Abstract
Background: Peritonitis, the most important limitation of peritoneal dialysis (PD), could be detected by biomarkers in dialysate effluent, representing a noninvasive method to indirectly assess the peritoneum status. The aim of our study was to test high mobility group box 1 (HMGB1) in PD patients, evaluating its role as precocious marker of peritoneum damage during peritonitis. Transforming growth factor (TGF)-β was correlated with peritoneal transport characteristics.
Methods: Six patients, treated by ambulatory PD, were enrolled. Samples were collected at the onset of peritonitis (T1) and every day until its resolution (T-end). Serum (s) and peritoneal (p) white blood cell (WBC) count was also evaluated. Peritoneal Equilibration Test evaluated the filter activity of peritoneum.
Results: In patients with acute peritonitis, the highest serum and peritoneal HMGB1 values (64 ± 3.6 and 70 ± 5.3 ng/mL, respectively) were assessed, with a progressive decrease of their levels at the resolution time (T-end: sHMGB1:36 ± 2.5; pHMGB1:30.5 ± 7.0 ng/mL). While no differences of sWBC and pWBC were observed between baseline and T-end values, pHMGB1 levels remained higher at T-end than those observed at T0 (pHMGB1:30.5 ± 7.0 versus 6.9 ± 3.6; p < 0.0001). TGF-β levels were higher in patients with low peritoneal permeability than in medium or high transporter patients (81 ± 15.5 versus 24.3 ± 7.5 pg/mL; p = 0.01). An inverse correlation was found between TGF-β levels and dialysate/plasmatic creatinine values (r = −0.83; p = 0.03).
Conclusion: HMGB1 represents a useful biomarker for peritoneum evaluation in PD patients. A prognostic role of this alarmin, as a marker of response to therapy, could be hypothesized. TGF-β could predict the peritoneal transport status and dialysis technique adequacy.
Introduction
Peritoneal dialysis (PD) is the preferred dialysis modality for children requiring renal replacement therapy, due to several advantages comparing to hemodialysis, such as its compatibility with schooling and social life, less difficulties to create and maintain an adequate access, elimination of pain related to punctures of the arteriovenous fistula and no need for anticoagulant use. Moreover, the efficacy of this technique in childhood is also based on the two-fold high peritoneal membrane surface per kilogram of body mass.Citation1
However, although the rate of PD-related peritonitis has been significantly reduced, it remains a major complication of PD, causing peritoneal membrane dysfunction and treatment drop out.Citation2 In recent years, the introduction of more biocompatible PD solutions has represented one of the most important enhancements of PD.Citation3 In fact, the peritoneal exposure to conventional poorly biocompatible peritoneal dialysis fluids (PDFs), characterized by non-physiological pH, high glucose concentrations, and high levels of glucose degradation products (GDPs), has been related to an accelerated peritoneal aging.Citation4,Citation5 Peritoneal biopsies allow clinicians to evaluate morphological peritoneal membrane alterations, but this invasive approach cannot be performed frequently. The measurement of pro- or anti-inflammatory and fibrotic new biomarkers in dialysate effluent, such as cancer antigen 125 or neutrophil gelatinase-associated lipocalin, could represent a noninvasive method to indirectly assess the peritoneum.Citation6
It is well known that peritoneal fibrosis, secondary to recurrent peritonitis and/or chronic peritoneal inflammation linked to the toxicity of dialysis fluids, represents another important cause of drop out to hemodialysis.Citation7
High mobility group box 1 protein (HMGB1), a 30 kDa nuclear and cytosolic ubiquitous protein, is actively secreted by innate immune cells and it has been shown to stimulate necrosis-induced inflammation.Citation8 Moreover, HMGB1, regulating the release of other cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and IL-8, is closely involved in inflammatory processes, including peritonitis.Citation9 Currently, no data are available about the role of this alarmin in pediatric patients treated by PD.
