Abstract
Objective. Patients with chronic renal failure (CRF) have an increased risk of death from cardiovascular diseases. The metabolic syndrome is a common risk factor for cardiovascular diseases. In the present study, it was aimed to evaluate the frequency of metabolic syndrome using the National Cholesterol Education Program Adults Treatment Panel III (NCEP-ATP III) and the International Diabetes Federation (IDF) definitions in patients with end-stage CRF undergoing hemodialysis (HD). Materials and Methods. A total of 222 cases undergoing HD were enrolled in the study. After obtaining medical history and physical examination, blood samples were collected from each patient for the measurements of fasting blood glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. Results. Among HD patients evaluated according to both IDF and NCEP-ATP III definitions, the diagnosis of metabolic syndrome was confirmed by IDF in 56.5% of those fulfilling the criteria for NCEP-ATP III. Similarly, 86% of the undiagnosed patients according to NCEP-ATP III were confirmed by IDF definitions. The sensitivity and positive predictive value of NCEP-ATP III for metabolic syndrome were 81.25% and 64.8%, respectively. The area under the Receiver Operating Characteristic (ROC) curve for NECP-ATP III and IDF was 0.730. False-positive rate and probability ratio for NECP-ATP III were 0.352 and 2.49, respectively. In other words, among the patients who were diagnosed with metabolic syndrome according to NCEP-ATP III definitions, the proportion of subjects whose diagnosis was confirmed by IDF definitions was 2.49-fold higher than those with unconfirmed diagnosis. Conclusion. It is logical to evaluate patients with CRF for metabolic syndrome and cardiovascular risk factors at the time of diagnosis and regularly thereafter due to the high ratio of metabolic syndrome in this population.
INTRODUCTION
Metabolic syndrome (MS) is considered a medical condition caused by over-nourishment and reduced physical activity as a consequence of industrial revolution and affluence, being the most frequently referred disorder in the recent literature. However, there is no general agreement regarding its denomination, and various synonyms exist in the literature such as Wohlstands syndrome, plurimetabolic syndrome, hormonal metabolic syndrome, syndrome X, insulin resistance syndrome, and hyperinsulinemia/insulin resistance syndrome.Citation[1],Citation[2]
Patients with chronic kidney disease (CKD) have an increased risk of death from cardiovascular diseases (CVD). They possess multiple metabolic abnormalities that may accelerate atherosclerosis, such as hypertension, insulin resistance, and dyslipidemia, along with other CKD-related risk factors. In addition, a considerable proportion of patients in advanced-stage CKD are malnourished, which represents MS with malnutrition.Citation[3]
The MS is characterized by a combination of obesity, insulin resistance, impaired glucose tolerance, type II diabetes mellitus (DM), dyslipidemia, and hypertension. More importantly, it is apparent that the MS presence results in a significant further increase in cardiovascular risk both in diabetic and non-diabetic patients, independently from the traditional risk factors. On the other hand, there are also several factors that may be associated with the MS. They include blood clotting abnormalities, autonomic neuropathy, hyperphagia, increased dietary fat content, reduced physical activity, smoking, excessive alcohol consumption, difficulty in coping with stress, altered adipose tissue physiology, gender, hormone abnormalities, reduced growth hormone levels, hypercortisolemia, and impaired glucocorticoid-receptor function.Citation[4–6]
There are several different definitions of MS. Although all of them include glucose intolerance, obesity, hypertension, and dyslipidemia as essential components, they differ in details. According to the definitions of the World Health Organization (WHO)Citation[7] and the European Group for the Study of Insulin Resistance (EGIR),Citation[8] glucose intolerance or insulin resistance are considered as essential MS components, whereas this is not the case for the National Cholesterol Education Program Adults Treatment Panel III (NCEP-ATPIII).Citation[9] The MS definition of the International Diabetes Federation (IDF) is the latest published.Citation[10]
The present study aimed to evaluate MS frequency among CKD patients undergoing hemodialysis (HD) using NCEP-ATP III and IDF definitions.
