Abstract
Backgrounds/Aims. Ghrelin, a recently discovered hormone, is released largely from stomach and might affect insulin secretion and glucose metabolism. The aim of this study was to determine the immunohistochemical localization of ghrelin in streptozotocin-induced diabetic rat kidneys. Methods. Fifty-four adult male Wistar rats were used in this study. All rats were divided into nine groups according to three time points of the study (2, 4, and 6 weeks) as control group, control group given 0.1 M phosphate-citrate, and diabetic group given 50 mg/kg streptozotocin intraperitoneally. The rats in all groups were decapitated at the end of 2, 4, and 6 weeks of the study. The kidneys of the rats were removed, and tissue samples were processed by using routine paraffin techniques. The samples were immunohistochemically stained using avidin-biotin-peroxidase method for ghrelin immunoreactivity. Results. There were no differences of ghrelin immunoreactivity between the control groups. Ghrelin immunoreactivity was observed in both distal tubulus and collecting ducts in the diabetic groups, while it was detected only in distal tubules of the control groups. The intensity of ghrelin immunoreactivity was increased at 4 and 6 weeks of the study in the diabetic groups. Conclusion. Increased ghrelin immunoreactivity in the diabetic rat kidney tissues suggests that ghrelin may contribute to the pathophysiological mechanism of diabetic nephropathy.
INTRODUCTION
Diabetic nephropathy is the major cause of end-stage renal failure in most Western nations and is associated with increased morbidity and mortality as compared to other causes of renal disease.[Citation1] Diabetic nephropathy occurs as a result of an interaction between hemodynamic and metabolic factors.[Citation2] However, the mechanisms behind the development of diabetic nephropathy are complex and not completely understood.[Citation3] Numerous studies have shown that increased circulating growth hormone might also play a significant role in the pathogenesis of diabetic nephropathy.[Citation4–6]
Increased circulating growth hormone levels may be caused by a number of factors. Ghrelin is a novel growth hormone secretagogue recently isolated from human and rat stomach that stimulates food intake and body weight gain.[Citation7,Citation8] Ghrelin gene expression has also been demonstrated in the kidney.[Citation9] Ghrelin immunoreactivities in the kidney have been determined to be more than 10 times abundant than those in the plasma, which indicate that ghrelin is also produced locally in the kidney.[Citation10] On the other hand, plasma total and active ghrelin levels are significantly increased in streptozotocin (STZ)-induced diabetic rats. Increased plasma ghrelin levels are found as well correlated with the increased serum growth hormone levels.[Citation8] These findings suggest the hypothesis that increased plasma ghrelin levels in diabetes mellitus may contribute to the pathogenesis of diabetic nephropathy via increased growth hormone levels. However, there have been no published data related with the intra-renal distribution of ghrelin in experimentally induced diabetes mellitus. The aim of this study was to determine the immunohistochemical localization of ghrelin in STZ-induced diabetic rat kidneys.
MATERIALS AND METHODS
Animal Procedures
Rats were obtained from the Faculty of Medicine Experimental Research Center, Firat University (FUDAM). Fifty-four adult male Wistar rats were used in this study. Animals were kept at room temperature and were exposed to alternate cycles of 12 h light and darkness. All animals were allowed free access to tap water and a standard pellet rat diet. All animal care and handling procedures conformed to the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care, and approval for the experimental study was obtained from the Local Ethics Committee for Animal Studies. Animals were divided into nine groups (n = 6) according to three time points of the study (2, 4 and 6 weeks) as control group (Control2, Control4, Control6), tampon-control group (given 0.1 M phosphate-citrate; Tampon-control2, Tampon-control4, Tampon-control6), and diabetic group (given 50 mg/kg streptozotocin intraperitoneally; Diabetes2, Diabetes4, Diabetes6). Following a 12-hour fasting period, blood samples of all the rats were obtained via the tail vein, and blood glucose levels were measured 72 hours after the initiation of the experiment. Rats with >250 mg/dL blood glucose were accepted as diabetic.
The rats in the groups (Control2, Tampon-control2, Diabetes2), (Control4, Tampon-control4, Diabetes4) and (Control6, Tampon-control6, Diabetes6) were sacrificed by decapitation under intraperitoneal (i.p.) ketamine (75 mg/kg)+xylazine (10 mg/kg) anesthesia at each time points of the study (2, 4, and 6 weeks, respectively). After decapitation, kidney and stomach (as positive control) tissues were rapidly removed. For each rat, left kidney weight/body weight ratio was determined. Kidney tissue specimens obtained from all groups were fixed in 10% formalin solution and processed by routine protocols for embedding in paraffin wax.