HMGB1 exerts its inflammatory actions through the interaction with different receptors, such as toll-like receptor (TLR) 2 or TLR4, and the receptor for advanced glycation end products (RAGE). This latter has been widely studied in association to its ligands advanced glycation end-products (AGEs), which participate to peritoneal membrane failure and aging.Citation10
However, the effects of these products on RAGE and its proinflammatory ligands, such as HMGB1, are unknown.
Similarly, transforming growth factor (TGF)-β, a polypeptide involved in the regulation of several cellular processes, plays immuno-modulatory actions, exerting both anti and pro-inflammatory functions, mediating the remodeling processes, and promoting tissue fibrosis.Citation11,Citation12
The aim of this preliminary, single-center, hypothesis generating study was to assess HMGB1 and TGF-β in pediatric patients treated by PD, evaluating their potential role as precocious markers of peritoneum damage. In particular we tested HMGB1 during a hyperacute stress, such as peritonitis, whereas TGF-β was related to effectiveness of the dialytic technique, analyzing the potential link between this cytokine and peritoneal transport characteristics.
Material and methods
Six patients, treated by ambulatory PD, who had been referred to the Section of Nephrology, Department of Pediatrics, University of Messina, were enrolled.
All patients were treated with 2-L bicarbonate/lactate-based PD solutions, at a concentration of 1.36% glucose (Physioneal 40, 1.36%; Baxter International Inc., Rome, Italy).
Exclusion criteria included acute or chronic exit site or tunnel infection, episode of peritonitis in the 6 months before the enrollment, antibiotic therapies performed in the last 3 months.
Blood samples and peritoneal fluids were collected, every 20 days after the enrollment (T0), to obtain baseline values of serum (s) and effluent peritoneal fluid (p) HMGB1. This strategy was adopted in order to detect potential precocious modification of HMGB1 levels before peritonitis events (T-pre).
Samples were then collected at the onset of peritonitis (T1) and every day until its resolution (T-end). At the same times, serum (s) and peritoneal (p) white blood cell (WBC) count was also evaluated.
We considered peritonitis any episode of turbid peritoneal effluent with a pathological cell count, defined as WBCs >100/mm3 with a polymorphonuclear neutrophil (PMN) percentage greater than 50%.Citation13
Cultures for bacteria, mycobacterium tuberculosis, and fungus were performed routinely at day 1 of peritonitis. Peritonitis was treated with the standard first-line intraperitoneal and systemic antibiotic regimen with cefazolin and ceftazidime. Antibiotics were modified once the culture results and sensitivities became available.
Blood samples for HMGB1 evaluation were collected in chilled vacutainer tubes containing potassium ethylenediaminetetraacetate, and the plasma was promptly analyzed. The injection ports of Tenckhoff’s catheter were disinfected with methanol and allowed to dry for 2 min. Ten milliliters of peritoneal liquid were centrifuged at 3000 rpm for 8 min and stored at −80 °C until assayed. HMGB1 was assessed by commercial ELISA kit (IBL Shino Test Corporation, Hamburg, Germany) according to the manufacturers’ instructions. The enzymatic reactions were quantified in an automatic microplate photometer. sHMGB1 and pHMGB1 levels were expressed as ng/mL.
Peritoneal Equilibration Test and TGF-β
Every patient did the Peritoneal Equilibration Test (PET) to evaluate the filter activity of peritoneum. All measurements were performed with the patients in euvolemic state and more than 2 months after an episode of peritonitis.
The evaluation of peritoneal permeability was performed through the PET of Twardowski and the determination of Dialysate (D)/plasmatic (P) creatinine to 4 h.Citation14
PET begins after 6 h from the completion of the PD session of the night, leaving a residual intra-peritoneal volume equal to 20 mL/kg of dialysis liquid, which must have a glucose percentage of 2.27%. Analysis on blood (glucose, urea, and creatinine), peritoneal fluid (glucose, urea, and creatinine), and dialysis fluid from the bag (to confirm the exact concentration of glucose that must be equal to 2.27%) are performed. Subsequently, we contemporary do the discharge of the intra-abdominal residual fluid and the load of 20 mL/kg of dialysis liquid with glucose at 2.27%. This phase corresponds to the time 0 (T0).
