Abstract
Approximately one-third of all dialysis patients have mild to moderate malnutrition, while 6–8% have severe malnutrition, which is associated with increased morbidity and mortality rates and numerous pre-existing factors directly correlated with, or existing prior to, replacement hemodialysis. However, moderate to severe malnutrition (present in 10–30% of dialysis patients) is a prevalent cause of death among the elderly. Many of these patients have a particularly unstable cardiovascular and metabolic status that, independent of any underlying uremia and/or dialysis, impacts negatively on both their quality of life and clinical status. Moreover, their condition is often further exacerbated by dialysis itself, with its acute (e.g., hypotension and sensorial alterations) and chronic complications, including an exacerbation of malnutrition and systemic vascular disease. Malnutrition can occur secondary not only to erroneous dietary choices or uremia, but it may also depend on the patient's level of tolerance to dialysis and on the dialysis modality. Despite the improvements made to dialysis techniques, the nutritional condition of elderly patients on dialysis for chronic renal failure remains a cause for concern. In this patient category, it is therefore mandatory to ensure the daily supervision of nutritional status and early control when the first signs of malnutrition appear.
INTRODUCTION
An altered nutritional status, frequently encountered in elderly patients with chronic renal failure (CRF), progressively deteriorates as renal function worsens. Findings made in the NHANES III study on elderly patients with creatinine clearance levels below 60 mL/min showed that 31% of patients were undernourished. As shown at multivariate analysis (OR: 3.6; 95% CI, 2–6.6), a creatinine clearance level (CL) below 30 mL/min plays an important role (OR:1.1; IC:2.1–3.7), comparable to that of heart failure, in determining malnutrition, whereas age does not appear to have a significant influence (OR:1.1 in patients aged 70–79 years and 1 in patients >80 years).Citation[1]
As CRF progresses, the dietary uptake of protein decreases, minimal levels being reached when renal function worsens (0.66 g/kg/day with creatinine clearance <24 mL/min).Citation[2]
In North American guidelines (Kidney Dialysis Outcome Quality Initiative, KDOQI), it is suggested that a periodical assessment should be made of nutritional status once every three months, utilizing as markers body weight (comparing it with average national values), albuminemia, and protein consumption. In subjects older than 60 years of age with limited physical activity, the recommended caloric intake is 30–35 kcal/kg/day, while for more active patients, it is identical to that of their younger counterparts, at > 35 kcal/kg/day. The presentation of clinical signs of undernourishment, suggesting a deterioration in the patient's condition, indicates that dialysis should soon be started.Citation[3]
In a retrospective study, Holland et al. found that among 362 undernourished pre-dialysis subjects, hospitalization was required in 57% cases before or at the same time of the start of dialysis.
At univariate analysis, it was found that advanced age, cardiovascular co-morbidities, vascular nephropathy, anemia, and CRF were significant predictive factors of non-elective hospitalization among pre-dialysis. The mortality rate during this pre-dialytic period is high, reaching about 10%.Citation[4]
In patients on dialysis—the elderly in particular—an insufficient caloric and protein intake is associated with an increase in morbidity and mortality rates. Death from cachexy is not rare among dialysis patients older than 70 years of age.
Numerous hormonal and depletive elements concur in the pathogenesis of malnutrition, and while some can be ascribed to dialysis itself, most occur secondary to an insufficient dietary intake. It is therefore of great importance to pay particular attention to the patient's diet, which should include at least 1 to 1.5 g of protein, 50% of which should have a high organic content, and an energy content of 35 Kcal/Kg/day in patients whose BMI is within the normal range.
Approximately one-third of all dialysis patients have mild to moderate malnutrition, while 6–8% have severe malnutrition, associated with increased morbidity and mortality rates, and with numerous pre-existing factors directly correlated with or existing prior to replacement hemodialysis. However, moderate to severe malnutrition, present in 10–30% of dialysis patients, is a prevalent cause of death among the elderly. Many of these patients have a particularly unstable cardiovascular and metabolic status which, independent of any underlying uremia and/or dialysis, impacts negatively on both their quality of life and clinical status. Moreover, their condition is often further exacerbated by dialysis itself, with its acute (e.g., hypotension and sensorial alterations) and chronic complications, including an exacerbation of malnutrition and systemic vascular disease.