Serum Glucose Measurements
Serum glucose values were measured with a Glucometer (GlukoDr Super Sensor, All Medicus Co. Ltd., Korea).
Immunohistochemistry
Five to six micrometer-thick sections were cut by microtome. Serial sections were collected on poly-L-lysine-coated slides, dewaxed, and rehydrated. They were placed in citrate buffer (pH 6.0), and an antigen-retrieval procedure was performed by heating the samples twice in a microwave oven at 750 W for 5 min each time. After cooling for 20 minutes at room temperature, the sections were washed in phosphate-buffered saline (PBS), pH 7.4, and then incubated in peroxidase block solution (Goat ImmunoCruz Staining System, Santa Cruz Biotechnology, Santa Cruz, California, USA) for 5 min to quench endogenous peroxidase activity and afterward washed with PBS. Non-specific staining was blocked by 20 min incubation with serum blocking solution (Goat ImmunoCruz Staining System, Santa Cruz Biotechnology), and samples were incubated with primary polyclonal antibody (Ghrelin Goat Polyclonal IgG, Santa Cruz Biotechnology) at a dilution of 1:100, overnight, at 4°C in a humid chamber.
Subsequently, the sections were incubated with biotinylated secondary antibody (Goat ImmunoCruz Staining System, Santa Cruz Biotechnology, USA) for 30 min and incubated with horseradish peroxidase streptavidin complex (Goat ImmunoCruz Staining System, Santa Cruz Biotechnology) for 30 min. Finally, the sections were washed with PBS three times for 5 min, and peroxidase activity was visualized using a substrate prepared by adding a drop of 3-amino–9-ethylcarbazole (AEC) chromogen (Ultravision Detection System, Lab Vision Corporation, TA- 125-HA, USA) and incubating with the sections for 7 min. The sections were counterstained with Mayer's hematoxylin. The samples were rinsed in PBS twice for 5 min and in distillated water for 5 min, and finally mounted in aqueous-mount (ScyTek Laboratories, Logan, Utah, USA).
Semiquantitative Analysis
Immunohistochemical ghrelin staining intensity in the kidney tissues of all groups was evaluated semiquantitatively. The intensity of ghrelin expression was scored as follows: no staining (−), low (+), moderate (++), or strong (+++).
RESULTS
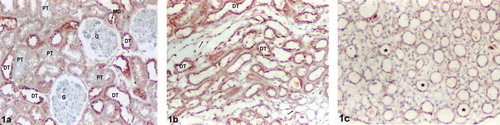
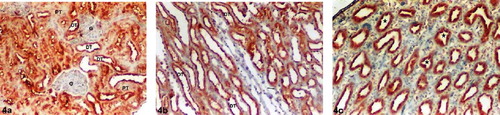
In the kidney sections, there were no differences between the Control and Tampon-control groups in terms of ghrelin immunoreactivity. Positive ghrelin immunoreactivity was observed in distal tubular epithelium, containing macula densa regions in control group sections (see ). Ghrelin immunoreactivity was also observed in distal tubules (DTs) of the renal medulla (see ). There was moderate (++) ghrelin immunoreactivity in the renal distal tubular epithelium of the controls (see and ). Positive staining was not observed in the glomeruli, proximal tubules (pars convoluta and pars recta), thin limbs of loop of Henle, or collecting ducts (CDs) (see –) in the renal sections of the controls.
Figure 1. Control group kidney sections. (a) Renal cortex, (b) medulla, and (c) papilla. Moderate (++) ghrelin immunoreactivity in the distal tubular epithelium, containing macula densa regions (a). Moderate (++) ghrelin immunoreactivity in the distal tubules (b). No positive staining in the glomeruli, proximal tubules, thin limbs of loop of Henle, and collecting ducts (a–c). Abbreviations: MD = Macula densa, G = glomeruli, PT = proximal tubule, DT = distal tubule, (→) = thin limbs of loop of Henle (☆) collecting duct. Magnification: ×10.
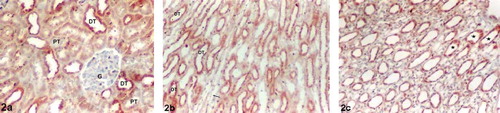
Ghrelin immunoreactivity in the Diabetes2 was similar to those of the controls in the renal cortex and medulla. Moderate (++) ghrelin immunoreactivity was observed in DTs in the renal cortex and medulla. There was no positive staining in the proximal tubules, thin limbs of loop of Henle, or glomeruli (see and ). However, a low (+) ghrelin immunoreactivity signal was observed in the CDs in the renal papilla sections as being different from the controls (see ).
