Abstract
A case of substantial debris captured by an embolic protection device placed in a patient's transplanted renal artery during common iliac artery angioplasty and stent placement is presented. To the authors knowledge, no previous literature exists pertaining to renal artery protection in patients with a history of a heterotopic renal transplant undergoing aortoiliac endovascular interventions.
INTRODUCTION
The most frequent vascular complication after renal transplantation is transplant renal artery stenosis, with a reported incidence of 2–8%.[Citation1–3] The treatment of choice for this complication is angioplasty with stent placement for recurrent or residual stenosis.[Citation4] More recently, embolic protection devices have been studied in patients with renal artery stenosis, but no consensus has been established guiding usage.[Citation5–7]
Stenosis of the iliac artery proximal to the transplant renal artery is less common, occurring in only 1.5% of transplant recipients.[Citation8] Iliac angioplasty with stenting has been performed in a small number of these patients with good results.[Citation8,Citation9] Currently, no literature exists on the use of EPDs during iliac artery angioplasty.
CASE REPORT
The patient is a 56-year-old African-American woman with a past medical history of hypertension, hyperlipidemia, coronary artery disease, type II diabetes, and end stage renal disease due to diabetic nephropathy. In 2003, she underwent a cadaveric kidney transplant with renal artery anastomosis to the left common iliac artery. She had primary nonfunction of the allograft in the initial post-operative period and required dialysis for several days until her renal function improved, eventually establishing her new baseline serum creatinine at 1.1 mg/dL. The patient's kidney function remained stable for approximately five years until an admission for an exacerbation of her congestive heart failure. At this time, her creatinine was 2.1 mg/dL with systemic signs and symptoms of volume overload.
Over the next four months, the patient's creatinine rose to 5.4 mg/dL despite close surveillance and adjustment of medications by the nephrology staff, and reinitiation of intermittent dialysis was required. During the patient's workup, a biopsy of the renal allograft was obtained that showed mild to moderate chronic allograft nephropathy and acute tubular necrosis. Although chronic allograft nephropathy was identified, the findings did not appear adequately severe to fully explain the profound renal dysfunction. Because of the patient's rather precipitous decline in renal function over the prior six months, an acute, potentially reversible cause was still felt to be present. Because both urinary obstruction and intrinsic renal parenchymal disease had been ruled out as primary etiologies for the renal dysfunction, renal circulation was then assessed by renal duplex scan. Tardus parvus pulses (prolongation of the systolic upstroke and attenuation of the systolic peak) with low amplitude waveforms were observed within the left external iliac artery as well as the main renal transplant artery, suggestive of left common iliac artery stenosis. The patient was then evaluated by the vascular surgery service, and arteriography with possible aortoiliac intervention was recommended.
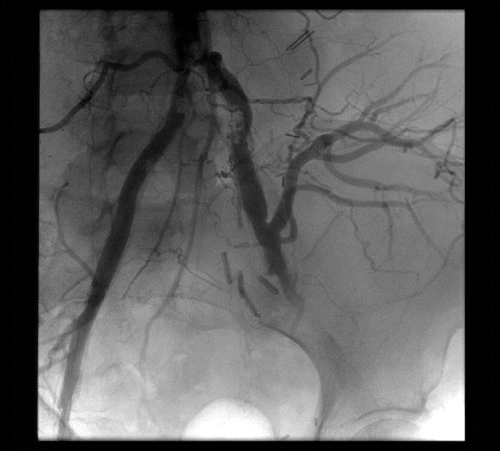
On the day of the procedure, after optimal medical management and intermittent dialysis, the patient's serum creatinine had decreased to a post dialysis value of 3.2 mg/dL. In the endovascular suite, bilateral retrograde access was obtained in the right and left common femoral arteries with 7-French sheaths. Hydrophilic wires were passed through the aorta, followed by a flush catheter. Flush aortogram was obtained in a gentle right anterior oblique projection. The abdominal aorta was heavily calcified with severe terminal aortic and bilateral common iliac artery stenosis (see ). The renal allograft was visualized off the distal common iliac artery and displayed a normal nephrogram. Distal to the transplant, the left internal iliac artery was occluded, and moderate stenosis was visualized in the left external iliac artery.
Figure 1. Aortogram with flush catheter in distal abdominal aorta demonstrating terminal aortic occlusive disease.
