Abstract
Aim. The objective of the present study was to investigate the effect of low-dose erytropoesis-stimulating agents (ESA) on the development of peritoneal fibrosis in chlorhexidine gluconate-induced peritoneal sclerosing rats and to assess the peritoneal tissue levels of MMP-2 and TIMP-2, which may be regarded as factors in the development of peritoneal fibrosis. Subjects and methods. Twenty-four Wistar albino rats were divided into three groups. The control group received 0.9% saline (3 ml/d) intraperitoneally, the CH group received 3 ml daily injections of 0.1% chlorhexidine gluconate (CH) intraperitoneally, and the CH+ESA group received 3 ml daily injections of 0.1% CH intraperitoneally and epoetin beta (3 × 20 IU/kg/week) subcutaneously. On the twenth-first day, rats were sacrificed, and parietal peritoneum samples were obtained from the left anterior abdominal wall. Pathological samples were examined using Hematoxyline & Eosin (HE) stains. The thickness, vascular proliferation, and inflammation were determined by light microscopy. MMP-2 and TIMP-2 were studied immunohistochemically by monoclonal antibody staining. Results. Inflammation, vascular proliferation, and fibrotic area percentages were not statistically significant between groups. Histopathologically control, CH, CH+ESA groups peritoneal thickness were 8.02 ± 2.89, 146.74 ± 26.1, and 48.12 ± 16.8 micrometers, respectively. The decrease in thickness of parietal peritoneum in CH+ESA group was statistically significant when compared to CH. Immunohistochemically, interferon was shown to decrease MMP-2 expression on parietal peritoneum than group CH, but has no effect on TIMP-2. Discussion. Low-dose ESA histopatologically reduces peritoneal fibrosis induced by chlorhexidine gluconate. However, from dosage and duration points of view, we need extended clinical and experimental studies.
INTRODUCTION
Peritoneal fibrosis develops as a result of different kinds of injuries to peritoneal dialysis patients, such as usage of inappropriate solutions, peritonitis attacks, uremia, and continuous inflammation.[Citation1] Uremia enhances the production of end products of peritoneal carbonyl stress and glycosylation.[Citation2] Peritoneal inflammation is constantly provoked by peritonitis attacks and PD solutions. The additive effect of these contributions, combined with the peritoneal mesothelial cells and the cytokines secreted from other cell clusters (e.g., peritoneal macrophages, fibroblasts, monocytes, and neutrophils), results in the development of peritoneal fibrosis. The main pathogenetic mechanism of peritoneal fibrosis is accumulation of an excess amount of extracellular matrix deposits by the overproduction of the mesothelial cells and the fibroblasts.[Citation1–3]
Erythropoiesis-stimulating agents (ESA) have been used in the treatment of anemia of millions of patients. Recently cytoprotective effect of ESA has also been shown.[Citation4]
It has been shown that in cases of head trauma, it diminishes the area of cerebral infarct by 75% due to subarachnoid bleedings.[Citation5] In cases of coronary ischemia, it corrects the functional outcome and left ventricle volume by 50% by decreasing cardiomyocyte loss.[Citation6] In addition, in cases of continuous cyclosporine toxicity, it has proven renoprotective effect in terms of interstitial fibrosis and inflammation.[Citation7]
It has been shown in several studies that ESA decreases the expression of TGF-b mRNA and apoptotic cell death, increases mitotic activity of the renal tubular cells, and, at low doses, does not alter hemoglobin or hematocrit levels.[Citation4,Citation[8],Citation9]
There is no study yet to show the effect of low-dose ESA on peritoneal fibrosis patients.
In this study, we plan to evaluate the effect of low dose ESA on peritoneal fibrosis and peritoneal tissue immunohistochemically over the action MMP-2 and TIMP-2 on rats with peritoneal fibrosis induced with the action of 15% ethyl alcohol and 0.1% KH dissolved in salty water.
MATERIALS AND METHODS
This study has been undertaken with the approval of the Ethical Committee of Experimental Animal Investigations. For homogenization of study groups, a total of 24 female Wistar albino rats weighing between 200–230 grams were used. The rats were housed in multidisciplinary laboratory for fourteen days in standard hutches, with four per hutch, and standard food and water provided.