MATERIALS AND METHODS
The present study was conducted in patients with end-stage renal disease undergoing HD as renal replacement therapy after obtaining approval of the local ethics committee. A total of 222 patients (103 female, 119 male) were included in the study. Patients with liver, thyroid, parathyroid, and adrenal insufficiencies; receiving hormone replacement therapy; or having history (within the last three months) of an acute event (e.g., cerebral or coronary), acute infection, major trauma, or surgery requiring anaesthesia were excluded from the study, as well as those who did not provide informed consent. Participants were instructed to fast for the 12 hours prior to study initiation. Demographic and clinical data, such as age, gender, health status, and current medications, were recorded.
All HD patients had a dietary intake of 30–35 kcal/kg/day, including 1.2 g/kg/day protein, 1000–1500 mg/day calcium, 600–700 mg/day phosphor, and 200–250 mg/day magnesium. Certain patients were also receiving calcium acetate, vitamin-B complex, iron supplements, and erythropoietin as phosphor-binding agents.
Waist circumferences were measured in a standing position during gentle exhaling at the area located midway between the lower costal margin and the iliac crest, in the narrowest section. Hip circumferences were measured at the level of the greater trochanter.
Subjects were asked to remove outer garments, except underwear, prior to measurements. The weight and height measurements were performed with a standard error mean of ± 0.5 kg and ± 0.5 cm, respectively. The body mass index (BMI) was calculated with the weight (kg)/height2 (m2) formula.
After HD, blood pressure was obtained from both arms in a sitting position after a 10-minute rest by placing a proper cuff attached to a mercury sphygmomanometer. Korotkoff I and IV sounds were taken into account. Two subsequent measurements with at least three-minute intervals were obtained and recorded. In the morning, prior to HD, blood samples were drawn between 8 a.m. and 10 a.m. to assess plasma glucose, serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides (TG).
Laboratory Methods
Plasma glucose levels were measured by Roche Cobas Integra 800 Analysis Device (Mannheim, Germany) based on enzymatic hexokinase method. Fasting glucose levels ≥7.0 mmol/L and plasma glucose levels in oral glucose tolerance test (OGTT) ≥11.1 mmol/L were considered as DM; fasting glucose levels ≥5.5 and <7.0 mmol/L were considered as impaired fasting glucose; and plasma glucose levels in OGTT ≥7.8–11.0 mmol/L were considered as impaired glucose tolerance.
Serum total cholesterol and TG were measured using standard commercial enzymatic kits (CHOD-PAP and GPO-PAP methods, Roche Diagnostics, Mannheim, Germany). HDL cholesterol levels were measured through the enzymatic colorimetric assay using a direct method (ADVIA 1650/2400, Bayer, Milano, Italy) after separation of cholesterol from non-HDL particles. LDL cholesterol concentration was calculated according to the Friedewald formula.Citation[11]
NCEP ATP III CriteriaCitation[9]
Three of the following five criteria were required:
abdominal obesity (waist circumference >102 cm in men and >88 cm in women);
fasting hypertriglyceridemia (≥ 1.7 mmol/L or 150 mg/dL);
low fasting HDL (<1.04 mmol or 40 mg/dL in men and <1.29 mmol/L or 50 mg/dL in women);
high blood pressure (≥130/85 mmHg) or current treatment with antihypertensive medication; and
high fasting glucose (≥6.1 mmol/L or 110 mg/dL) or current treatment with anti-diabetic medication.
IDF CriteriaCitation[10]
Central obesity (waist circumference ≥94 cm for European men and ≥80 cm for European women, with ethnicity-specific values for other groups) was required, as well as two of the following four factors:
raised TG levels (>150 mg/dL or 1.7 mmol/L) or specific treatment for this lipid abnormality;
reduced HDL cholesterol (<40 mg/dL or 0.9 mmol/L in males and <50 mg/dL or 1.1 mmol/L in females) or specific treatment for this lipid abnormality;
raised blood pressure (systolic ≥130 or diastolic ≥85 mmHg) or treatment for previously diagnosed hypertension; and
raised fasting plasma glucose (≥100 mg/dL or 5.6 mmol/L), or previously diagnosed type 2 DM.