The plasma albumin level is the most important known prognostic factor for survival in patients on dialysis. It has been demonstrated that plasma albumin levels of < 4 g/dL are associated with an increased risk of death. Therefore, preventing malnutrition in dialysis patients not only incurs decreasing morbidity but also increasing survival rates, with a consequent reduction in the social costs of chronic kidney disease. Furthermore, according to the 1997 guidelines of the National Kidney Foundation Dialysis Outcome Quality Initiative (DOQI), albumin should be used to assess the efficacy of dialysis and should also play a role in determining the appropriate moment to start dialysis.
Findings made in epidemiologic studies demonstrate that malnutrition is linked, especially in elderly patients, to an increase in morbidity, mortality, and hospital stay, with a consequent increase in healthcare costs, whatever the underlying disease.
Dialysis registries are of little help in assessing the role of cachexy and malnutrition as causes of death, as these conditions are rarely registered as such. However, even in registries that allow for them to be recorded, such as the ERA/EDTA Registry, they are seldom mentioned, probably because their role in causing death is underestimated.
It has been suggested that because the numerous factors involved in malnutrition can be considered the result of two main underlying mechanisms, malnutrition itself can be divided into two main types of mechanism:
Type 1. Malnutrition occurring secondary to reduced caloric and protein uptake and associated with the CKD per se or with its related factors; and
Type 2. Malnutrition due to chronic systemic inflammation and linked to dialysis-related co-morbidities and complications (e.g., recurrent infection, heart failure), which can be considered the consequence of the “non-physiological” nature of the treatment itself.Citation[5]
In both cases, and in the many overlapping conditions, a reduction is found in the fat-free mass (FFM), which normally accounts for 75–80% of body weight. It has been demonstrated that a decreased FM, rather than an increased fat mass (FM), is the cause of the higher morbility and mortality rates in the above patient category. Moreover, whatever the co-morbidities, in each malnutrition type it is possible to distinguish between factors related to uremia and those related to dialysis.
UREMIA-RELATED FACTORS
Already present during the course of conservative treatment, uremia-related factors include a reduced appetite, a negative nitrogen balance, and a reduced muscle mass. Consequently, many patients starting on replacement therapy already present clear signs of malnutrition. Moreover, during replacement therapy, some subjects mistakenly continue to adhere to pre-dialytic dietary habits, overestimating the advantages of a hypoproteic diet, without professional counseling being provided by a dietician with a view to achieving the correct nutritional balance. In addition, many patients develop anorexia due the gastroenteric side effects (e.g., nausea, esophagitis, gastroparesis, constipation) of pharmacological treatment.
A spontaneous reduction in protein consumption acts in synergy with a persistent catabolic state caused by metabolic acidosis and some hormonal factors. Metabolic acidosis, typically an early-stage complication of renal failure exacerbated by an increase in the levels of blood potassium and phosphates, tends to worsen as renal failure progresses, and its negative effects on protein catabolism are only partially controlled by dialysis.
In uremic patients, several quantitative and qualitative hormonal alterations influence protein synthesis. Leptin levels in particular, typically augmented during kidney failure (due to a reduced renal or dialytic clearance), have been considered as possible factors contributing to malnutrition. In addition, the close correlation found between leptin levels and fatty mass, also present in patients on dialysis, suggests that leptin might be used as another “marker” in assessing body composition.Citation[6]
DIALYSIS-RELATED FACTORS
Patients undergoing dialytic treatment require an increased carbo-proteic intake. While the nitrogen balance can be maintained in healthy subjects by a protein consumption of 0.5 g/kg/die, dialytic patients should ingest at least 1g/Kg/die of protein in order to maintain a positive nitrogen balance. Because periodic dialysis worsens the catabolic processes typical of the pre-dialytic phase, it causes increased cardiac output, alters thermal balance (hence requiring enhanced thermogenesis), and increases energy consumption. Furthermore, many nutrients (e.g., amino acids, proteins, and glucose) are lost during a dialysis session.