After 2 h (T1), there is the discharge of 10 mL/kg of the intra-abdominal fluid which is analyzed (glucose, urea, and creatinine). After further 2 h (T2), there is the discharge of the remaining intra-abdominal fluid.
The ultra-filtrate calculation and the same exams on blood and peritoneal fluid done at T0 are performed at T2. The main parameter that we evaluate is the ratio between the concentrations of creatinine in the dialysate with the corresponding concentrations in plasma at 4 h (T2) from the test start (D/P creatinine). Through the D/P creatinine, we identify three categories of peritoneal permeability (categories modified from the original formulation of Twardowski): low permeability (D/P creatinine less than 0.6); medium permeability (D/P creatinine between 0.6 and 0.8); high permeability (D/P creatinine greater than 0.8).Citation15
TGF-β was assessed at T0 by commercial ELISA kit (Quantikine High Sensitivity; R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions. The enzymatic reactions were quantified in an automatic microplate photometer. TGF-β levels were expressed as pg/mL. The study protocol was approved by the Hospital’s Ethics Committee. Written informed consent was obtained from parents and informed assent from children and adolescents. Statistical analyses were performed using Medcalc 8.0 for Windows package and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). Data were presented as mean ± standard deviation for normally distributed values (at Kolmogorov–Smirnov test) and median (IQ range) for non-normally distributed values. Differences between groups were established by ANOVA followed by Bonferroni’s test for normally distributed values and by Kruskal–Wallis analysis followed by Dunn’s test for nonparametric values. p < 0.05 was considered to be statistically significant.
Results
The main axiological and biochemical data of patients are summarized in .
Table 1. Clinical characteristics and laboratoristic data of cohort study.
The study group included 3 females and 3 males, with a mean chronological age of 9 ± 6 years. 2 patients were affected by urethral valves, 2 children had Joubert syndrome, whereas renal dysplasia was the cause of end-stage renal disease in 2 patients.
At enrollment phase, serum creatinine was 3.5 ± 0.5 mg/dL, whereas blood urea nitrogen concentration was 49.3 ± 8.8 mg/dL. The mean age of dialysis treatment was 41 ± 17.5 months and all patients were treated with 1.36% glucose dialysate fluid. The mean daily ultrafiltration (UF) peritoneal volume was 816 mL ±451 mL.
Four patients experienced a peritonitis event, with a complete resolution after antibiotic therapy and not requiring the drop out of the treatment. In 2 cases, gram positive bacteria have been isolated.
At baseline, patients were characterized by serum and peritoneal HMGB1 values of 9.5 ± 1.0 and 6.9 ± 3.6 ng/mL, respectively. At the same time, sWBC values were 4796 ± 325 cells/mm3, whereas 8.3 ± 3.3 WBCs/mm3 has been revealed in peritoneal fluid.
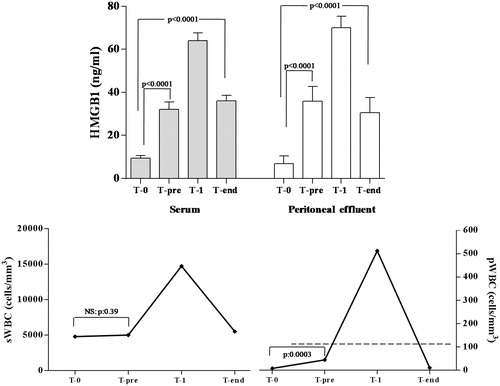
Higher levels of HMGB1 were assessed just before the diagnosis of peritonitis (T-pre), than those observed at baseline (sHMGB1: 32.1 ± 3.3 versus 9.5 ± 1.0 ng/mL; p < 0.0001; pHMGB1: 35.8 ± 6.9 versus 6.9 ± 3.6 ng/mL; p < 0.0001). sWBC values did not differ at these two time points (4800 ± 320 versus 5008 ± 303.4 cells/mm3, p = 0.39), whereas high pWBC levels were detected, but not enough to diagnose a peritonitis event (44.6 ± 10.5 cells/mm3; p = 0.0003).