The development of infections and systemic reactions other than peritoneal sclerosis or any other reason of morbidity comprises the exclusion criteria.
The rats included in the study were weighed before study and divided into three groups (i.e., eight rats per group). The duration of the study was 12 days. To induce peritoneal fibrosis, we used a model described by Ishii and et al.[Citation10] that composed of 0.1% CH and 15% ethyl alcohol dissolved in saline prepared in aseptic era. Epoetin beta (Neorecormon, F. Hoffmann-La Roche Ltd., Basel, Switzerland) was used as 1000 IU/0.3 ml. The three groups were as follows:
Control group. Rats were given intraperitoneal injection of 0.9% saline (3 mL/200g) through a 21-gauge needle for twenty days.
Chlorhexidine gluconate group (CH). Rats were given daily intraperitoneal injections of 15% ethyl alcohol/0.1% CH in saline (3 mL/200g) through a 21-gauge needle for twenty days.
Erythropoiesis-stimulating agent group (ESA). Rats were given the same as the CH group plus epoetin beta (3×20 IU/kg/week) subcutaneously through a 21-gauge needle.
All intraperitoneal applications were performed through the right lower quadrant of abdomen.
On the twenty-first day, rats were sacrificed by using ether in toxic dose. Afterward, they were weighed, and parietal peritoneum was sampled from the left anterior abdominal wall. Samples were divided into two, and the samples for pathological examination were fixed with 10% formaldehyde. The samples for biochemical examination were kept in tubes at −80°C.
Histopathologic Analysis
From formaldehyde-fixed samples, vertical sections of 3 mm thickness were prepared, embedded in paraffin, and stained with hematoxylin-eosin (H&E). The thickness of parietal peritoneum, vasculopathy, and inflammation were examined by light microscopy.
Light Microscopic Examination
To measure peritoneal thickness at sections stained with H&E, microscopic images were transferred to a computer through a camera (Olympus BX50, Olympus Optical Co, Tokyo, Japan). Median of thicknesses of parietal peritoneum at ten sites was measured using image analysis software.
Inflammation was scored semi-quantitatively in H&E stained sections accordingly: 0, no inflammation; 1, mild; 2, moderate; and 3, severe. Vasculopathy was scored as follows, modified from Williams et al.[Citation5]: 0, normal; 1, subendothelial hyalinization; 2, luminal irregularity and stenosis; 3, luminal obstruction.[Citation11–13]
Immunohistochemical Analysis
Sections prepared on slides with poly-L-lysine were stained with MMP-2 Neomarkers (72 kDa collagenase IV) Ab-1 (Clone CA-4001) (1: 100, 200 mcg/mL), and TIMP-2 Ab-5 Neomarkers (prediluted, Clone 3A4) (Cat MS-1485-R7 7 ml) immunohistochemical stains. Placental tissue for MMP-2 and colonic tumoral tissue for TIMP-2 were used as positive controls. The expression was evaluated by light microscopy, by giving scores for the density and intensity from 0 (none) to 4 (extensive-strong). The multiplication of these gave a score from 1 to 16. Values equal and less than 4 represented weak staining and higher values represented intense staining.[Citation14]
Statistical Analysis
We used nonparametric methods for statistical analysis, as rat numbers in the groups were low. Kruskal-Wallis variance analysis was used to determine the difference between groups. Mann-Whitney U test was used in the case of significant difference to determine which group caused the difference. p < 0.05 denoted statistical significance. Values were presented as mean ± standard deviation.
RESULTS
Evaluation of the Study Groups
All rats included completed the study. On the first day of the study, ten minutes after injection of the first dose, rats in the CH and ESA groups developed tachypnea, somnolence, and difficulty in walking. Almost one hour later, respiration patterns and walking improved. From the second day on, after each application, they developed the same symptoms for three or four hours. We did not observe these findings in control group.
We observed macroscopically parietal peritoneal thickness and adhesions over the liver and abdominal wall in rats in CH and ESA groups compared to control group.
Histopathological Analysis
Peritoneal thicknesses, inflammation scores, and vascularization scores were shown as mean ± SD in .