In particular, during dialysis, there is a rapid, progressive, and prolonged loss of amino acids, secondary to simple diffusion in the dialysate. In order to maintain amino acid homeostasis, catabolic processes, mainly involving muscle mass, are triggered in an attempt to normalize the circulating amino acid pool. The regular repetition of this mechanism may be partly responsible for the irreversible muscle wasting found in elderly patients on chronic dialysis.Citation[7]
Veeneman et al. have studied the effects of an orally administered nutritive bolus supplement (yogurt enriched with 45g of protein and 1100 kcal once every 30 minutes during dialysis) on protein metabolism by applying stable isotope infusion techniques using [1-13C] valine as a tracer.
Patients undergoing this treatment were found to have a positive protein balance during the dialysis session.Citation[8]
Other dialysis-linked factors influencing protein balance include the dose of dialysis, the liquids used, and the biocompatibility of the treatment. A direct relationship has been found between dialysis dose, measured with the Kt/V index, and the rate of protein catabolism; this suggests that the dialysis dose may influence the patient's nutritional status. However, it is important to bear in mind that protein catabolism is not always a reliable marker of protein intake. Reduced treatment biocompatibility is also an important cause of malnutrition. In addition, the patient on dialysis is also at risk of exposure to numerous inflammatory stimuli, due to the contact between the patient's blood and the dialysis membranes.
Any endotoxins present in the dialysis fluids may reach the bloodstream, especially if highly permeable membranes are used, thus causing mononucleate cells to secrete cytokine, IL-6 in particular. The presence of chronic inflammation, the reduced synthesis of albumin, and the increased production of protein in the acute phase can thus contribute to the onset of malnutrition.Citation[6]
A question of crucial importance, which has been neglected in literature, is whether irregular eating habits linked to the rhythm of dialysis can influence protein metabolism in elderly patients. In one study, protein consumption was 10% greater on dialysis-free than on dialysis days, and Bellizzi et al. have demonstrated that, in some elderly patients, the seventh interdialytic day is associated with a reduction in food consumption in the region of 40%.Citation[9]
EVALUATION OF NUTRITIONAL STATUS
Unfortunately, the current lack of methods or bio-humoral indices allowing the straightforward and accurate identification of nutritional status of the patient hinders any satisfactory evaluation of the nutritional status of patients on dialysis; the use of any single parameter is inadequate for the diagnosis of malnutrition, as several are required.
Traditionally, a distinction is made between biochemical parameters for studying protein status and the nutritional uptake of energy and protein and anthropometric parameters for evaluating the body composition.
Biochemical Parameters
The most frequently used and tested biochemical parameters are serum concentrations of albumin and prealbumin; also, slight reductions in these proteins are statistically associated with an increase in mortality. Albumin is influenced by the hepatic capacity for synthesis, has a half-life of about 20 days, and is therefore a late marker of malnutrition in patients on dialysis; pre-albumin has a shorter half-life (i.e., two days) and is therefore an early indicator, albeit widely questioned, because it is metabolized and excreted mainly by the kidney. One of its limitations depends on the fact that hypo-albuminemia may be caused by other co-morbid factors associated with an increased mortality rate.
However, notwithstanding the numerous factors that can influence albuminemia, it depends first and foremost on an insufficient protein uptake. In order to ensure a correct nutritional status, the daily protein uptake must be regularly controlled by the dietician; a consumption of at least 1 g of protein (1.2–1.3 g for patients on peritoneal dialysis) per kg ideal body weight is required, with an overall caloric intake of no less than 35 Kcal per kg of body weight. The correction of hypo-albuminemia takes time, even when high-quality dialysis is used and nutrition is carefully controlled, and it can call for the long-term provision of oral and parenteral nutritional supplements, with variable results.
In the general population, a high index of body mass (BMI, expressed as Kg/m) is associated with an increased incidence of cardiovascular disease and all the causes of mortality. However, the effect of over-weight (BMI ranging from 25 to 30) or obesity (BMI >30) in elderly patients with chronic renal failure who undergo maintenance hemodialysis therapy is, paradoxically, characterized by an opposite effect; for example, a high BMI is associated with a longer survival.Citation[10] Possible causes of this may be a greater hemodynamic stability, alterations in circulating cytokines, and interactions between endotoxins and lipoproteins, with the induction of the complex malnutrition–inflammation syndrome. This inverse epidemiology may have important implications in the management of dialysis in elderly patients, as this type of population has a high mortality rate. An investigation into the causes and effects of the inverse epidemiology of obesity in patients on dialysis may lead to the evaluation of and research into other interesting possible epidemiological paradoxes, including classical risk factors such as arterial hypertension, serum levels of cholesterol, and blood concentrations of homocysteine, being evaluated in populations at a high risk, such as the elderly, and subjects with heart failure, cancer, or AIDS.Citation[11]
Another sensitive and early indicator of malnutrition is atransferrinemia, a parameter revealed in many dialysis centers in the common evaluation of the iron status; however, the variations linked to malnutrition during therapy with erythropoietin and iron administration have not yet been well documented. Another available biochemical parameter is the growth factor (IGF-1); values of less than 300 mg/L are highly specific indicators of severe malnutrition.
