echocardiograms had been performed prior to 2001, the hemoglobin at the time of echocardiogram reflects the absence of erythropoietin. As expected, hemoglobins were higher in PD patients (p < 0.001) and serum albumin higher in HD patients (p = 0.004). Total serum cholesterol was within normal limits in this population with a traditionally low prevalence of atherosclerosis, although it was significantly higher in PD compared to HD patients (p < 0.001). Systolic blood pressures were higher in HD compared to PD patients (p = 0.038). Medication use at time of echocardiogram was available in 115 patients: angiotensin-converting enzyme inhibitors were prescribed in 64 (54%) patients, beta blockers in 49 (43%), calcium channel blockers in 90 (78%), alpha blockers in 44 (39%), and diuretics in 9 (8%). A greater proportion of patients were receiving PD in the initial time periods (see , p = 0.006), reflecting the policy in South Africa to attempt to use PD in all patients initiating dialysis. More echocardiograms were therefore performed early in PD compared to HD patients as an initial workup for listing for transplantation (see , p = 0.002). During the study period, five patients underwent coronary angiography: four black male patients with diabetes mellitus and one white female patient with severe mitral valve disease and reduced EF. Four of the five patients had no evidence of coronary artery disease, with the remaining patient (male, 51 yr, insulin-dependent diabetes, EF 70%) having triple vessel disease.
Table 3 Demographic and clinical data on patients in whom echocardiograms were available
Table 4 Ejection fraction and LVH by dialysis vintage (n = 132)
Lack of Association between Ejection Fraction and Duration of Dialysis
Echocardiograms were performed at varying times after initiation of dialysis, as shown in and , at a median of 12 months from initiation (range 1–204 months). Indications for echocardiogram were predominantly for transplant work-up; others were performed when clinically indicated. Systolic dysfunction (EF < 50%) was present in 12.1% (16 patients), LVH in 68% (90 patients), and ventricular dilatation in 3.8% (5 patients). Fifty-nine patients had a baseline echocardiogram performed within three months of initiation of dialysis, median time one month (range 0–3 months). Mean baseline EF was 62.9 ± 9.9%. Four patients (6.8%) had an EF < 50%, 43 (72.9%) had an EF 50–70%, and 12 (20.3%) had an EF ≥ 70%. LVH was present in 43 (72.9%) baseline echocardiograms, 5 (8.5%) had no LVH, and in 10 (16.9%) there was no comment. One patient had left ventricular dilatation at baseline, which improved significantly after several months of dialysis. Two patients had significantly reduced EF later, one with an EF of 30% at 156 months after diagnosis of ESRD from FSGS associated with malaria. This patient had dilated cardiomyopathy with some restrictive features; he returned to dialysis after having a transplant for 10 years and died three years later of sepsis. The second patient had an EF of 28% at 46 months after initiation of dialysis for polycystic kidney disease; he had severe valvular dysfunction likely secondary to rheumatic heart disease, and remained asymptomatic.
Figure 1. Scattergram of ejection fraction (EF) versus time from initiation of dialysis in 132 dialysis patients. Systolic dysfunction was defined as an EF < 50%.
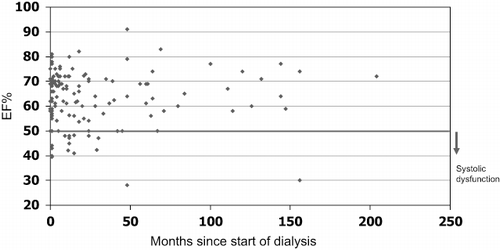
In this cross-sectional study, EF (i.e., systolic function) was not significantly different among patients with differing dialysis duration (see ). The prevalence of LVH tended to increase with dialysis vintage, but failed to reach statistical significance. The proportion of patients with systolic dysfunction, EF < 50%, was also not statistically significantly different among the groups. Among the 16 patients with EF < 50%, significant valvular abnormalities were described in 8, ventricular dilatation in 3, and pulmonary hypertension in 1. Using the more rigorous cut off of an EF ≤ 40%, systolic dysfunction was present in 1 patient (1.7%) at baseline, 2 (2.8%) in the 0–12-month group, 0 in the 13–24-month group, 1 (4.5%) in the 25–60-month group, and 1 (5.6%) in the >60 month group. Conversely, the proportion of patients with EF > 70% tends to increase with time, likely reflecting the increasing LVH and possible diastolic dysfunction. Diastolic dysfunction was not routinely measured in all echocardiograms and could not be independently examined. Among 64 echocardiograms in which diastolic function was reported, this was present in more than 50% of patients within each group. There was no significant association between EF, time on dialysis, hemoglobin, albumin, PTH, calcium-phosphate product, or blood pressures.
