Abstract
Metformin, belonging to a class of drugs called biguanides, is the recommended first-line treatment for overweight patients with type 2 diabetes mellitus. It has multiple mechanisms of action, such as reduction of gluconeogenesis, increases peripheral uptake of glucose, and decreases fatty acid oxidation. However, a potential serious complication, defined metformin-associated lactic acidosis (MALA), is related to increased plasma lactate levels, linked to an elevated plasma metformin concentrations and/or a coexistent condition altering lactate production or clearance. The mortality rate for MALA approaches 50% and metformin has been contraindicated in moderate and severe renal impairment, to minimize its potential toxic levels. Nevertheless, metformin prescription or administration, despite the presence of contraindications or precipitating factors for MALA, was a common topic highlighted in all reviewed papers. Routine assessment of metformin plasma concentration is not easily available in all laboratories, but plasma metformin concentrations measured in the emergency room could ensure the correct diagnosis, eliminating metformin as the cause of lactic acidosis if low plasma levels occurred. Renal replacement therapies have been successfully employed to achieve the correction of metabolic acidosis and rapidly remove metformin and lactate, but the optimal treatment modality for MALA is still controversial.
Introduction
Metformin, belonging to a class of drugs called biguanides, oral agents used in the management of non-insulin dependent diabetes mellitus, is the recommended first-line treatment for overweight patients with type 2 diabetes mellitus (T2DM), and accounts for one-third of all orally active diabetes drugs prescribed in the USA. The biguanide class also included phenformin and buformin, withdrawn from most pharmaceutical markets due to the elevated risk of causing lactic acidosis.Citation1
Metformin has multiple not been completely elucidated mechanisms of action. It reduces gluconeogenesis, increases peripheral uptake of glucose, and decreases fatty acid oxidation. From a pharmacokinetic point of view, metformin is little associated with plasma proteins and unmetabolized excreted in the urine, without direct nephrotoxic action. The half-life is approximately 6.5 h in individual with normal renal function, extending in patients with severe renal failure.Citation2
Recent studies have shown beneficial pleiotropic effects of metformin in patients with polycystic ovary syndromeCitation3 and metabolic syndrome.Citation4 Moreover, in experimental gentamicin-related renal injury, nephrotoxicity was significantly decreased by metformin administration, confirming its pleiotropic effects.Citation5
However, its use in clinical practice is limited to side effects, such as lactic acidosis.Citation6
Two different types of lactic acidosis, related to metformin therapy, have been proposed. In particular, MALA (metformin-associated lactic acidosis), is caused by metformin accumulation in presence of precipitating factors, such as acute kidney injury or dehydration; less common is MILA (metformin-induced lactic acidosis), when metformin seems to be the only cause of lactic acidosis without apparent associated pathology and is usually related to acute intoxication.Citation7
MALA, strictly defined by arterial lactate >5 mmol/L and blood pH <7.35 within the context of recent metformin exposure, represents a rare but worrisome complication, with a mortality rate ranging from 10% to 45%.Citation8
Identified risk factors for MALA include acute kidney injury, hypoxemia, sepsis, alcohol abuse, liver failure, radiological contrast media administration, myocardial infarction, and shock.Citation9
Moreover, medications that interfere with renal hemodynamic autoregulation (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and non-steroidal anti-inflammatory drugs) and volume depletion, secondary to gastrointestinal losses, are frequently implicated in generating acute kidney injury leading to MALA.Citation10
Furthermore, the excellent efficacy and safety profile of metformin use in patients with diabetes have resulted in poor patient selection and ignoring safety guidelines, with recent reports suggesting increasing numbers of cases of MALA around the world.
The aim of our study was to better analyze this complication, frequently involving intensivists and nephrologists’ activity.