Table 1 Statistical analysis of groups for peritoneal thicknesses, inflammation score, and vascularization score area percentages as mean ± SD
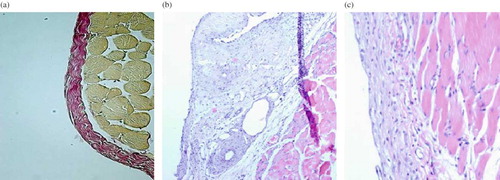
Peritoneal thickness was significantly less in the ESA group compared to the CH group (p < 0.05). Increase in thickness of parietal peritoneum in bot ESA and CH groups was significant compared to control group (p < 0.001). Histopathologic images of peritoneal thickness were shown (see ).
Figure 1. Histopathologic images of peritoneal thickness: (A) Control group, ×20, (B) CH group, ×20, (C) ESA group original magnification, ×20.
Analysis of inflammation score revealed that in the CH group rats, there was mild inflammation in two, moderate inflammation in four, and severe inflammation in two rats. In the ESA group rats, there was mild inflammation in five and moderate inflammation in three of rats. There was no inflammation in control group rats. The difference between CH and ESA groups compared to control group was statistically significant (p < 0.001).
Vasculopathy was detected in two CH group rats in the peritoneal tissue, and in one ESA group rat. We did not detect vasculopathy in the control group. There was no difference between groups according to mean vascularization scores (p > 0.05).
Immunohistochemical Analysis
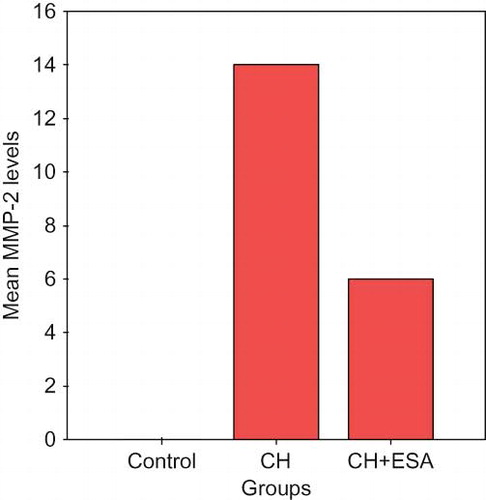
The increase in MMP-2 scores at peritoneal tissue in CH and ESA groups was significant statistically compared to the control group (p < 0.001). MMP-2 was suppressed in ESA group compared to CH group (p < 0.05; see ).
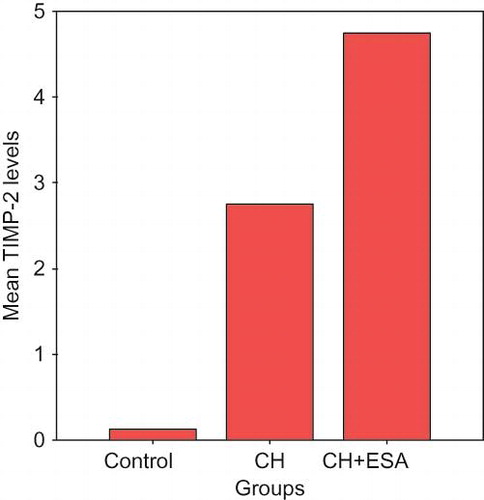
The level of increase in TIMP-2 scores was significant in ESA and CH groups compared to the control group (p < 0.001). There was no difference between ESA and CH groups (p > 0.05; see ).
DISCUSSION
In our study, we demonstrated that low-dose ESA decreased parietal peritoneal thickness histopathologically compared to chlorhexidine group but had no effect on inflammation and vascularization scores. It decreases the thickness of peritoneum but has no effect on inflammation, and vascularization score can be explained by the fact that these findings occur later on the course. In this study, the changes seen in parietal peritoneum showed no difference histopathologically and biochemically.
Erythropoiesis-stimulating agents were not pure as a molecule in our study. Epoetin beta is used as adjunct to some ingredients such as urea, sodium chloride, polysorbate 20, sodium dihydrogen phosphate, monohydrogen sodium phosphate, calcium chloride, glassine, lösin, isolysin, trionym, glutamic asit, and phenylalanine. In the conclusion of the study, because low-dose ESA caused a decrease of peritoneal fibrosis, we suggested that neither low-dose ESA nor other adjunctive materials had an effect on the development of peritoneal fibrosis. If ever either ESA or any of adjunctives cause peritoneal fibrosis, this also might be prevented. Low-dose ESA is introduced as a compound and clinically used subcutaneously so we could not use ESA as pure; therefore, the effect of adjunctive substances of the compound on peritoneal fibrosis could not be estimated.
