Abstract
Urofacial (Ochoa) syndrome is a rare autosomal-recessive disorder that features an unusual “inverted” facial expression, such that patients appear to be crying when they smile. This syndrome also involves serious urinary tract disorders, though the diagnosis may be missed because of variability of these problems and failure to recognize the characteristic facial grimacing. The urinary issues usually result in enuresis, urinary tract infection, and hydronephrosis, and some severely affected patients become hypertensive and progress to end-stage renal disease. Early diagnosis is very important for management of urinary problems and best prognosis in these patients. We report the first published case of urofacial syndrome in Turkey. The patient was diagnosed at 16 years of age, after having been followed with the diagnosis of recurrent urinary tract infection and vesico-ureteral reflux. Physicians should keep this syndrome in mind for any patient who presents with dysfunctional voiding, particularly in countries with high rates of consanguineous marriage.
INTRODUCTION
Urofacial (Ochoa) syndrome (UFS) is a rare autosomal-recessive disorder. Although a potential gene for this syndrome has been mapped to chromosome 10q23–q24, the pathogenesis remains unknown.[Citation1,Citation2] Approximately 65% of patients with UFS have moderate to severe constipation; consequently, this condition is also considered a dysfunctional voiding or dysfunctional elimination syndrome.[Citation3] The characteristics of UFS are typical facial expression, dysfunctional voiding that may result in enuresis, urinary tract infection, and hydronephrosis, leading to renal damage.
The facial dysmorphism is a characteristic “inverted” expression such that, when these patients smile, they appear to be crying. This unusual facial expression (grimacing) is highly diagnostic, and the most important clue to identifying UFS. This syndrome was considered extremely rare; however, it may be more common than previously thought, and it is likely that cases are missed because of failure to recognize the characteristic grimacing. Here we report the first case of UFS to be diagnosed in Turkey.
CASE REPORT
A 16-year-old girl was referred to our hospital for evaluation of facial dysmorphism, mandibular prognathism, and urinary problems. She had been born via spontaneous vaginal delivery at term after an uncomplicated pregnancy. During infancy, she had been healthy and had met all developmental milestones. The patient's father and mother were consanguineous (first cousins), and were 46 and 38 years old, respectively.
From 1 year of age through 10 years of age, the patient had had recurrent urinary tract infections. At age 10, she had been admitted to a nephrology unit at another hospital for evaluation of urinary tract infection and enuresis. Ultrasonography at that time showed moderate bilateral hydronephrosis with the left kidney more severely affected. Voiding cystourethrography revealed a trabeculated bladder and urinary retention, and cystoscopic examination showed grade V reflux on the left. Intravenous pyelography revealed delayed excretion of contrast material via the left system and left hydroureteronephrosis and trabeculation of the bladder wall. Radioisotopic scintigraphy with dimercaptosuccinic acid was also done, and this showed asymmetry with greatly reduced uptake of the isotope by the left kidney (29.5%) compared to the right (70.5%). On the suspicion of bladder dysfunction, urodynamic studies were performed. These revealed a poorly compliant and hyperactive bladder, high voiding pressures, and dyssynergia between the detrusor and sphincter muscles.
At age 10, the patient underwent an operation for vesicoureteral reflux. She was then started on a maintenance regime of clean intermittent catheterization (CIC), antibiotic prophylaxis, anticholinergics, and muscle relaxants. Although these steps were followed, several episodes of urinary tract infection ensued. All of these responded to antibiotic treatment. The problem of enuresis diurna resolved completely, and the frequency of nighttime bedwetting decreased.
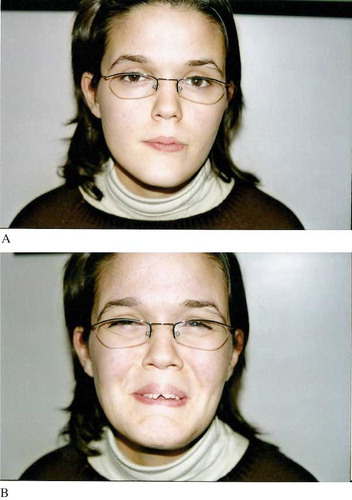
At time of referral to our institution (16 years of age) for facial dysmorphism, mandibular prognathism, and urinary problems, the patient showed the characteristic inverted facial expression (see ). However, there was no clinical evidence of eyelid weakness, and examination showed normal facial nerve function. Facial nerve electromyography also revealed nothing abnormal. Spinal and cranial magnetic resonance imagings were also done, and no cranial or spinal neuropathology was evident. Ultrasonography (USG) showed bilateral hydroureteronephrosis, with the left kidney more advanced. Post-voiding USG revealed dilatation of the left and right renal pelvis and calyces, but this was less prominent than before voiding. Bladder distension was also prominent after voiding. Radioisotopic scintigraphy with dimercaptosuccinic acid showed normal cortical uptake of the isotope in the right kidney (64%) and reduced cortical uptake of the isotope in the left kidney (36%). The left kidney was smaller than the right and exhibited scar formation. The patient's glomerular filtration rate was 167 mL/min/1.73 m2. Routine biochemistry results, including urea and creatinine levels, were within normal limits. For management, she was carrying out self-CIC four times daily and taking antibiotic prophylaxis and anticholinergic treatment (propantheline bromide 3 mg/kg/day).
DISCUSSION
Urofacial (Ochoa) syndrome was first described in 1979 by Elejelde and Ochoa in the Colombian population.[Citation4,Citation5] Reports from other countries and regions, such as Kuwait,[Citation6] the United States,[Citation7] and Europe,[Citation8,Citation9] were later published. In addition, researchers have demonstrated genetic homogeneity in the reported North America and French families.[Citation7,Citation8] Our patient is the first case reported from Turkey.