Other indicators that should be evaluated in order to obtain an objective picture of the patient's nutritional status are predialytic values of azotemia, creatininemia, kaliemia, phosphatemia, and cholesterolemia; low pre-dialysis levels of azotemia and creatininemia should always be interpreted in the context of dialytic dose and intradialytic variations in the concentrations of urea. Predialysis hypocholesterolemia, hypopotassemia, and hypophosphoremia are usually valid indicators of malnutrition, for which, of course, there are no iatrogenic determinants. Finally, an important index is dietary protein intake (DPI), which can be obtained from the patient's diary or at an interview conducted to obtain information on the patient's diet; in the stable patient, it is correlated with the equivalent protein nitrogen appearance (PNA) or with the protein catabolic rate (PCR) normalized for kg of body weight.
Anthropometric Parameters
The principal anthropometric measures and tools used to evaluate the body composition in the population on hemodialysis are well known, but their sensitivity and reliability in detecting the early stages of nutritional disturbance have not yet been well documented. Because the percentages determined for body composition vary markedly, depending on the technique used, it is important to use the same evaluation system in each patient throughout the course of treatment. The principal instrumental analyses, dual X-ray photon absorptiometry (DEXA) and bioimpedenziometry (BIA), allow the determination of the thin mass, the fatty mass, and total body water. Unlike DEXA, which requires recourse to complex tools, BIA is easy to perform, non-invasive, and can be undertaken routinely and directly at the patient's bedside. The method has proven useful in evaluating variations in the corporeal composition, although it accurately determines only body water content. In fact, in patients on hemodialysis, the measures used are influenced by the different states of hydration in each case, and therefore must be accompanied by anthropometric measures appropriate for patients on dialysis. As they are easy to perform, anthropometric measures are currently the most widely used in determining corporeal composition. They are evaluated using plicometry which, however, is limited by the subjective nature of the evaluation and the quality of the picometer utilized. Another method, subjective global assessment (SGA), is clinically valid and useful in evaluating the proteic-energetic nutritional status of patients on dialysis, based on an objective examination and clinical history. The dietary history has been found useful, above all because it reveals the most deficient caloric quota in patients and, moreover, because it allows the identification not only of malnourished patients, but also, and above all, those at risk of developing malnutrition.
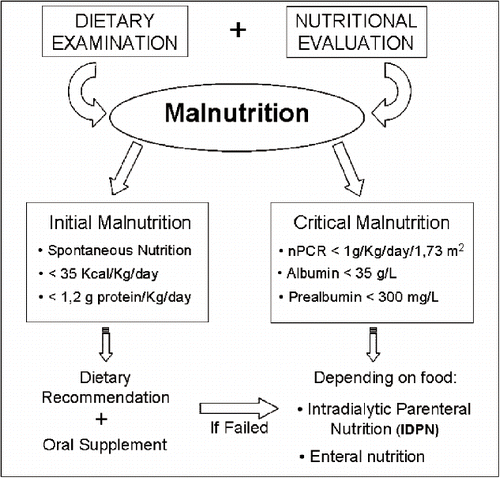
THERAPEUTIC APPROACH
Therapeutic approaches for preventing or treating malnutrition must be applied in the pre-dialysis phase of conservative therapy. The available strategies are numerous: some of them are simple, while some are more complex (see ).
Simple Approaches
These include dietary prescriptions, correction of acidosis, adequate therapy for co-morbid conditions, and the maintenance of an adequate dialysis dose. With an adequate or optimal dialysis dosage, anorexia and catabolism can be reduced, this being achieved through the correction of acidosis and/or resistance to anabolic hormones such as insulin, IGF-1, and GH. Optimal dialysis calls for a Kt/V greater than 1.2 and probably nearer to 1.4.