MALA: from epidemiologic data to physiopathological mechanisms
The incidence of MALA widely differs in published studies. A recent meta-analysis found no evidence of increased incidence of lactic acidosis in patients treated with metformin (4 per 100,000 person-years) when compared to other anti-hyperglycemic treatments (5 per 100,000 person-years).Citation11
Furthermore, retrospective studies demonstrated higher incidence of MALA than those reported in clinical trials, probably secondary to a better prescription of the drug, accordingly to contraindications or underlying conditions, such as renal failure.Citation12
In fact, MALA incidence drops when the contraindications and correct rules of prescription are respected, such as hypoxic conditions, impaired lactate clearance, and impaired metformin clearance.
In clinical practice, chronic renal failure represents the main limitation to metformin use. Its catabolism exclusively depends on kidneys through tubular secretion, with its progressive hematic accumulation in case of glomerular filtration rate (GFR) lower than 30 mL/min. Current guidelines recommend that the dose of metformin should be reviewed if GFR is <45 mL/min/1.73 m2 and that metformin should be discontinued in patients in whom GFR falls to <30 mL/min/1.73 m2.Citation13
No limitation for its use is required for patients with stable chronic renal failure, with positive effects on cardiovascular mortality and myocardial infarction risk.Citation14–16
Moreover, patients with type 2 diabetes on insulin randomized to the addition of either metformin or placebo had a 39% reduction in macro-vascular events.Citation17
The relationship between metformin and lactic acidosis is currently a subject of debate; despite the recent Cochrane review in which no contraindications were provided against administering metformin,Citation11 several authors have provided evidence for a relationship between this drug and the development of this important complication, when metformin was added.
Vecchio et al. recently demonstrated that metformin plasma concentrations were closely correlated with creatinine, pH, and plasma lactate levels, whereas lactate and metformin mean values were not statistically different in surviving and deceased patients.Citation18
However, low arterial blood pH and normal renal function were predictors of death in MALA patients, confirming that the accumulation of the drug is less dangerous than other coexisting risk factors for lactic acidosis.Citation19
In addition, hypotension, elevated anion gap, hyperglycemia, and coma may be prognostic of severe or fatal outcome, as well as prothrombin activity, reflecting liver activity.Citation20
Mortality in MALA patients ranged between 10.8% and 45% in the largest case series reported. Therefore, it can be confirmed that MALA is frequently fatal, depending on a correct and prompt diagnosis and aggressive therapy.
Reported cases of metformin overdose provide insight about mechanisms linking metformin accumulation, increased plasma lactate levels and development of lactic acidosis. A retrospective analysis demonstrated a strong correlation between increased circulating metformin concentrations and decreased arterial pH. In particular, patients who died had 100% higher circulating plasma metformin levels, 30% higher plasma lactate concentrations and lower arterial pH than survivors. In addition, excessive dose of metformin, in the absence of an intercurrent precipitating event, could cause MALA even in healthy individuals.Citation21
The physiopathological mechanisms of MALA are not well known. This anti-diabetic drug exerts its prevailing glucose-lowering effect inhibiting hepatic gluconeogenesis by reducing hepatic lactate reuptake and opposing the action of glucagon. The inhibition of mitochondrial complex I results in defective cyclic adenosine monophosphate (cAMP) and protein kinase A signaling in response to glucagon.Citation22
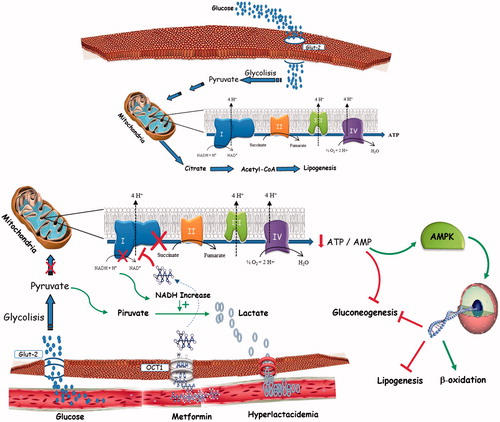
However, high hematic levels of metformin (4 mg/L is considered the high side of the target range) interfere with mitochondrial respiratory chain complex and then with the oxidative metabolism of lactate, inducing a non-hypoxic lactic acidosisCitation23 ().