As noted, the main feature of UFS is inverted facial expression, which was clearly evident in our patient. This abnormality is not a structural facial defect; it is dysfunctional facial expression. The face of a typical UFS patient appears similar to that of an unaffected individual at rest, but when a UFS patient laughs, their expression suggests they are crying, sad, or feeling discomfort or pain. When a UFS patient is feeling sad or cries, this is not evident in the face, and they appear be feeling fine. The facial expression abnormality has been postulated to be due to facial muscle weakness secondary to bilateral partial nerve paresis, but the precise relationship is not yet well understood.[Citation1,Citation[5],Citation9] None of the UFS cases described in the literature have featured any detectable neurological abnormality. Thus, it seems that the facial alterations in UFS constitute localized or specific dysfunction of facial expression. As well, the more in-depth neurological and EMG studies performed on some UFS patients have failed to demonstrate any neuromuscular deficits or abnormalities.[Citation1] We performed cranial magnetic resonance imaging and facial nerve EMG in our case and found no neurological pathology.
Dysfunctional emptying (urine and/or feces) is another frequent component of UFS, and is also referred to as dysfunctional elimination syndrome. Affected patients exhibit bladder voiding dysfunction due to lack of coordination of detrusor muscle contraction and urethral sphincter relaxation.[Citation1,Citation9] Authors have suggested that because the urinary and gastrointestinal tracts share the same embryologic origin (endoderm) and the same innervation (sacral pelvis plexus), any condition that affects one system can significantly affect the other.[Citation1,Citation10] Micturition is a reflex coordinated by the autonomic and somatic systems and originating from the brainstem and the sacral portion of the spinal cord. Dyscoordination of these systems results in detrusor paralysis and detrusor-sphincter dyssynergia, also known as “neurogenic bladder.” However, a lack of coordination of detrusor contraction and sphincter relaxation can also occur in the absence of neurological problems and result in functional obstruction of the bladder outlet. This “non-neurogenic neurogenic bladder” is what UFS patients exhibit. The exact cause and pathogenesis of this condition are not well understood.[Citation1] Based on studies of patients with pathological laughing and crying, authors have suggested that the so-called “laughing and crying center” must be located above the facial and respiratory nuclei, somewhere in the upper pons and midbrain.[Citation11] Evidence strongly suggests that facial expression is under central nervous system control at brainstem level. Although most reported cases of dysfunctional voiding in UFS have had no identifiable organic neurological cause, the anatomical proximity of the micturition center and facial nerve origin in the brainstem have led to the theory that there may be a common neurological cause at this site.[Citation1]
The goals of treatment for UFS cases is to restore balanced bladder emptying and prevent further damage to the upper urinary tract. Urodynamic studies are very important for detecting abnormalities and guiding drug therapy, including anticholinergics and antibiotic prophylaxis. Additionally, CIC is very important in these patients.
There are several important clues to the diagnosis of UFS, namely, dysfunctional voiding and its complications (such as vesicoureteral reflux), family history of the syndrome, consanguineous parents, and typical facial characteristics. The facial gestalt may be an immediate diagnostic signal, and early recognition of this syndrome is key to adequately treat or prevent damage to the upper urinary tract. Physicians should consider UFS as a possible diagnosis in any case of dysfunctional voiding, particularly in countries with high rates of consanguineous marriage.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was presented as a poster at the 14th Congress of the International Pediatric Nephrology Association in Budapest, Hungary, 31 August–4 September, 2007.
REFERENCES
- Ochoa B. Can a congenital dysfunctional bladder be diagnosed from a smile? The Ochoa syndrome updated. Pediatr Nephrol. 2004; 19: 6–12
- Wang CY, Davoodi-Semiromi A, Shi JD, et al. High resolution mapping and mutation analyses of candidate genes in the urofacial syndrome (UFS) critical region. Am J Med Genet. 2003; 119A: 9–14
- Gorlin RJ, Cohen MM, Jr, Hennekam RCM. Syndromes with unusual facies: Other syndromes. Syndromes of the head and neck.4th. Oxford University Press, Inc, Oxford 2001; 1054–1055
- Elejalde BR. Genetic and diagnostic considerations in three families with abnormalities of facial expression and congenital urinary obstruction: The Ochoa syndrome. Am J Med Genet. 1979; 3: 97–108
- Ochoa B, Gorlin R. Urofacial (Ochoa) syndrome. Am J Med Genet. 1987; 27: 661–667
- Teebi AS, Hassoon MM. Urofacial syndrome associated to hydrocephalus due to aqueduct stenosis. Am J Med Genet. 1991; 40: 199–200
- Wang C-Y, Shi J-D, Huang Y-Q, et al. Construction of a physical and transcript map for a 1-Mb genomic region containing the urofacial (Ochoa) syndrome gene on 10q-23q24 and localization of the disease gene within two overlapping BAC clones (<360 kb). Genomics. 1999; 60: 12–19
- Chauve X, Missirian C, Malzac P, et al. Genetic homogeneity of the urofacial (Ochoa) syndrome confirmed in a new French family. Am J Med Genet. 2000; 95: 10–12
- Garcia-Minaur S, Oliver F, Yanez JM, Soriano JR, Quinn F, Reardon W. Three new European cases of urofacial (Ochoa) syndrome. Clin Dysmorphol. 2001; 10: 165–170
- Feng WC, Churchill BM. Dysfunction elimination syndrome in children without obvious spinal cord diseases. Pediatr Clin North Am. 2001; 48: 1489–1504
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughing and crying. Brain. 2001; 124: 1708–1719