Echocardiograms were available in 118 of 163 patients who survived (73.4%) and in 14 of 39 patients who died (35.9%). Median time of echocardiogram from start of dialysis was 13 months (range 1–156) in those who died and 12 months (range 1–204) in those who survived (p = NS). Comparison of echocardiograms between patients who died and those who survived revealed significantly higher EFs among those who died (p = 0.023). LVH was present in all patients who died, but numbers were too small for statistical analysis (see ).
Table 5 Comparison between patients who died or survived
Repeat Echocardiograms
Forty-four patients had echocardiograms performed more than once since the initiation of dialysis. Of these, 29 patients had repeat echocardiograms greater than 12 months apart, at a median interval of 27 months (range 12–103). None of these patients had an EF < 50% on the first study, and 4 patients experienced a drop in EF below 50% by the time of the second study, at 15, 22, 60 and 103 months, respectively, after the first echocardiogram. The repeat EF was 47–49% in all four patients, and no patient had symptomatic cardiac failure. Of these 44 patients, only two had an EF of < 45% on repeat echocardiogram. One developed symptomatic cardiac dysfunction, with the EF dropping from 59% at month 4 of dialysis to 41% at month 15. This patient had severe mitral valve disease. The second patient initiated hemodialysis with an EF of 25% documented at month 4 of dialysis, which was thought to be due to advanced uremia, that improved to 42% after seven months of dialysis. This patient was clinically very well.
DISCUSSION
The prevalence of baseline systolic dysfunction and left ventricular dilatation in this cohort of black African dialysis patients without co-morbid disease was low, whereas the prevalence of LVH was high. In addition, the prevalence of patients with systolic dysfunction and LV dilatation did not increase with dialysis duration, suggesting that cardiac function does not appear to worsen in this population despite the high prevalence of LVH. These observations are in contrast to those observed in other, predominantly Caucasian dialysis populations in which systolic dysfunction and left ventricular dilatation are present from 16–26% and 27–38%, respectively, at initiation of dialysis.Citation[3],Citation[16],Citation[34–36] In these populations, cardiac abnormalities often worsen progressively and are associated with high morbidity and mortality.Citation[2],Citation[4],Citation[6],Citation[16],Citation[34] Up to 17% of dialysis patients develop de novo heart failure, and up to 10% develop de novo ischemic heart disease on dialysis.Citation[3],Citation[10] A subset of patients, however, does remain free of cardiac dysfunction, and those with persistently normal echocardiograms or who undergo improvement in left ventricular parameters have the best survival.Citation[3],Citation[17],Citation[36],Citation[38]
The high prevalence of cardiac abnormalities in dialysis patients worldwide is in part attributed to the frequent presence of traditional cardiovascular risk factors and pre-existing cardiac disease.Citation[3],Citation[8–13],Citation[15],Citation[–39] These risk factors are shared with the general population, but have a greater impact and are associated with worse outcomes in the dialysis population. A retrospective analysis of long-term dialysis survivors in the United States found a low prevalence of cardiac risk factors compared to the general USRDS population, highlighting the impact of cardiovascular morbidity on patient survival.Citation[14] Superimposed on traditional cardiac risk factors, uremia, and/or dialysis are also associated with additional cardiac stressors (e.g., volume overload, anemia, arteriovenous fistula, inflammation, hyperparathyroidism).Citation[8] The relative impacts of traditional, compared to dialysis-related, risk factors in ESRD patients are not yet clear. Subtle changes in diastolic function have been described in ESRD patients that correlate with age, diabetes mellitus, hypertension, and left ventricular mass, rather than with ESRD alone, dialysis adequacy, or endothelial dysfunction.Citation[39] Although the long-term impact of these changes is still unknown, they underscore the contribution of non-dialysis-specific risk factors to cardiac function.