Figure 1. Lactate metabolism in physiological condition and in MALA patients.MALA may be attributed to mitochondrial impairment and subsequent generation of lactate, which finally effluxes into the circulation rather than being oxidized further.Metformin raises lactic acid levels by affecting the redox potential and promoting anaerobic metabolism, leading to a decrease in the ATP:ADP ratio and an increase in the NADH:NAD ratio. This can result in an accumulation of pyruvate (by inhibition of pyruvate dehydrogenase) that is later converted into lactate.
Treatment options
Measurement of metformin plasma concentration could confirm the involvement of the drug in patients with suspected MALA. The mean plasma concentrations of metformin in healthy subjects fluctuate between about 0.5 and 1 mg/L while fasting and 1 to 2 mg/L after a meal.Citation24
Metformin concentrations in erythrocytes may be more useful than plasma levels because it better represents tissue accumulation.Citation25
Moreover, the slow decline in erythrocyte metformin concentration may therefore contribute to retrospective diagnosis of metformin accumulation.
Even though low metformin blood concentration could exclude MALA, the contrary is not necessarily true. In fact, patients may present a wide clinical spectrum in which metformin levels do not correlate well to symptoms. However, high metformin levels are commonly associated with more severe lactic acidosis.Citation26
Repeated measurements of plasma or erythrocyte metformin levels lactate and pH will not only assist in the diagnosis of MALA, but also help its management. These measures could indicate when dialysis should be started or stopped.
After diagnosis, treatment should rapidly involve alkalinization, vital function support, treatment of underlying conditions, forced diuresis, and/or hemodialysis (HD) to rapidly eliminate the drug.Citation27,Citation28
Sodium bicarbonate could be administered to treat the metabolic acidosis, but it must be carefully managed, due to potential iatrogenic hypernatremia, hyperosmolality, volume overload, decreased cardiac contractility and even alkalosis.
It is well known that metformin, characterized by a molecular weight lower than 500 Da, is highly water soluble and weakly bound to proteins. Moreover, a large volume of distribution (3.1 L/kg), secondary to intracellular penetration, characterized the drug kinetic,Citation29 explaining the difficult to effectively dialyze the substance and achieve an adequate and rapid elimination.
In fact, it was estimated that dialysis may be most effective if it is performed before metformin redistributes into the tissues. Furthermore, due to a two-compartment model of distribution, adequate metformin elimination requires prolonged dialysis sessions (duration greater than 4 h).Citation1
The optimal treatment modality for MALA is controversial and relies on nonspecific supportive measures.
Renal replacement therapies, including conventional HD and continuous veno-venous hemofiltration, have been successfully employed, allowing for both isovolemic correction of the metabolic acidosis as well as removal of metformin and lactate.
Bicarbonate HD is recommended to decrease metformin levels and correct acidosis, but its optimal duration has not been determined.Citation30
Sequential measurements of metformin levels during HD confirm a bi-compartmental elimination model. Moreover, 15-h HD session was associated with the normalization of hematic metformin value within its therapeutic range.Citation31
Moreover, Nguyen, dosing pre and post-HD drug levels, assessed that a single dialysis session could remove about 60% of total amount of metformin.Citation32
Beyond HD, continuous renal replacement therapy (CRRT) (veno-venous hemofiltration or hemodiafiltration), has been proposed as therapy option of MALA, especially in patients with hemodynamic instability.
Keller et al. demonstrated that CRRT might be a superior choice for MALA, rapidly correcting metabolic disorders, gradually removing solutes with a prolonged physiologically steady state.Citation33
However, there are some concerns about using renal replacement therapy to manage MALA. In fact, it is not certain whether rapid metformin elimination is an appropriate end-point in studies of MALA therapy, due to the lack of relation between metformin, lactate concentrations, and prognosis.
Large series reports and controlled studies may better determine the optimal duration and best dialysis technique in these patients.