In the present study, we induced experimental peritoneal fibrosis by using 15% ethyl alcohol and 0.1% chlorhexidine gluconate dissolved in saline. Experimental peritoneal fibrosis can be induced by intraperitoneal injection of chlorhexidine gluconate.[Citation15] It is a chemical agent that is not used clinically except to generate experimental peritoneal fibrosis. There are other methods to induce experimental peritoneal fibrosis, and the studies have been ongoing to prevent peritoneal fibrosis.[Citation16,Citation17]
Junor and colleagues first described the role of gluconate and alcohol in the pathogenesis of peritoneal fibrosis in 1985.[Citation10] They reported the development of clinical ESP when peritoneal catheter gets sterilized by chlorhexidine gluconate in alcohol. Upon this finding, Ishii and colleagues showed the development of peritoneal fibrosis in test animals by using 0.1% chlorhexidine gluconate and 15% alcohol dissolved in saline.[Citation10]
Symptoms like tachypnea, somnolence, and difficulty in walking observed in test animals after induction of peritoneal fibrosis by 15% ethyl alcohol and 0.1% chlorhexidine gluconate had not been reported before. We suggested that high-dose ethyl alcohol and chlorhexidine gluconate induced acute metabolic acidosis, and neurologic effects of alcohol or hypotension might have contributed to these symptoms.
At the end of study, we observed peritoneal adhesions over liver and anterior abdominal wall in rats with chemical peritonitis. This proved the success of the method.
Currently it was demonstrated that ESAs have other effects than therapy of chronic renal failure-related anemia. They inhibited apoptosis in an experimental acute renal failure model made by bilateral ischemic reperfusion, increased tubular epithelial regeneration, and accelerated gain of renal functions.[Citation18] They also decreased interstitial fibrosis and tubular injury.
On an experimental study of peritoneal fibrosis showing the decreasing effect of erythropoietin on peritoneal fibrosis, the dosage of ESA was 5000 U/kg intraperitoneal three days a week, which cannot be used clinically.[Citation19]
Matrix metalloproteinases and their natural inhibitors play a role in the ongoing process of peritoneal fibrosis.[Citation1,Citation20] MMPs and TIMPs are produced by peritoneal mesothelial cells, fibroblasts and macrophages in peritoneum, and also in peritoneal tissue cultures MMP-2, MMP-3, and MMP-9, and TIMP-1 and TIMP-2 activity levels were shown to be increased. TIMPs not only suppress MMPs but also increase their activity at lower tissue concentrations.[Citation20–22] Recent experimental animal studies have shown that use of MMP suppressors prevent neovascularization and inflammatory cell accumulation and thus hinder peritoneal fibrosis.[Citation15,Citation[22],Citation23]
In this study, immunohistochemically MMP-2 levels in the peritoneum were shown to be suppressed in the low-dose ESA group compared to the CH group, but increased compared to the saline group. On the other hand, biochemically the ratio of active to pro MMP-2 in the peritoneum was high in the ESA group compared to the CH group. Immunohistochemically, MMP-2s in the peritoneum represent both pro- and active forms of the molecule. Accordingly we can conclude that ESA increases total MMP-2 level, and accelerates pro-MMP-2 conversion to active MMP-2 as well. We thought that the effect of ESA decreasing development of peritoneal fibrosis induced by CH in rats was due to increase in active MMP-2 level. Immunohistochemically peritoneal TIMP-2 levels were significantly high in both ESA and CH groups compared to saline group, but no difference was observed between the ESA and CH groups. In our findings, TIMP-2 levels nonsignificantly were high in the ESA group compared to the CH group. This could be due to ongoing inflammation or low sample numbers in groups.
In light of these findings, we conclude that ESA has its action on the activation and increase of peritoneal TIMP-2 and the acceleration of the conversion of pro-MMP-2 to active MMP-2 to prevent peritoneal fibrosis. Other mechanisms may also seem to play a role in preventing peritoneal fibrosis in CH-induced peritoneal fibrosis in rats. We need further prospective studies to elucidate the mechanisms responsible for development of peritoneal fibrosis.