The presence of LVH has been described as risk factor for death, whereas its regression is associated with improved outcomes in the dialysis population.Citation[17],Citation[35],Citation[40],Citation[41] Most of the patients included in our cohort had ESRD associated with severe hypertension. LVH is known to be highly prevalent in black patients with hypertension.Citation[42],Citation[43] The prevalence of LVH in our dialysis patients was high and tended to increase with duration of dialysis, but was not associated with worsening cardiac function. Similarly, a Brazilian study of ESRD patients with low prevalence of co-morbidities did not find an association with LVH and mortality.Citation[44] It is possible, therefore, that LVH in healthier patients may have a less unfavorable prognosis. Consistent with this possibility, increasing the prevalence of LVH is seen in long-term dialysis survivors in diverse populations.Citation[3],Citation[37],Citation[45]
Dialysis-related cardiac risk factors (e.g., anemia, hyperparathyroidism, hypertension, and hypoalbuminemia) were present in our dialysis population but did not have a significant association with systolic function.Citation[46–49] The higher blood pressures in our cohort may have been protective, consistent with reverse epidemiology of cardiac risk factors in the dialysis population.Citation[50] We also did not find any significant difference in cardiac function between patients on HD compared to PD. As our patients were predominantly non-diabetic and had no known coronary artery disease, this is consistent with published data.Citation[28] A high proportion of our patients were receiving cardio-protective medications compared to published studies, which may have had an impact in preserving cardiac function.Citation[51–55] Therefore, in our patient population, dialysis and uremia do not appear to result in progressive cardiac functional deterioration.
The lack of longitudinal prospective data in our cohort is a weakness and limits the strength of our conclusions because we cannot exclude survivor bias. The preservation of good cardiac function in our longer-term dialysis patients, however, does not appear to be related to cumulative loss of patients with poor cardiac function. Among patients who died, EFs were greater than in those who survived, although there was a non-significant trend toward a greater prevalence of LVH in patients who died. As not all patients who died had echocardiograms, cardiac function in most patients who died remains unknown. Importantly, however, no patient died of an ischemic cardiac event. Taken together, these data suggest that cardiac function was unlikely to be worse in those who died, supporting our finding of preservation of cardiac function in the majority of our dialysis population over time.
Another potential weakness, which could be viewed as strength, is that echocardiograms were not performed specifically for the study. Our echocardiograms were performed in a single well-respected cardiology center by staff cardiologists using the same machines and techniques, and reflect the reality of reports received in a daily basis during clinical practice. In addition, a potential confounder in our study is the fact that not all patients had echocardiograms available. If echocardiograms were performed for clinical indications, one would expect a bias towards poorer cardiac function. This is contrary to what we found, and therefore not likely relevant.
The dialysis population included in the present study is not typical, in that the patients are predominantly black and have a low prevalence of traditional cardiovascular risk factors and other co-morbidities. Our findings are not therefore uniformly generalizable. We do, however, strongly suggest that our observations are informative as to cardiac function in healthy dialysis patients and suggest that dialysis and ESRD themselves are not always major contributors to the development of cardiac dysfunction. A further suggestion that requires investigation is whether rigorous optimization of cardiac function and risk factors prior to dialysis initiation may have a beneficial impact on morbidity and mortality in the general dialysis population.