Statistical Analysis
Receiver operating characteristic (ROC) curves were generated to evaluate the specificity and sensitivity of NECP and IDF for MS diagnosis in HD patients. The differences between these methods in diagnosing were evaluated through the χ2 McNemar's test. Student's t-test was used to compare age, weight, waist circumference, total cholesterol, TG, and mean LDL and HDL levels, while χ2 test was used to compare gender, hypertension, and DM between MS-diagnosed and undiagnosed patients in both methods. All of these parameters (age, gender, weight, waist circumference, total cholesterol, TG, HDL and LDL cholesterol, hypertension, and DM) were re-analyzed through the backward stepwise logistic regression analysis. The software Statistics Package for the Social Sciences (SPSS) version 11.5 was used for the statistical procedures, and p values <0.05 were considered as statistically significant.
RESULTS
Of the 222 patients included in the study, 53.6% (119) were male and 46.4% (103) were female. The mean age was 54.2 ± 16.9 years (range: 19–91 years) and the median age was 55 years. The mean body weight was 64.91 ± 13.46 kg, ranging between 32–112 kg. Mean waist circumference was 84.47 ± 14.67 cm (range: 50–120 cm). CRF etiologies of the HD patients are shown in .
Table 1 Distribution of chronic renal failure causes in hemodialysis patients
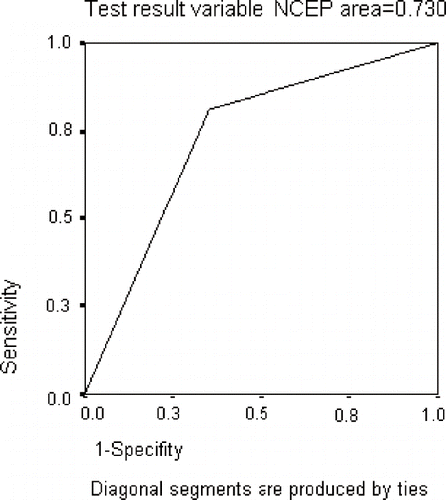
After evaluations according to IDF and NCEP-ATP III definitions, the diagnosis of MS was confirmed by IDF in 56.5% of the patients fulfilling NCEP-ATP III criteria. Similarly, 86% of the undiagnosed patients according to NCEP-ATP III were confirmed by IDF definitions. The sensitivity and positive predictive value of NCEP-ATP III for MS were 81.25% and 64.8%, respectively. The area under the ROC curve for NECP-ATP III and IDF was 0.730 (see ). The rate of false-positive results and probability ratio for NECP-ATP III were 0.352 (1–0.648 = 0.352) and 2.49 (0.812/0.352 = 2.49), respectively. In other words, among the patients who were diagnosed with MS using NCEP-ATP III definitions, the proportion of subjects whose diagnosis was confirmed by IDF was 2.49-fold higher than those with unconfirmed diagnosis.
A strong significant difference was observed between IDF and NCEP-ATP III definitions in the diagnosis of MS in HD patients (p = 0.000). The ratio of MS diagnoses was higher for NCEP-ATP III (51.8%) compared to IDF definitions (36.0%; see ).
Table 2 Distribution of metabolic syndrome diagnosis according to IDF and/or NCEP criteria in hemodialysis patients
summarizes CRF etiologies of the patients diagnosed with MS (65 patients) according to both NCEP-ATP III and IDF definitions.
Table 3 Causes of renal failure in patients with metabolic syndrome according to NCEP and/or IDF criteria undergoing hemodialysis
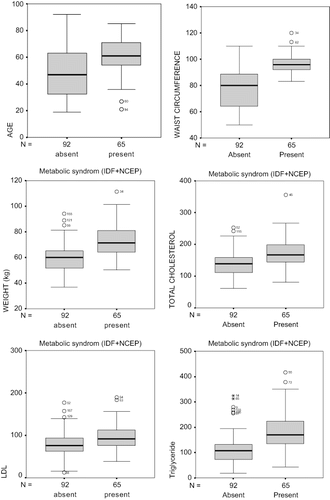
When all of the parameters were individually analyzed, the patients diagnosed with MS according to both NCEP-ATP III and IDF definitions (65 patients) had higher mean age, weight, waist circumference, total cholesterol, TG, and LDL cholesterol levels compared to those without MS (92 patients, p < 0.001; see and ). No statistically significant difference was found in HDL cholesterol levels (p > 0.05). Female patients represented 66.2% (n = 43) of the patients fulfilling both MS criteria and 37.0% (n = 34) of those not meeting any criteria. DM was present in 40 (58.46%) of the 65 patients with MS according to both criteria versus 13 (14.13%) of the 92 non-MS patients (p = 0.001). Hypertension was also identified in 25 (38.4%) patients with MS according to both criteria versus 30 (32.6%) of those without MS (p = 0.499). When all of these parameters (gender; age; weight; waist circumference; presence of DM or hypertension; total, HDL, and LDL cholesterol; and TG) were analyzed with stepwise backward logistic regression analysis, the waist circumference, TG levels, and proportion of females and patients with DM were found to be higher among patients fulfilling both criteria compared to those who did not meet any (p = 0.000, constant = 0.000; see ). There were no significant differences regarding age; total, HDL, and LDL cholesterol; and presence of hypertension between the groups (p > 0.05).