Regarding the type of dialysis given, it is known that the risk of inducing or worsening proteic malnutrition is increased in patients on peritoneal dialysis, due to the loss of proteins through the peritoneum. In fact, while proteic losses are minimal in patients on hemodialysis, they reach 5–15 g/die in CAPD, further increasing to as much as 20–25 g/die in case of peritonitis; following peritonitis, the reintegration of the losses can call for a period of as long as eight weeks. A category of patients at risk of developing malnutrition consists of the so-called “high-transporters”; it has been demonstrated that these patients lose a larger quantity of proteins with the peritoneal effluent, and are therefore at a higher risk of malnutrition than “low-transporters.” It is important to bear in mind that an inadequate dialysis dose can induce malnutrition.
Moderate and Complex Interventions
These consist of nutritional interventions and drug therapy. An attempt has been made to improve nutritional status by increasing the dietary uptake with recourse to dietary counselling and proteic-caloric supplementation. However, efforts are often thwarted by anorexia and altered palatability and gustatory acuity for foodstuffs. The dietary limitations for the elderly patient on dialysis should be reduced to a minimum, and hyperpotassemia and hyperphosphatemia should not be corrected by making dietary restrictions, which can limit the proteic uptake, but by evaluating the adequacy of dialysis and vascular access and utilizing pharmacologic therapy (phosphate binders). Patients on a hypoproteic diet during the pre-dialytic phase should be instructed regarding the need to increase their proteic consumption to a minimum of 1 to 1.3 g/kg/die.
Oral dietary supplementation has been examined in some studies, but the use of these supplementary food products is limited due to their poor palatability and high, non-refundable, costs.Citation[11]
Enteral nutrition has the advantage of allowing the daily administration of essential nutrients according to specific needs, and the possibility of utilizing concentrated formulas in order to obviate water overload. The findings reported in a meta-analysis conducted by Stratton et al. demonstrate the benefits of these pre-packed supplements in elderly patients on dialysis.Citation[12]
A European prospective randomized multicenter is now underway to evaluate patients on hemodialysis who have an insufficient diet and biological indicators of initial malnutrition in order to ascertain the efficacy of a concentrated supplement (Renilon, 2 kcal/mL–500 kcal/day); the findings should be available before the end of 2008.
Parenteral nutrition given during the dialysis session also has a positive effect, improving the nutritional status and reducing the mortality rate of patients on treatment.Citation[13]
Pharmacological Treatment
Numerous drugs have been utilized in the treatment of malnutrition in elderly patients, though without always yielding evident benefit.
Anti-emetics
Methoclopramide, which stimulates upper GI tract motility without affecting gastric, biliary, and pancreatic secretion, enhances the tonus of the lower esophageal sphincter and promotes gastric voiding. It is particularly appropriate for the gastro-paretic diabetic patient.
Hormones
The administration of rhIGF-1 twice a day is of potential toxicity (joint pain, nausea, hypoglycemia, cardiac arrhythmia). This has led to a preference for rhGH, which is of low toxicity and can be administered three times a week; in cases of infection, however, patients can become resistant to the anabolic action of rhGH. In some cases, rhGH has been associated with intradialytic parenteral nutrition.