Figure 2. Diabetes2 group kidney sections. (a) Renal cortex, (b) medulla, and (c) papilla. Moderate (++) ghrelin immunoreactivity in the distal tubules (a) and (b). No positive staining in the glomeruli, proximal tubules, or thin limbs of loop of Henle (a, b). Low (+) ghrelin immunoreactivity in the collecting ducts in the renal papilla (c). Abbreviations: G = glomeruli, PT = proximal tubule, DT = distal tubule, (→) = thin limbs of loop of Henle (☆) collecting duct. Magnification: ×10.
There were significant differences relative to ghrelin immunoreactivity in the kidney tissues in Diabetes4 and Diabetes6. The intensity of ghrelin immunoreactivity was increased in both Diabetes4 and Diabetes6. A strong (+++) ghrelin immunoreactivity was observed in DTs in the renal cortex and medulla (see , , , and ). There was no positive staining in the proximal tubules, thin limbs of loop of Henle, or glomeruli in both the Diabetes4 and Diabetes6 groups. Ghrelin immunoreactivity was moderate (++) in CDs of the renal papilla in the Diabetes4 group (see ). On the other hand, a strong (+++) ghrelin immunoreactivity was determined in CDs of the renal papilla in the Diabetes6 group (see ).
Figure 3. Diabetes4 group kidney sections. (a) Renal cortex, (b) medulla, and (c) papilla. Strong (+++) ghrelin immunoreactivity in the distal tubules (a) and (b). No positive staining in the glomeruli, proximal tubules, or thin limbs of loop of Henle (a, b). Moderate (++) ghrelin immunoreactivity in the collecting ducts in the renal papilla (c). Abbreviations: G = glomeruli, PT = proximal tubule, DT = distal tubule, (→) = thin limbs of loop of Henle (☆) collecting duct. Magnification: ×10.
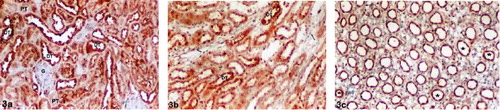
Figure 4. Diabetes6 group kidney sections. (a) Renal cortex, (b) medulla, and (c) papilla. Strong (+++) ghrelin immunoreactivity in the distal tubules (a) and (b). No positive staining in the glomeruli, proximal tubules, or thin limbs of loop of Henle (a, b). Strong (+++) ghrelin immunoreactivity in the collecting ducts in the renal papilla (c). Abbreviations: G = glomeruli, PT = proximal tubule, DT = distal tubule, (→) = thin limbs of loop of Henle (☆) collecting duct. Magnification: ×10.
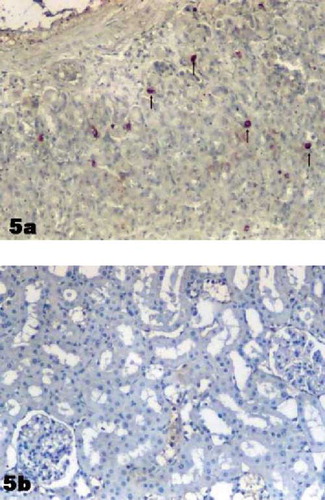
In the stomach (positive control tissue), ghrelin immunoreactive cells were determined from the neck to the base of rat gastric gland in the mucosa layer (see ). Immunoreactivity was not detected in negative control staining (see ).
DISCUSSION
Diabetic nephropathy is an important cause of morbidity and mortality in diabetic patients. Various mechanisms have been suggested for the pathophysiology of diabetic nephropathy, such as abnormalities in glucose transport mechanisms, activation of protein kinase C isoforms, increased activity of specific intracellular metabolic pathways, generation of reactive oxygen species, increased production of advanced glycation end products, and altered activity of a variety of growth factors and cytokines.[Citation11] Several clinical and experimental studies have shown that growth hormone-insulin like growth factor (GH-IGF-I) system may play a significant role in the pathogenesis of diabetic nephropathy.[Citation4–6,Citation12] Increased circulating growth hormone levels have been reported in rodents with diabetic nephropathy.[Citation13] In some experimental studies, growth hormone receptor antagonist's treatment protected the animals against development of renal changes.[Citation14,Citation15] Although it is well known that ghrelin is a growth hormone secretagogue, there have been no studies that investigate the relationship between increased ghrelin and growth hormone in diabetic nephropathy. In this study, we demonstrated that there was increased ghrelin immunoreactivity in kidney tissues of the STZ-induced diabetic rats.