In patients with acute peritonitis, sWBC levels were 14,750 ± 1109 cells/mm3 associated with 512 ± 331.6 cells/mm3 of WBC detected in peritoneal fluid. At this time, the highest serum and peritoneal HMGB1 values (64 ± 3.6 and 70 ± 5.3 ng/mL, respectively) were assessed, with a progressive decrease of their levels at the resolution time occurred after 4.2 ± 0.9 days (T-end: sHMGB1: 36 ± 2.5; pHMGB1: 30.5 ± 7.0 ng/mL).
However, while no differences of sWBC and pWBC were observed between baseline and T-end values, HMGB1 levels remained higher at T-end than those observed at T0 (pHMGB1: 30.5 ± 7.0 versus 6.9 ± 3.6; p < 0.0001) (.
Figure 1. High mobility group box 1 (HMGB1) and white blood cell (sWBC) count in sera and peritoneal effluent before, during, and after a peritonitis event.
Dividing our patients according to the D/P creatinine value, we found that TGF-β levels were higher in patients with low peritoneal permeability (low transporter, LT), when compared with values observed in medium transporter (MT) or high transporter (HT) (81 ± 15.5 versus 24.3 ± 7.5 pg/mL; p = 0.01) patients.
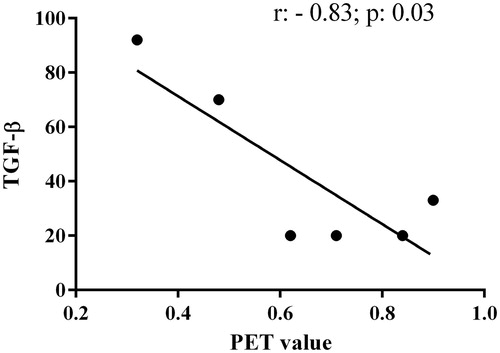
An inverse correlation was then highlighted between TGF-β levels and D/P creatinine values (r = −0.83; p = 0.03) (.
Figure 2. Univariate correlation between transforming growth factor (TGF)-β levels and dialysate/plasmatic creatinine values.
Furthermore, patients characterized by the highest levels of this cytokine had not events of peritonitis in their medical history, whereas low peritoneal TGF-β levels were detected in patients with more episodes of infective events during the dialytic treatment.
In particular, only one HT patient, who suffered from several peritonitis events in the last 3 years, had a slightly increased, borderline TGF-β level (33 pg/mL).
No correlations were found with the duration of dialysis and the underlying renal disease.
Discussion
Our data clearly demonstrate that HMGB1 represents an useful biomarker for peritoneum evaluation in PD patients. In particular, both serum and peritoneal HMGB1 levels arise during a peritonitis event, providing additional evidence to support the diagnosis and prompt for immediate antimicrobial therapy. Moreover, a gradually reduction of this marker was also observed after effective antibiotic treatment, hypothesizing a prognostic role of this alarmin, such as marker of therapy response.
It is well known that HMGB1 is actively secreted by natural immune cells or passively released by necrotic or apoptotic cells, as response to several inflammatory insults.Citation16,Citation17
Moreover, peritoneal HMGB1 also derived by a direct production of mesothelial cells, as demonstrated by Cao et al who revealed that, in cultured human peritoneal mesothelial cell line, lipopolysaccharide actively induced HMGB1 nuclear-cytoplasmic translocation, and release in a time and dose-dependent fashion.Citation18
The high levels of pHMGB1, contrasted to normal levels of pWBC assessed during the early phase of peritonitis and at the end of the infectious process, could highlight a source beyond immune cells. We have demonstrated that pHMGB1 began to rise in the absence of clinical and classic laboratoristic signs of peritonitis, allowing early detection of a subclinical inflammatory-infective process involving the peritoneum.
Moreover, in the resolution period, while pWBC returned to basal levels after about a week, pHMGB1 levels normalized more slowly, due to its continuous production by mesothelial cells, still “stunned” by inflammatory and infectious processes.