Systemic heparin was given at a dose of 70 units/kg. A 6 mm embolic protection device (SpideRX, eV3 Inc., Minneapolis, Minnesota, USA) was passed from the left transfemoral sheath and deployed into the left renal transplant artery (see ). Bilateral angioplasty of the common iliac arteries and the terminal aorta were then undertaken using 6- × 40-mm balloons (Opta-pro, Corids Endovascular, Warren, New Jersey, USA). Bilateral terminal aortic and common iliac artery stenting was accomplished with bilateral 8- × 57-mm balloon-expandable stents (Express LD, Boston Scientific Corporation, Natick, Massachusetts, USA). Completion aortography showed appropriate positioning of the stents and resolution of the terminal aortic disease (see ). The filter was removed at the conclusion of the intervention. There was visible atheroembolic material within the filter.
Figure 2. Deployment of EPD in the left renal transplant artery; bilateral balloon angioplasty of the terminal aorta and common iliac arteries.
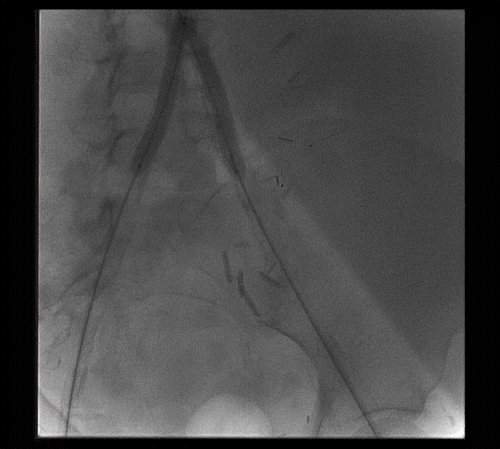
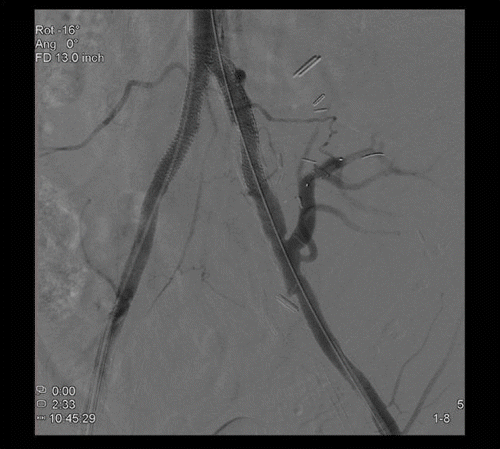
Figure 3. Completion aortogram demonstrating successful angioplasty and stent deployment with a good technical result.
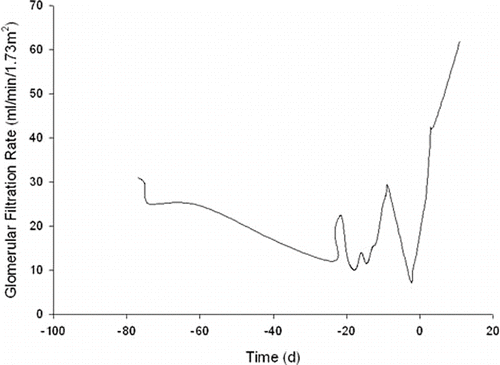
The patient tolerated the procedure well, and 12 hours post-procedure, her creatinine had decreased from 3.18 mg/dL to 2.71 mg/dL then 2.62 mg/dL at 24 hours. On post-procedure day 2, the creatinine was 1.9 mg/dL and continued to decline until her baseline creatinine of 1.1 mg/dL was reached 10 days after the intervention. A graphic depiction of the patient's estimated glomerular filtration rate (eGFR) around the time of intervention is shown (see ).
DISCUSSION
Stenosis of the iliac artery proximal to the transplant renal artery is an uncommon but treatable disease. As the age of renal transplant patients continues to increase, it can be assumed that the incidence of aortoiliac atherosclerosis will also increase in these patients.[Citation10] Blood supply to the graft should be restored to stabilize or improve renal function and arterial hypertension. Endovascular therapy is the treatment of choice for unilateral or bilateral common iliac artery stenosis based on the Trans-Atlantic Inter-Society Consensus (TASC) guidelines.[Citation11]
Intuitively, EPDs are appealing for their potential to prevent embolic debris from reaching and causing distal obstruction. Currently there are eight distal or EPDs approved by the U.S. Food and Drug Administration for marketing in the United States. These devices are commonly used during endovascular modalities, especially in coronary and carotid artery interventions where the ability to trap embolic material and reduce complications has been clearly demonstrated. Utility of EPD prophylaxis in other vascular beds has not been substantiated.