An important caveat to our conclusions is the possibility that with a relatively short follow-up, we may have missed later development of symptomatic cardiac dysfunction in our patients. A “lag time” has been described between the occurrence of echocardiographic abnormalities and the subsequent development of symptomatic ischemic heart disease or congestive heart failure.Citation[3],Citation[17] In these studies of serial echocardiograms in the same patients, however, major changes in cardiac function were evident within the first year after initiation of dialysis, although time to development of symptoms was many months later.Citation[2],Citation[17] In 46% of our patients, the latest echocardiogram was performed after 12 months of dialysis, and EF was preserved in the majority. It is therefore unlikely that these patients would develop symptomatic cardiac dysfunction in the future in the absence of current echocardiographic changes. Furthermore, in the smaller cohort of patients with repeat echocardiograms, very few patients had deterioration of EF, and clinical cardiac function remained excellent.
Very few studies have evaluated cardiac function in black patients in dialysis. To our knowledge, there has been one cross-sectional study of cardiac function in a predominantly black dialysis and CKD population.Citation[56] In these 45 patients, with a mean age of 43 years, the predominant causes of CKD were hypertension, glomerulonephritis, and diabetes. Systolic dysfunction, EF < 50%, was present in 23% of patients and diastolic dysfunction in 34%. The two-year mortality was 28.3%. The high proportion of patients with systolic dysfunction in this study may be related to their higher prevalence of diabetes compared to our cohort. In other studies, African American dialysis patients have lower risks of incident myocardial infarction, heart failure, and death compared with Caucasian patients after adjustment for major co-morbidities.Citation[57–59] The reasons for this difference are not well explained. In the general United States population, black patients have a higher prevalence of traditional Framingham risk factors, but a lower prevalence on initiation of dialysis.Citation[58] This observation may reflect greater cardiac death before the development of ESRD in the black population, under-diagnosis of pre-existing cardiac disease in the black ESRD population, or genetic polymorphisms (e.g., of TGFβ) that accelerate renal injury but protect against atherosclerosis.Citation[57–59] Intriguingly, a recent publication has described a gene variant that is more prevalent in the African American population and is protective against chronic heart failure.Citation[60] The gene GRK5 encodes a G-protein-coupled receptor kinase that participates in signaling via β adrenergic receptors, and the more active variant GRK5-Leu41 is common in African Americans (25%) and rare in Caucasians (2%). The active variant effectively reduces signaling via the β adrenergic receptor, analogous to being on a β-blocker.Citation[60] The prevalence of this genetic variant among black dialysis patients has not been studied, but if—as the authors show—this variant results in better survival, black patients reaching ESRD may have a higher prevalence of this variant and thereby be more protected than Caucasian patients, whereas those without the variant may have succumbed to cardiac disease before developing ESRD.
In summary, in a highly selected population of South African black dialysis patients with little co-morbidity, ejection fraction is not associated with time on dialysis. This finding suggests that in the absence of pre-existing cardiac disease, dialysis and uremia do not necessarily worsen cardiac function. Given the variability in cardiac survival between black and white patients in other dialysis populations, more prospective studies are needed to confirm this hypothesis to further understand the mechanisms underlying these differences as well as to study the impact of optimization of cardiac function prior to dialysis in all populations.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The results presented in this paper have not been published previously in whole or part, except in abstract format. There was no outside source of funding for this work. All authors have no disclosures.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Dr. Mark Hopley in early discussions.