These data could indicate a better sensitivity and specificity of pHMGB1 than those achievable with sHMGB1. Furthermore, this latter could be influenced by chronic inflammation status that typically characterized uremic patients, leading to the well-known malnutrition/inflammation/athero-sclerosis (MIA) syndrome.Citation19,Citation20
High pHMGB1 levels after a peritonitis could be referred to an impairment of peritoneal host defense and alterations of cellular components within the peritoneal cavity secondary to the chronic exposure to PD solutions, leading to disturbances in proinflammatory and anti-inflammatory mediator release.Citation21
We have also revealed that TGF-β was associated with low peritoneal transport status, regardless of underlying disease, duration of dialysis, and number of peritonitis events from the start of dialysis.
The major findings, about TGF-β and PD have been detected in animal studies, whereas few studies have been conducted in humans, always involving adult patients.
In particular, Stompòr et al., evaluating 32 adult PD patients, found a positive correlation between serum TGF-β concentration and dialysate-to-plasma ratio for creatinine in PET. Serum TGF-β levels did not correlate with acute inflammatory markers, such as IL-6, C-reactive protein, fibrinogen, or other growth factors, as well as basic fibroblast growth factor and vascular endothelial growth factor.Citation22
However, TGF-β levels were not assessed in peritoneal fluid.
Our data revealed a closely connection between peritoneal TGF-β and peritoneal transport characteristics in children treated by PD, assessing that low transporters were characterized by the highest levels of TGF-β in peritoneal effluent. Furthermore, an inverse correlation was assessed between TGF-β levels and D/P creatinine values.
However, serum TGF-β concentrations were not determined in our study, and no direct proof exists that TGF-β production could be intra-peritoneal, although few studies suggested a local TGF-β production.Citation23
Furthermore, only a small portion of TGF-β is in soluble form and TGF-β must be activated to have biological effect.
Nevertheless, Wong et al. confirmed our data in more than 100 adult patients treated by PD, revealing higher TGF-β values in peritoneal fluid of patients with lower D/P creatinine ratio at 4 h, independently of peritonitis events, hypothesizing a potential influence of TGF-β concentrations by the baseline solute transport characteristics.Citation24
The role of TGF-β levels with regard to longitudinal change in peritoneal transport requires further analyses.
This study certainly had limitations. This was a single-center, generating hypothesis study based on slightly limited patients’ number. The applicability of our findings to the pediatric PD population is limited and a prospective, large scale, trial is mandatory.
We did not evaluate inflammatory markers, such as C-reactive protein, whose levels are closely related to malnutrition and inflammation. Further studies should compare this marker to HMGB1 and TGF-β, in order to better describe the physiopathological processes occurring during peritonitis or chronically in PD patients.
However, our study demonstrated a significant increase of HMGB1 in serum and peritoneal fluid in the first phases of peritoneal inflammation, even before the positivity of classic symptoms and laboratoristic signs of peritonitis, confirming the hypothesis that HMGB1 could represent a promising early noninvasive biomarker of peritonitis in PD patients.
Moreover, it has been demonstrated that ultrasonographic measurement of peritoneal membrane thickness is a simple and noninvasive method in chronic PD children, enabling to assess peritoneal structure and function and representing another promising noninvasive tool to evaluate PD patients.Citation25
Further studies are necessary to understand the possible use of TGF-β as predictive marker of peritoneal transport status and dialysis technique adequacy.
Disclosure statement
The authors have no conflicts of interest to disclose that could be perceived as prejudicing the impartiality of the research reported.
References
- Schaefer F, Borzych-Duzalka D, Azocar M, et al. Impact of global economic disparities on practices and outcomes of chronic peritoneal dialysis in children: Insights from the International Pediatric Peritoneal Dialysis Network Registry. Perit Dial Int. 2012;32:399–409.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393–423.
- Chaudhary K, Khanna R. Biocompatible peritoneal dialysis solutions: Do we have one? Clin J Am Soc Nephrol. 2010;5:723–732.
- Amore A, Cappelli G, Cirina P, et al. Glucose degradation products increase apoptosis of human mesothelial cells. Nephrol Dial Transplant. 2003;18:677–688.