In conclusion, low-dose ESA caused a histopathologic decrement of peritoneal fibrosis induced chemically by CH. Immunohistochemically, it suppressed peritoneal tissue MMP-2 compared to CH group yet had no effect on TIMP-2. The decrease in peritoneal fibrosis by ESA can be attributed to its stimulatory effect on TIMP-2 activity. Other pathways may also play a role. We can say that ESA does not cause peritoneal fibrosis, and perhaps even prevents it, but we need further clinical and experimental studies concerning dose and duration.
REFERENCES
- Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int. 2003; 23: 530–541
- Miyata T, Devusyst O, Kurukowa K, Van Ypersele de Strihou C. Toward beter dialysis compatibility advances in the biochemistry and pathophysiology of the peritoneal membranes. Kidney Int. 2002; 61: 375–386
- Dobbie JW, Anderson JD. Ultrastructure, distribution, and density of lamellar bodies in human peritoneum. Perit Dial Int. 1996; 16: 482–487
- Johnson DW, Pat B, Vesey DA, et al. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006; 69: 1806–1813
- Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000; 97: 10526–10531
- Calvillo L, Latini R, Kajstura J, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA. 2003; 100: 4802–4806
- Lee SH, Li C, Lim SW, et al. Attenuation of interstitial inflammation and fibrosis by recombinant human erythropoietin in chronic cyclosporine nephropathy. Am J Nephrol. 2005; 25: 64–76
- Park SH, Choi MJ, Song IK, et al. Erythropoietin decreases renal fibrosis in mice with ureteral obstruction: Role of inhibiting TGF-beta-induced epithelial-to-mesenchymal transition. J Am Soc Nephrol. 2007; 18: 1497–1507
- Nishiya D, Omura T, Shimada K, et al. Effects of erythropoietin on cardiac remodeling after myocardial infarction. J Pharmacol Sci. 2006; 101: 31–39
- Ishii Y, Sawada T, Shimizu A, et al. An experimental sclerosing encapsulating peritonitis model in mice. Nephrol Dial Transplant. 2001; 16: 1262–1266
- Sis B, Sarioglu S, Sokmen S, et al. Desmoplasia measured by computer assisted image analysis: An independent prognostic marker in colorectal carcinoma. J Clin Pathol. 2005; 5: 32–38
- Sarioglu S, Sis B, Celik A, et al. Quantitative digital histochemistry with methenamine silver staining in renal allograft biopsies excluding pure chronic allograft nephropathy cases. Transplant Proc. 2006; 38: 490–491
- Sis B, Sarioglu S, Celik A, et al. Renal medullary changers in renal allograft recipients with raised serum creatinine. J Clin Pathol. 2006; 59: 377–381
- Sokmen S, Lebe B, Sarioglu S, et al. Prognostic value of CD44 expression in colorectal carcinomas. Anticancer Res. 2001; 21: 4121–4126
- Hirahara I, Inoue M, Okuda K, et al. The potential of matrix metalloproteinase–2 as a marker of peritoneal injury, increased solute transport, or progression to encapsulating peritoneal sclerosis during peritoneal dialysis—a multicentre study in Japan. Nephrol Dial Transplant. 2007; 22: 560–567
- Hirahara I, Umeyama K, Shofuda K, et al. Increase of matrix metalloproteinase–2 in dialysate of rat sclerosing encapsulating peritoneal model. Nephrology 2002; 7: 161–169
- Ersoy R, Celik A, Yilmaz O, et al. The effects of irbesartan and spironolactone in prevention of peritoneal fibrosis in rats. Perit Dial Int. 2007; 27: 424–431
- Johnson DW, Pat B, Vesey DA, et al. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006; 69: 1806–1813
- Mondello S, Mazzon E, Di Paola R, et al. Erythropoietin suppresses peritoneal fibrosis in rat experimental model. Eur J Pharmacol. 2009; 604: 138–149
- Martin J, Yung S, Robson RL, et al. Production and regulation of matrix metalloproteinases and their inhinbitors by human peritoneal mesotelial cells. Perit Dial Int. 2000; 20: 524–533
- Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995; 77: 863–868
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002; 90: 251–262
- Nagase H, Woessner JF, Jr. Matrix metalloproteinases. J Biol Chem. 1999; 274: 21491–21494