Table 4 Age, weight, waist circumference, and laboratory results of HD patients with or without metabolic syndrome according to both NCEP and IDF criteria
Table 5 Factors affecting metabolic syndrome in patients diagnosed by both NCEP and IDF criteria
DISCUSSION
In the present study, MS diagnosis in patients with CRF undergoing HD was compared by two different methods using the latest DM criteria defined by the American Diabetic Association.Citation[12]
After evaluation with both IDF and NCEP-ATP III definitions, the MS diagnosis was confirmed by IDF definitions in 56.5% of the patients fulfilling the criteria for NCEP-ATP III. Similarly, 86% of the undiagnosed patients according to NCEP-ATP III were confirmed by IDF definitions. Moreover, the sensitivity and positive predictive value of NCEP-ATP III for MS was 81.25% and 64.8%, respectively. There was a strong significant difference between IDF and NCEP-ATP III criteria in diagnosing MS to HD patients. The ratio of MS diagnoses was higher for NCEP-ATP III (51.8%) compared to IDF criteria (36.0%).
In 1998, the WHO defined MS as the presence of DM, impaired fasting glucose, impaired glucose tolerance, and insulin resistance together with at least two of the following risk factors: hypertension (>140/90 mmHg), hyperlipidemia, central obesity, and microalbuminuria.Citation[13] The NCEP prepared the report of Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel, ATP III) in 2001.Citation[9]
Apart from these diagnostic criteria, ATP III also defined other MS components. Two of these components, although not included in the criteria, are attracting an increasing amount of interest in recent studies. They are proinflammatory and prothrombotic statuses. In a data analysis by the National Health and Nutrition Examination Survey (NHANES), no significant difference was found regarding CVDs between patients diagnosed with MS according to WHO criteria and those according to ATP III criteria.Citation[14] The latest definition of MS was elaborated by IDF.Citation[10]
The present study aimed to compare the frequency of MS among patients with CRF undergoing HD by using NCEP-ATP III and IDF definitions, which are considered applicable and comprehensible methods in these patients.
In patients diagnosed with MS both through NCEP-ATP III and IDF definitions (65 patients), the mean age, weight, waist circumference, total cholesterol, TG, and LDL cholesterol levels were higher compared to those not meeting any definition. No statistically significant difference was found in HDL cholesterol levels. Females represented 66.2% of the patients fulfilling both criteria, and 37.0% of those not meeting any criteria.
Waist circumference, an important component of MS, and obesity are considered as a low-grade inflammatory state. Chronic subclinical inflammation constitutes part of the MS. Besides C-reactive protein (CRP), the levels of fibrinogen and plasminogen activator inhibitor-1 are elevated, which also occurs with uric acid and microalbuminuria. Adipose tissue is not only the energy storage, but also an endocrine organ releasing various peptides and cytokines into the circulation. Abdominal obesity is associated with insulin resistance and hypertension.Citation[15] Various active molecules released from adipose tissue and referred to as adipocytokines, such as tumor necrosis factor-α, interleukin-6, CRP, plasminogen-activator inhibitor, leptin, adiponectin, and resistin, are believed to be involved in hypertension and atherosclerosis.Citation[16],Citation[17] For MS, a blood pressure above 130/85 mmHg was accepted as hypertension according to the NCEP-ATP III definition. Insulin resistance can also be observed in non-obese hypertensive patients. High levels of TG and low levels of HDL cholesterol are apparent findings in MS. Although LDL cholesterol is not elevated, the small dense LDL, which is prone to oxidation and hence manifests atherogenic features, is increased.Citation[18]
In the present study, 40 of 65 patients (58.46%) with MS (according to both definitions) and 13 of 92 patients (14.13%) without MS had DM. Furthermore, hypertension was identified in 25 (45.5%) patients of the former group and in 30 (54.5%) of the latter. When all of these parameters (gender; age; weight; waist circumference; presence of DM or hypertension; total, HDL, and LDL cholesterol; and TG) were analyzed with backward stepwise logistic regression analysis, the waist circumference, TG, and the proportion of females and patients with DM were found to be higher among patients fulfilling both criteria compared to those who did not meet any. Age; total, HDL, and LDL cholesterol; and presence of hypertension were not significantly different between the groups.