Conclusive opinions
The worldwide prevalence of T2DM has risen dramatically over the past two decades and metformin represents the mainstay of anti-hyperglycemic therapy, providing numerous beneficial effects.Citation34 A rare but severe adverse reaction is the development of MALA, associated to high mortality rate. However, its prevalence is high variable and potentially underestimated. In fact, this condition normally develops with nonspecific symptoms, such as general discomfort and abdominal symptoms, including diarrhea, nausea, and vomiting, representing common critical triggers ().
Figure 2. Triggers for metformin associated lactic acidosis.Lactic acidosis may be secondary to relatively small changes in hydration, kidney function, plasma concentrations of metformin or tissue oxygenation leading to severe lactic acidosis.
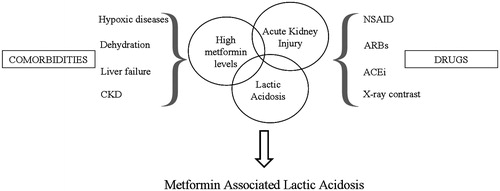
The worsening of renal function often depends on hypovolemic status and pharmacological nephrotoxicity, caused by metformin accumulation. Moreover, the concomitant administration of nephrotoxic drugs, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and non-steroidal anti-inflammatory drugs, and associated illness, as well as liver and cardiac diseases, could play a detrimental role.
We have assessed that most MALA cases are related to an inappropriate use of metformin in patients with risk factors. Metformin prescription or administration, despite the presence of contraindications or precipitating factors for MALA, was a common issue highlighted in all reviewed papers.
We believe that in the absence of absolute contraindications, there is no obvious reason to deny patients clinical benefits of metformin therapy. Clinicians who start metformin treatment should assess renal function and instruct patients to discontinue the medication and consult their doctor in case of severe vomiting and/or diarrhea, or other hypovolemic conditions.
A correct prescription of this dug represents the main explanation of data provided by the COSMIC trial and a Cochrane review, which evaluated more than seventy thousand patients treated with metformin, reporting no increase of lactic acidosis incidence and prevalence.Citation11,Citation35
However, MALA has very poor prognosis if it is not diagnosed and treated early.
Routine assessment of metformin plasma concentration is not easily available in all laboratories. Plasma metformin concentrations, ideally measured in the emergency room, ensure the correct diagnosis, eliminating metformin as the cause of lactic acidosis in patients with low plasma levels. However, the concentration of metformin in erythrocytes may be more useful, since it better reflects tissue accumulation that cannot be revealed by plasma evaluation.
Renal replacement therapies have been successfully employed to achieve the correction of metabolic acidosis and rapid removal of metformin and lactate.
There is no consensus about the best replacement therapy modality, but dialysis appears to be the first-line treatment in association with symptomatic organ failure treatment.
In fact, whereas sodium bicarbonate must be managed carefully, the dialytic therapy could correct electrolytes and metabolic alterations without further risks, except the risk of central venous catheterization.
Moreover, precipitating and underlying conditions of metformin accumulation should be early recognized and immediately corrected, in addition to dialysis therapy.
Renal replacement therapy can improve the prognosis of severe cases, if started early. A close monitoring in intensive care unit, together with prompt and repeated dialysis sessions, can lead to favorable outcomes, but the management will vary accordingly the severity of each patient.
Larger and prospectively designed studies are clearly needed to draw firm recommendations and strategies for MALA management.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Guo PY, Storsley LJ, Finkle SN. Severe lactic acidosis treated with prolonged hemodialysis: Recovery after massive overdoses of metformin. Semin Dial. 2006;19:80–83.
- Frid A, Sterner GN, Löndahl M, et al. Novel assay of metformin levels in patients with type 2 Diabetes and varying levels of renal function: Clinical recommendations. Diabetes Care. 2010;33:1291–1293.
- Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35.
- Bellinghieri G, Bernardi A, Piva M, et al. Metabolic syndrome after kidney transplantation. J Ren Nutr. 2009;19:105–110.