The use of these protective devices during renal artery intervention has yielded mixed results with regard to improved renal function.[Citation5,Citation6] However, in one of the most commonly referenced studies of EPD use during renal artery stent placement, the authors noted renal function improved or stabilized in 95% of patients compared to 75% of patients with stent alone.[Citation11] In this same study, the incidence of retrievable debris was reported to occur in 65% of cases; other authors have observed rates of 100%.[Citation12–14] Embolic signals have also been observed via Doppler ultrasound at the level of the common femoral artery in 90% of patients undergoing iliac artery angioplasty despite the authors attempt to limit this complication with pre-procedure aspirin and intra-procedure heparin administration.[Citation15]
Due to the lack of randomized prospective studies, the benefit provided by an EPD is uncertain. Early experience has demonstrated that the device can be used safely and effectively in patients with appropriate anatomy. In this rapidly expanding specialty, future device improvements should aid in achieving technical success in a greater number of patients. Lower-profile devices should also allow accurate placement with less disruption of plaques and fewer atheroembolic particles. Such improvements should render procedures using EPDs in the renal arteries safer and offer the potential to further improve renal function outcomes. While the early data on EPDs is promising, much remains to be learned about these devices in the renal arteries, especially in situations where the intervention required is more proximal, as in our case.
Given the critical importance of renal function preservation in our patient with a failing renal transplant, we elected to use an EPD. Furthermore, we recognized that the bulky terminal aortic disease in this patient put her at high risk for atheroembolic complications during the intervention. The fact that visible atheromata were captured in the EPD supported our theory, and undoubtedly the patients' renal function would have been further compromised without its use.
ACKNOWLEDGMENTS
All authors declare no conflict of interest.
REFERENCES
- Patel NH, Jindal RM, Wilkin T, et al. Renal arterial stenosis in renal allografts: Retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology 2001; 219(3)663–667
- Wong W, Fynn SP, Higgins RM, et al. Transplant renal artery stenosis in 77 patients—does it have an immunological cause?. Transplantation 1996; 61(2)215–219
- Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick AC. Post-transplant renal artery stenosis: Impact of therapy on long-term kidney function and blood pressure control. J Urol. 1996; 155(6)1860–1864
- Beecroft JR, Rajan DK, Clark TW, Robinette M, Stavropoulos SW. Transplant renal artery stenosis: Outcome after percutaneous intervention. Journal of Vascular and Interventional Radiology. 2004; 15(12)1407–1413
- Cooper CJ, Haller ST, Colyer W, et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008; 117(21)2752–2760
- Dubel GJ, Murphy TP. Distal embolic protection for renal arterial interventions. Cardiovasc Intervent Radiol. 2008; 31(1)14–22
- Hagspiel KD, Stone JR, Leung DA. Renal angioplasty and stent placement with distal protection: Preliminary experience with the FilterWire EX. Journal of Vascular and Interventional Radiology. 2005; 16(1)125–131
- Voiculescu A, Hollenbeck M, Plum J, et al. Iliac artery stenosis proximal to a kidney transplant: Clinical findings, duplex-sonographic criteria, treatment, and outcome. Transplantation 2003; 76(2)332–339
- Aslam S, Salifu MO, Ghali H, Markell MS, Friedman EA. Common iliac artery stenosis presenting as renal allograft dysfunction in two diabetic recipients. Transplantation. 2001; 71(6)814–817
- Danovitch GM, Cohen DJ, Weir MR, et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant. 2005; 5(4 Pt 2)904–915
- Galaria II, Davies MG. Percutaneous transluminal revascularization for iliac occlusive disease: Long-term outcomes in transatlantic inter-society consensus A and B lesions. Ann Vasc Surg. 2005; 19(3)352–360
- Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: Early experience. Journal of Vascular Surgery. 2003; 38(5)962–968
- Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int. 2006; 70(5)948–955
- Henry M, Klonaris C, Henry I, et al. Protected renal stenting with the PercuSurge GuardWire device: A pilot study. Journal of Endovascular Therapy: Official Journal of the International Society of Endovascular Specialists 2001; 8(3)227–237
- Al-Hamali S, Baskerville P, Fraser S, Walters H, Markus HS. Detection of distal emboli in patients with peripheral arterial stenosis before and after iliac angioplasty: A prospective study. Journal of Vascular Surgery 1999; 29(2)345–351