Wilson et al.Citation[19] failed to demonstrate an improvement in the Framingham scoring system when adding (age, gender, total cholesterol, diabetes, HDL cholesterol) or removing (obesity, TG) specific MS factors to the Framingham algorithm. Moreover, when they removed increased blood glucose from MS definition, they found that a 10-year risk for CVD did not reach the baseline risk found in ATP III, suggesting a critical role for glucose intolerance in the predictive power of MS.Citation[9] Stern et al.Citation[20] also suggested that the Framingham scoring system had a superior predictive power. From San Antonio, a total of 2570 Spanish and non-Spanish Caucasian subjects without a previous history of DM or CVD were included in the study. An eight-year monitoring was performed for CVD. The authors concluded that the sensitivity of the Framingham scoring system was superior to MS and, when combined together, no further increase occurred in the predictive power. In a multivariable study, it was demonstrated that the MS sensitivity in predicting CVD was 55%, with a 22% false-positive ratio, whereas the Framingham scoring system sensitivity was 69%, with a 22% false-positive ratio. In another investigation,Citation[21] the efficiency and predictive power of MS was found to be equivalent to the Framingham scoring system.
Imbalanced contribution of glucose intolerance (impaired glucose fasting, impaired glucose tolerance, and DM) was suggested by Malik et al. to be included in the definition of the syndrome. In their study, which included the participants of NHANES II,Citation[22] DM alone (risk of CVD hazardous ratio [HR] = 5, risk of coronary artery disease [CAD] HR = 3.6, and risk of overall mortality HR = 2.1) was considered to be indicative of higher risk compared to the presence of MS (HR = 3.5, 2.7, and 2.5, respectively). When DM was combined with a previous history of cardiac disease, the predicting power for the overall mortality risk at the end of the 13-year follow-up period was substantially increased (HR = 11.3, 7.9, and 2.9, respectively). Similarly, when Stern et al. included DM in the syndrome's definition, those patients having CVD demonstrated an increased risk of all-cause and CVD mortality; however, after adjustment for DM, MS was not associated with an increased risk.Citation[23] Finally, Hunt et al.Citation[24] found that impaired fasting glucose values > 6.1 mmol/L alone were strong predictors of CVD compared to the syndrome presence or the presence of other criteria. These findings lead one to question DM's inclusion in the definition, although glucose intolerance practically provides the entire power of the syndrome to predict CVD.
Because MS does not include every known risk factor, it demonstrates different and independent results from other traditional risk factors (i.e., LDL cholesterol, age, smoking habit, family history). However, it is not clear the level of global (total) risk the syndrome leads to. It would be valuable to determine the predictive power of combined risk factors from those in the CVD list. Thus, comparison of MS with models holding different risk factorsCitation[16],Citation[25] or new combinations could ultimately create the ideal predicting model for CVD.