- Morales AI, Detaille D, Prieto M, et al. Metformin prevents experimental Gentamicin-Induced Nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–869.
- Scheen AJ, Paquot N. Metformin revisited: A critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013;39:179–190.
- Lalau JD, Race JM. Lactic acidosis in metformin therapy: Searching for a link with metformin in reports of ‘metformin-associated lactic acidosis’. Diabetes Obes Metab. 2001;3:195–201.
- Lalau JD. Lactic acidosis induced by metformin: Incidence, management and prevention. Drug Saf. 2010;33:727–740.
- Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008;5:157–167.
- Rocha A, Almeida M, Santos J, et al. Metformin in patients with chronic kidney disease: Strengths and weaknesses. J Nephrol. 2013;26:55–60.
- Salpeter SR, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(4):CD002967.
- Vecchio S, Protti A. Metformin-induced lactic acidosis: No one left behind. Crit Care. 2011;15:107.
- Klachko D, Whaley-Connell A. Use of metformin in patients with kidney and cardiovascular diseases. Cardiorenal Med. 2011;1:87–95.
- Pilmore HL. Review: Metformin: Potential benefits and use in chronic kidney disease. Nephrology. 2010;15:412–418.
- Nye HJ, Herrington WG. Metformin: The safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118:380–383.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589.
- Kooy A, Jager J, Lehert P, et al. Long term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625.
- Vecchio S, Giampreti A, Petrolini VM, et al. Metformin accumulation: Lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol (Phila). 2014;52:129–135.
- Renda F, Mura P, Finco G, et al. Metformin-associated lactic acidosis requiring hospitalization. A national 10 year survey and a systematic literature review. Eur Rev Med Pharmacol Sci. 2013;17:45–49 [Database].
- Spiller A, Quadrani DA. Toxic effects from metformin exposures. Ann Pharmacother. 2004;38:776–780.
- Al-Abri SA, Hayashi S, Thoren KL, Olson KR. Metformin overdose-induced hypoglycemia in the absence of other antidiabetic drugs. Clin Toxicol (Phila). 2013;51:444–447.
- Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174.
- Protti A, Lecchi A, Fortunato F, et al. Metformin overdose causes platelet mitochondrial dysfunction in humans. Crit Care. 2012;16:R180.
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98.
- Lalau JD, Lacroix C. Measurement of metformin concentration in erythrocytes: Clinical implications. Diabetes Obes Metab. 2003;5:93–98.
- Perrone J, Phillips C, Gaieski D. Occult metformin toxicity in three patients with profound lactic acidosis. J Emerg Med. 2011;40:271–275.
- Pan LT, Maclaren G. Continuous venovenous haemodiafiltration for metformin-induced lactic acidosis. Anaesth Intensive Care. 2009;37:830–832.
- Buemi M, Lacquaniti A, Bolignano D, et al. Dialysis and the elderly: An underestimated problem. Kidney Blood Press Res. 2008;31:330–336.
- Sambol NC, Chiang J, O’Conner M, Liu CY, Lin ET, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–1021.
- Gan SC, Barr J, Arieff AI, Pearl RG. Biguanide-associated lactic acidosis. Case report and review of the literature. Arch Intern Med. 1992;152:2333–2336.
- Seidowsky A, Nseir S, Houdret N, et al. Metformin-associated lactic acidosis: A prognostic and therapeutic study. Crit Care Med. 2009;37:2191–2196.
- Nguyen H. Metformin intoxication requiring dialysis. Hemodial Int. 2011;15:S68–S71.
- Keller G, Cour M, Hernu R, et al. Management of metformin-associated lactic acidosis by continuous renal replacement therapy. PLoS One. 2011;6:e23200.
- Lacquaniti A, Donato V, Pintaudi B, et al. “Normoalbuminuric” diabetic nephropathy: tubular damage and NGAL. Acta Diabetol. 2013;50:935–942.
- Cryer DR, Nicholas SP, Henry DH, et al. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care. 2005;28:539–543.