REFERENCES
- Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. Jan, 1995; 47(1)186–192
- Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int. Nov, 1998; 54(5)1720–1725
- Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. Jul, 1999; 10(7)1606–1615
- Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. Aug, 1989; 36(2)286–290
- Collins AJ, Li S, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. Oct, 2001; 38(4 Suppl. 1)S26–S29
- Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int. Mar, 1995; 47(3)884–890
- Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. May, 2008; 3(3)920–929
- Locatelli F, Covic A, Chazot C, Leunissen K, Luno J, Yaqoob M. Hypertension and cardiovascular risk assessment in dialysis patients. Nephrol Dial Transplant. May, 2004; 19(5)1058–1068
- Hase H, Joki N, Ishikawa H, et al. Independent risk factors for progression of coronary atherosclerosis in hemodialysis patients. Ther Apher Dial. Aug, 2006; 10(4)321–327
- Hase H, Tsunoda T, Tanaka Y, et al. Risk factors for de novo acute cardiac events in patients initiating hemodialysis with no previous cardiac symptom. Kidney Int. Sep, 2006; 70(6)1142–1148
- Kessler M, Zannad F, Lehert P, et al. Predictors of cardiovascular events in patients with end-stage renal disease: An analysis from the Fosinopril in dialysis study. Nephrol Dial Transplant. Dec, 2007; 22(12)3573–3579
- Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol. Jul, 2002; 13(7)1918–1927
- Nishimura M, Tsukamoto K, Hasebe N, Tamaki N, Kikuchi K, Ono T. Prediction of cardiac death in hemodialysis patients by myocardial fatty acid imaging. J Am Coll Cardiol. Jan 15, 2008; 51(2)139–145
- Owen WF, Madore F, Brenner BM. An observational study of cardiovascular characteristics of long-term end-stage renal disease survivors. Am J Kidney Dis. Dec, 1996; 28(6)931–936
- McCullough PA, Jurkovitz CT, Pergola PE, et al. Independent components of chronic kidney disease as a cardiovascular risk state: Results from the Kidney Early Evaluation Program (KEEP). Arch Intern Med. Jun 11, 2007; 167(11)1122–1129
- Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. Jul, 1996; 11(7)1277–1285
- Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol. May, 2000; 11(5)912–916
- Locatelli F, Marcelli D, Conte F, et al. Survival and development of cardiovascular disease by modality of treatment in patients with end-stage renal disease. J Am Soc Nephrol. Nov, 2001; 12(11)2411–2417
- Barnes E, Dutka DP, Khan M, Camici PG, Hall RJ. Effect of repeated episodes of reversible myocardial ischemia on myocardial blood flow and function in humans. Am J Physiol Heart Circ Physiol. May, 2002; 282(5)H1603–H1608
- Bos WJ, Bruin S, van Olden RW, et al. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis. May, 2000; 35(5)819–826
- Covic A, Goldsmith DJ, Panaghiu L, Covic M, Sedor J. Analysis of the effect of hemodialysis on peripheral and central arterial pressure waveforms. Kidney Int. Jun, 2000; 57(6)2634–2643
- McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. Jan, 2008; 3(1)19–26
- Selby NM, Fonseca S, Hulme L, Fluck RJ, Taal MW, McIntyre CW. Automated peritoneal dialysis has significant effects on systemic hemodynamics. Perit Dial Int. May–Jun, 2006; 26(3)328–335
- Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial. May–Jun, 2007; 20(3)220–228
- Verbeke F, Van Biesen W, Pletinck A, Van Bortel LM, Vanholder R. Acute central hemodynamic effects of a volume exchange in peritoneal dialysis. Perit Dial Int. Mar–Apr, 2008; 28(2)142–148
- Cooper HA, Braunwald E. Clinical importance of stunned and hibernating myocardium. Coron Artery Dis. Aug, 2001; 12(5)387–392
- Vonesh EF, Moran J. Mortality in end-stage renal disease: A reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. Feb, 1999; 10(2)354–365
- Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol. Feb, 2003; 14(2)415–424
- Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int. Sep, 2003; 64(3)1071–1079
- Kronenberg F, Lingenhel A, Neyer U, et al. Prevalence of dyslipidemic risk factors in hemodialysis and CAPD patients. Kidney Int Suppl. May, 2003, 84: S113–S116
- Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): A cohort study. Lancet. Mar 15, 2008; 371(9616)915–922
- Katz I. Kidney and kidney related chronic diseases in South Africa and chronic disease intervention program experiences. Adv Chronic Kidney Dis. Jan, 2005; 12(1)14–21
- Naicker S. End-stage renal disease in sub-Saharan and South Africa. Kidney Int Suppl. Feb, 2003, 83: S119–S122
- Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int. May, 1996; 49(5)1428–1434
- London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol. Dec, 2001; 12(12)2759–2767
- Zoccali C, Benedetto FA, Mallamaci F, et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol. Apr, 2004; 15(4)1029–1037
- Takeda K, Nakamoto M, Baba M, et al. Echocardiographic evaluation in long-term continuous ambulatory peritoneal dialysis compared with the hemodialysis patients. Clin Nephrol. May, 1998; 49(5)308–312
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. Jun, 1995; 5(12)2024–2031
- Fathi R, Isbel N, Haluska B, Case C, Johnson DW, Marwick TH. Correlates of subclinical left ventricular dysfunction in ESRD. Am J Kidney Dis. May, 2003; 41(5)1016–1025
- de Simone G. Left ventricular geometry and hypotension in end-stage renal disease: A mechanical perspective. J Am Soc Nephrol. Oct, 2003; 14(10)2421–2427
- Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. May, 2001; 12(5)1079–1084
- Lepira FB, Kayembe PK, ’Buyamba-Kabangu M, JR, Nseka MN. Clinical correlates of left ventricular hypertrophy in black patients with arterial hypertension. Cardiovasc J S Afr. Jan–Feb, 2006; 17(1)7–11
- Skudicky D, Sareli P, Libhaber E, et al. Relationship between treatment-induced changes in left ventricular mass and blood pressure in black African hypertensive patients: Results of the Baragwanath Trial. Circulation. Feb 19, 2002; 105(7)830–836
- De Lima JJ, Sesso R, Abensur H, et al. Predictors of mortality in long-term haemodialysis patients with a low prevalence of comorbid conditions. Nephrol Dial Transplant. 1995; 10(9)1708–1713
- Covic A, Mardare NG, Ardeleanu S, Prisada O, Gusbeth-Tatomir P, Goldsmith DJ. Serial echocardiographic changes in patients on hemodialysis: an evaluation of guideline implementation. J Nephrol. Nov–Dec, 2006; 19(6)783–793
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. Oct, 2001; 12(10)2131–2138
- Moon KH, Song IS, Yang WS, et al. Hypoalbuminemia as a risk factor for progressive left-ventricular hypertrophy in hemodialysis patients. Am J Nephrol. Sep–Oct, 2000; 20(5)396–401
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. Jul, 1996; 28(1)53–61
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. May, 1996; 49(5)1379–1385
- Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. Mar, 2003; 63(3)793–808
- Bouchard J, Madore F. Inadequate treatment of congestive heart failure in dialysis patients. Semin Dial. Sep–Oct, 2007; 20(5)383–386
- Gowdak LH, Arantes RL, de Paula FJ, Krieger EM, De Lima JJ. Underuse of American College of Cardiology/American Heart Association Guidelines in hemodialysis patients. Ren Fail. 2007; 29(5)559–565
- Roy P, Bouchard J, Amyot R, Madore F. Prescription patterns of pharmacological agents for left ventricular systolic dysfunction among hemodialysis patients. Am J Kidney Dis. Oct, 2006; 48(4)645–651
- Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol. May 7, 2003; 41(9)1438–1444
- Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int. Oct, 2006; 70(7)1318–1324
- Chung A, Iheonunekwu N, Gilbert DT, Barton EN. Cardiac disease in dialysis patients in a Jamaican hospital: Echocardiographic findings that predict mortality. West Indian Med J. Jun, 2007; 56(3)305–308
- Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol., 2008 Jul;19(7):1403–1410. E-pub 2008 Apr 2.
- Volkova N, McClellan W, Soucie JM, Schoolwerth A. Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant. Aug, 2006; 21(8)2202–2209
- Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. May, 2006; 69(9)1691–1698
- Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. May, 2008; 14(5)510–517