There are no randomized clinical trials on MS treatment. Management of all MS components, along with DM, hypertension, and CVD, constitutes the main objective. Primarily, lifestyles should be altered. Through weight loss, a decline in all-cause and CVD mortality has been observed by improvement of each MS parameter. The Diabetes Prevention Program demonstrated that effective changes in lifestyle could reduce in 50% the development of DM in the pre-diabetic population (from 11% to 4.8%).Citation[26]
Although there is no drug class among antihypertensives specifically targeting the MS, the angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers, and alpha-blockers are advantageous over diuretics and beta-blockers. Calcium-channel blockers are neutrally effective in terms of metabolic effect. Moxonidine and rilmenidine are suggested to be of benefit by acting on imidazoline receptors and decreasing sympathetic nervous system activation. Through a reduction of LDL cholesterol and CRP levels, statins may be a useful entity in inflammation suppression. In large-scale, randomized, controlled trials, statins have been shown to reduce mortality and morbidity in patients with DM or with impaired fasting glucose levels. Fibrate treatment may be considered to reduce TG and to increase HDL cholesterol levels.Citation[27] The combination of statin-fenofibrate is a recommended approach whenever circumstances apply. Metformin and thiazolidinediones act to decrease insulin resistance. Currently, studies on dual peroxisome proliferator-activated receptor agonists (PPAR) are being performed. These agents, acting on both PPARγ and PPARα, are believed to affect insulin resistance, glucose intolerance, high TG, and reduced HDL cholesterol levels. Low-dose aspirin administration is recommended in primary and secondary protection to preclude thrombosis.
In conclusion, because a high proportion of MS was observed among CRP patients, who are also prone to an associated CVD risk, it is suggested that an evaluation regarding MS and CVD risk must be performed both at the time of diagnosis and thereafter.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hansen BC. The metabolic syndrome X. Ann NY Acad Sci. 1999; 892: 1–24
- Reaven GM. Banting lecture 1988: Role of insulin resistance in human disease. Diabetes. 1988; 37: 1595–1607
- Shoji T, Nishizawa Y. Chronic kidney disease as a metabolic syndrome with malnutrition—need for strict control of risk factors. Internal Medicine. 2005; 44: 179–187
- Bonora E, Kiechl S, Willeit J, et al. Metabolic syndrome: epidemiology and more extensive phenotypic description. Cross-sectional data from the Bruneck Study. Int J Obes Relat Metab Disord. 2003; 27(10)1283–1289
- Zimmet P, Boyko EJ, Collier GR, De Courten M. Etiology of the metabolic syndrome: Potential role of insulin resistance, leptin resistance, and other players. Ann NY Acad Sci. 1999; 892: 25–44
- Nieuwdorp M, Stroes ES, Meijers JC, Büller H. Hypercoagulability in the metabolic syndrom. Current Opin Pharmacol. 2005; 5: 155–159
- World Health Organization Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. WHO, GenevaSwitzerland 1999
- Camastra S, Bonora E, Del Prato S, Rett K, Weck M, Ferrannini E, for EGIR (European Group for the Study of Insulin Resistance). Effect of obesity and insulin resistance on resting and glucose-induced thermogenesis in man. Int J Obes Relat Metab Disord. 1999; 23: 1307–1313
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285: 2486–2497
- http://www.idf.org, Alberti K, Zimmet P, Shaw J. The IDF consensus worldwide definition of the metabolic syndrome. Lancet, 2005. International Diabetes Federation
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without the use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499–502
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004; 27: S5–S10
- Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications, Part 1. Diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998; 15: 539–553
- Ford ES, et al. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care. 2003; 26: 575–581
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities: The role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996; 334: 374–381
- Festa A, D'Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000; 102: 42–47
- Das UN. Is obesity an inflammatory condition?. Nutrition. 2001; 17: 953–966
- Brunzell JD, Hokanson JE. Dyslipidemia of central obesity and insulin resistance. Diabetes Care 1999; 22(Suppl. 3)C10–C13
- Wilson PW, D'Agostino RB, Levy D, Be-langer AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97: 1837–1847
- Stern MP, Williams K, Gonzalez-Villal-Pando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease?. Diabetes Care. 2004; 27: 2676–2681, [erratum in Diabetes Care 2005;28:238]
- McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities Study. Diabetes Care. 2005; 28: 385–390
- Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease and on all causes in United States adults. Circulation. 2004; 110: 1245–1250
- Stern MP, Williams K, Hunt KJ. Impact on diabetes/metabolic syndrome in patients with established cardiovascular disease. Atheroscler Suppl. 2005; 6: 3–6
- Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004; 110: 1251–1257
- Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003; 108: 414–419
- Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003; 26: 557–562
- Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999; 341: 410–418