Yabuki et al.[Citation16] reported the ghrelin immunoreactivity in the epithelium of distal tubules in normal rodent kidneys. Similarly, moderate (++) ghrelin immunoreactivity was also observed in the renal distal tubular epithelium of the controls and Diabetes2 group in the present study. However, the intensity of ghrelin immunoreactivity was increased from moderate (++) to strong (+++) in the distal tubules of the Diabetes4 and Diabetes6 groups. While there has been no ghrelin immunoreactivity out of the distal tubules in the controls, we interestingly determined gradually increased ghrelin immunoreactivity in the CDs in Diabetes2, Diabetes4, and Diabetes6 groups. A gradual increase of ghrelin immunoreactivity in both distal tubules and CDs during the experimental period indicates that ghrelin may undertake a task in the process of diabetic nephropathy development.
In patients with diabetes, hypertension is approximately twice as frequent compared with patients without the disease.[Citation17] Distal tubules and CDs where a strong (+++) ghrelin immunoreactivity was determined in this study are also considered as the regions of blood pressure regulation in the kidney. Epithelial sodium channel in the distal convoluted tubule to the collecting duct sodium handling in this part of the renal tubule may be involved in blood pressure mechanisms.[Citation18] The probable roles of ghrelin in the progression of diabetic hypertension should also be investigated.
In conclusion, increased ghrelin immunoreactivity in kidney tissues of the STZ-induced diabetic rats suggests that ghrelin might be involved in the pathogenesis of diabetic nephropathy.
ACKNOWLEDGMENTS
This research was supported by Firat University Scientific Research Projects Management Unit (FUBAP) project number 1309.
REFERENCES
- Boner G, Cooper ME. Diabetic nephropathy. Diabetes Technol Ther. 1999; 1: 489–496
- Cooper ME. Interaction of metabolic and hemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001; 44: 1957–1972
- Saxena U, Timmer RT, Pillarisetti S. New approaches for treatment of diabetic nephropathy: The endothelium as a target for drug discovery. Expert Opin Ther Targets. 2001; 5: 539–545
- Lundbaek K, Christensen NJ, Jensen VA, Johansen K, Olsen TS, Hansen AP, Orskov H, Osterby R. Diabetes, diabetic angiopathy, and growth hormone. Lancet. 1970; 2: 131–133
- Esposito C, Liu ZH, Striker GE, Phillips C, Chen NY, Chen WY, Kopchick JJ, Striker LJ. Inhibition of diabetic nephropathy by a GH antagonist: A molecular analysis. Kidney Int. 1996; 50: 506–514
- Flyvbjerg A. Role of growth hormone, insulin-like growth factors (IGFs) and IGF-binding proteins in the renal complications of diabetes. Kidney Int Suppl. 1997; 60: 12–19
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402: 656–660
- Masaoka T, Suzuki H, Hosoda H, Ota T, Minegishi Y, Nagata H, Kangawa K, Ishii H. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Lett. 2003; 541: 64–68
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002; 87: 2988–2991
- Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett. 2000; 486: 213–216
- Liu Y, Freedman BI. Genetics of progressive renal failure in diabetic kidney disease. Kidney Int Suppl. 2005; 99: 94–97
- Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000; 43: 1205–1223
- Segev Y, Eshet R, Rivkis I, Hayat C, Kachko L, Phillip M, Landau D. Comparison between somatostatin analogues and ACE inhibitor in the NOD mouse model of diabetic kidney disease. Nephrol Dial Transplant. 2004; 19: 3021–3028
- Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999; 48: 377–382
- Segev Y, Landau D, Rasch R, Flyvbjerg A, Phillip M. Growth hormone receptor antagonism prevents early renal changes in nonobese diabetic mice. J Am Soc Nephrol. 1999; 10: 2374–2381
- Yabuki A, Taharaguchi S, Ichii O, Kojima M, Nishi Y, Mifune H, Kamimura R, Matsumoto M, Suzuki S. Immunohistochemical localization of ghrelin in rodent kidneys. Histochem Cell Biol. 2006; 126: 231–238
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension. 2001; 37: 1053–1059
- Matsubara M. Renal sodium handling for body fluid maintenance and blood pressure regulation. Yakugaku Zasshi. 2004; 124: 301–309