Recently, moreover, the anabolic properties of androgens have been demonstrated in elderly patients with renal failure. Fatigue and physical capacity have been ameliorated with nandrolone.In elderly hemodialysis patients administered erythropoietin, Gascon et al. randomized subjects for the administration of 200 mg nandrolone a week for six months, interrupting erythropoietin in a group of 14 patients, and giving erythropoietin without nandrolone to another group of 19 patients. Patients who received nandrolone presented an increase in muscle mass of 2 kg and hemoglobin levels of 96 to 110 g/L. Plasma albumin levels decreased in the erythropoietin group, but were constant in the nandrolone group. In the nandrolone group, the lipid balance was modified, with an increase in triglycerides and a diminution in HDL-cholesterol. On the other hand, Lp-a, an important vascular risk factor, diminished. These results demonstrate that androgens have an effect on cardiovascular risk, and cause hirsutism, vocal change, and an increase in hepatic enzymes. Therefore, elderly men should undergo serial controls of prostatic markers, and androgens are clearly contraindicated in patients with prostate cancer.Citation[14]
Levocarnitine
Carnitine, an important co-factor in the intermediate metabolism, is accumulated in patients with terminal uremia in the form (inactive) of acylcarnitine; the losses with the dialytic fluid increase the secondary deficit in carnitine, which is expressed by a reduction in the cardiac ejection fraction, muscular exercise, and functional activity, and calls for an increase in the dose of erythropoietin. Scant data in the literature bear out that levocarnitine should be used in patients on hemodialysis. Notwithstanding the fact that the administration of levocarnitine can improve subjective symptoms (malaise, muscle weakness, intradialytic cramps, and hypotension) and quality of life, current evidence is insufficient to recommend its routine use. Therefore, while awaiting results from further randomized controlled clinical studies, the most valid available approach is to treat the resistance to erythropoietin.Citation[15]
Effects of Hemodialysis
Malnutrition can occur secondary to not only erroneous dietary choices or uremia, but may also depend on the level of tolerance of the patient to dialysis. In fact, the frequent finding of intradialytic episodes or in the immediate post-dialysis that negatively condition the hours following treatment can cause a reduction in appetite on the day of dialysis and lead to malnutrition. Several studies have demonstrated that there is an increased incidence of proteic-caloric malnutrition and depletion of the subcutaneous fat reserve in patients on dialysis, also in the presence of an apparently stable condition, and the protein and calories intake has been found to diminish as the anagraphic age increases. Moreover, although adequate hemodialysis treatment is a prerequisite for maintaining an adequate nutritional status, not all well-dialyzed patients are well nourished. To bear this out, an evaluation was made of hemodialysis patients' daily dietary intake, and it was found that it diminished on the day in which patients underwent dialysis. Moreover, when dietary intake was evaluated on the basis of grade of tolerance of dialysis, a progressive reduction was recorded, overall in the caloric quota, the reduction being inversely proportionate to an increase in score.
The effects of dialysis on malnutrition are contradictory. On the one hand, “dialytic dose” is an important factor that influences nutritional status in uremic patients; on the other, factors connected with dialysis contribute to the increased intake of proteins with respect to the duration of chronic renal failure. Different mechanisms underlie hemodialysis-induced protein metabolism alterations, such as loss of substrates associated with dialysis and the promotion of net/marked catabolism induced by the dialytic process.
The hemodialysis-induced catabolism is due to a reduction in protein synthesis probably occurring in the muscle rather than to an increase in proteolysis. A reduction in synthesis is probably related to the reduced availability of amino acids in the tissues during treatment, which occurs during the dialytic session. However, Lofberg et al. observed no significant variations in the muscular concentrations of amino acids during hemodialysis, suggesting that other factors may underlie the reduction in proteic synthesis.Citation[16]
No direct correlation has yet been found between albuminemia and dialytic dose, nor have interventions been proven effective in increasing plasma concentrations; however, it is likely that an increased prevalence of hypoalbuminemia in the same population of patients expresses a diffuse under-dialysis. Epidemiological studies have demonstrated a correspondence between increased mortality and low dialysis doses, while an improvement in survival and a simultaneous increase in patients' albuminemia occurred when the dialytic dose was gradually modified until it was adequate. It is therefore possible that, in the long term, inadequate dialysis causes a state of proteic malnutrition, which concurs in increasing the mortality rate.
Effects of Peritoneal Dialysis
Although substantial glucose absorption occurs during the course of peritoneal dialysis, the frequency of proteic malnutrition with the preservation or increase in body fat may be similar to or even higher in patients on peritoneal dialysis than that in those on hemodialysis. Patients on peritoneal dialysis show blood and tissue levels of essential amino acids that are even lower than those present in subjects on hemodialysis. This suggests that a response occurs in the proteic turnover to a state of depletion or that there is a reduced liberation in the circulation of amino acids from the tissues consequent to hyperinsulinemia. During hyperinsulinemia, there is a reduction in proteolysis, and the net proteic balance is similar to that of controls. When insulin is infused together with amino acids, proteic synthesis increases, and the proteic balance becomes positive. Overall, the data from these studies suggest that the response to the proteic turnover in patients on CAPD is expressed in the activation of mechanisms that can minimize the protein loss, and that the proteic metabolism response to insulin and amino acids is normal.