- Conti G, Amore A, Cirina P, et al. Glycated adducts induce mesothelial cell transdifferentiation: Role of glucose and icodextrin dialysis solutions. J Nephrol. 2008;21:426–437.
- Lacquaniti A, Chirico V, Mondello S, et al. Neutrophil gelatinase-associated lipocalin in peritoneal dialysis reflects status of peritoneum. J Nephrol. 2013;26:1151–1159.
- Williams JD, Craig KJ, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479.
- Chirico V, Lacquaniti A, Salpietro V, et al. High-mobility group box 1 (HMGB1) in childhood: From bench to bedside. Eur J Pediatr. 2014;173:1123–1136.
- Arrigo T, Chirico V, Salpietro V, et al. High-mobility group protein B1: A new biomarker of metabolic syndrome in obese children. Eur J Endocrinol. 2013;168:631–638.
- Opatrna S, Popperlova A, Kalousová M, Zima T. Low glucose degradation product peritoneal dialysis regimen is associated with lower plasma EN-RAGE and HMGB-1 proinflammatory ligands of receptor for advanced glycation end products. Ther Apher Dial. 2014;18:309–316.
- Cernaro V, Lacquaniti A, Lupica R, et al. Relaxin: New pathophysiological aspects and pharmacological perspectives for an old protein. Med Res Rev. 2014;34:77–105.
- Cernaro V, Lacquaniti A, Donato V, Fazio MR, Buemi A, Buemi M. Fibrosis, regeneration and cancer: What is the link? Nephrol Dial Transplant. 2012;27:21–27.
- Warady BA, Bakkaloglu S, Newland J, et al. Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int. 2012;32:32–86.
- Twardowski ZJ, Nolph KO, Khanna R, et al. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138–147.
- van Biesen W, Heimburger O, Krediet R, et al. Evaluation of peritoneal membrane characteristics: Clinical advice for prescription management by the ERBP working group. Nephrol Dial Transplant. 2010;25:2052–2062.
- Chirico V, Lacquaniti A, Leonardi S, et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: High-mobility group box 1 (HMGB1) between inflammation and infection. Clin Microbiol Infect. 2014;21:368.e1–369.
- Chirico V, Lacquaniti A, Piraino B, et al. Thalassaemia major and infectious risk: High mobility group box-1 represents a novel diagnostic and prognostic biomarker. Br J Haematol. 2015;171:130–136.
- Cao S, Li S, Li H, et al. The potential role of HMGB1 release in peritoneal dialysis-related peritonitis. PLoS One. 2013;8:e54647.
- Zhu N, Yuan W, Zhou Y, et al. High mobility group box protein-1 correlates with microinflammatory state and nutritional status in continuous ambulatory peritoneal dialysis patients. J Artif Organs. 2011;14:125–132.
- Lacquaniti A, Bolignano D, Campo S, et al. Malnutrition in the elderly patient on dialysis. Ren Fail. 2009;31:239–245.
- Vidal E, Edefonti A, Puteo F, et al. Encapsulating peritoneal sclerosis in paediatric peritoneal dialysis patients: The experience of the Italian Registry of Pediatric Chronic Dialysis. Nephrol Dial Transplant. 2013;28:1603–1609.
- Stompór T, Zdzienicka A, Motyka M, Dembińska-Kieć A, Davies SJ, Sulowicz W. Selected growth factors in peritoneal dialysis: Their relationship to markers of inflammation, dialysis adequacy, residual renal function, and peritoneal membrane transport. Perit Dial Int. 2002;22:670–676.
- Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT. Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med. 1999;134:124–132.
- Wong TY, Szeto CC, Lai KB, Lam CW, Lai KN, Li PK. Longitudinal study of peritoneal membrane function in continuous ambulatory peritoneal dialysis: Relationship with peritonitis and fibrosing factors. Perit Dial Int. 2000;20:679–685.
- Yavaşcan Ö, Aksu N, Alparslan C, et al. The importance of ultrasonographic measurement of peritoneal wall thickness in pediatric chronic peritoneal dialysis patients. Ren Fail. 2015;37:381–386.