The diminution of muscle protein synthesis, correlated with a fall in the blood levels of essential amino acids, is obviated if use is made of solutions containing both glucose and amino acids. These studies indicate that the hyperinsulinemia occurring during peritoneal dialysis after the absorption of glucose causes an antiproteolitic action that is, however, ineffective because it is counterbalanced by a parallel diminution in the availability of amino acids for protein synthesis. The consequent proteic turnover is diminished and less efficient. On the other hand, the combined use of dextrose and amino acids results in a cumulative effect after the suppression of proteolysis (induced by insulin) and the stimulation of the muscle protein synthesis (induced by the non-reduced availability of amino acids). These data suggest that in patients on peritoneal dialysis, a reduced uptake of nutrients leads to a greater maintenance of muscle mass if dialysis liquids containing both glucose and amino acids are administered.Citation[17],Citation[18]
Despite the improvements made to dialysis techniques, the nutritional condition of elderly patients on dialysis for chronic renal failure remains a cause for concern. In this patient category, it is therefore mandatory to ensure the daily supervision of nutritional status and early control when the first signs of malnutrition appear.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Garg AX, Blake PG, Clark WF, et al. Association between renal insufficiency and malnutrition in older adults: Results from the NHANES III. Kidney Int. 2001; 60: 1867–1874
- Clèdes J, Perrichot R, Hanrotel C, Smiela JP. Alteration de l'etat nutritionnel au cours de l'aggravation de l'insuffisance rénale chronique des sujets agés. Néphrologie. 1999; 20(Suppl. 1)111
- National Kidney Foundation. Kidney Disease Outcome Quality Initiatives (DOQI). Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 2000; 35(Suppl. 2)S56–S65
- Holland DC, Lam M. Predictors of hospitalisation and death among pre-dialysis patients: A retrospective cohort study. Nephrol Dial Transplant. 2000; 15: 650–658
- Stevinkel P, Heimburger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation, and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000; 15: 953–960
- Manno C, Pertosa G, Montinaro V, et al. I livelli plasmatici di leptina aumentano nei pazienti in emodialisi indipendentemente dalla malnutrizione. Giorn It Nefrol. 2001; 1: 24–49
- Raj DS, Welbourne T, Dominic EA. Glutaminekinetic and protein turnover in end stage renal disease. Am J Physiol Endocrinol Metab. 2005; 288: E37–E46
- Veeneman JM, Kingma HA, Boer TS. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Met. 2003; 284: E954–E965
- Bellizzi V, Di Iorio BR, Terracciano V. Daily nutrient intake represents a modifiable determinant of nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2003; 18: 1874–1881
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. Mar, 2005; 81(3)543–554
- Hiroshige K, Iwamoto M, Kabashima N, Mutoh Y, Ohtani A. Prolonged use of intradialytic parenteral nutrition in elderly malnourished chronic hemodialysis patients. Nephrol Dial Transplant. 1998; 13: 2081–2088
- Stratton RJ, Bircher G, Fouque D. Multinutrient oral supplements and tube feeding in maintenance dialysis: A systematic review and meta-analysis. Am J Kidney Dis. 2005; 46: 387–405
- Cano N. Intradialitic parenteral nutrition: Where do we go from here?. J Renal Nutr. 2004; 14: 3–5
- Gascon A, Belvis JJ, Erisa F. Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis. Geriatr Nephrol Urol. 1999; 9: 67–72
- Manno C, Strippoli GFM, Pertosa G, et al. Valutazione e trattamento della malnutrizione in emodialisi. Giornale Italiano di Nefrologia. 2001; 18(5)542–551
- Lofberg E, Essen P, McNurlan M, et al. Effect of hemodialysis on protein synthesis. Clin Nephrol 2000; 54: 284–294
- Castellino P, Luzi L, Giordano M, et al. Effects of insulin and amino acids on glucose and leucine metabolism in CAPD patients. J Am Soc Nephrol. 1999; 10: 1050–1058
- Buemi M, Senatore M, Corica F, Aloisi C, Romeo A. Tramontana D, Frisina N. Diet and arterial hypertension: Is the sodium ion alone important?. Med Res Rev. Jul, 2002; 22(4)419–428