Abstract
Objectives
Concerns are increasing about the clinical characteristics of gram- negative bacterial peritonitis for providing reference for clinical diagnosis, treatment and prevention.
Methods
A retrospective analysis was performed examining patients who developed peritoneal dialysis-related peritonitis (PDRP) from 1 January 2009 to 31 December 2018.
Results
Among 898 PD patients, 677 episodes of peritonitis occurred in 344 patients. Over 10 years, the proportion of gram-negative bacterial peritonitis increased from 0% to 26.15% (p = .045). E. coli was the leading cause (38.51%) of the 148 cases of gram-negative bacterial peritonitis. The increase of E. coli peritonitis between the first 5 and the last 5 years was obvious (20.45% vs. 46.15%). The antimicrobial sensitivity of gram-negative organisms to cefotaxime decreased from 71.43% to 55.84% (p = .017). In the gram-negative group, the effluent white cell count (WCC) on the first day was larger (OR: 1.374;95%CI: 1.248–1.563; p < .001), the time required for the WCC to normalize was longer (OR: 1.100;95%CI: 1.037–1.189; p = .003), and the level of C-reactive protein (CRP) was higher (OR: 1.038;95%CI: 1.026–1.042; p < .001) than those in the gram-positive group. The complete cure rate and treatment failure rate of gram-negative bacteria peritonitis were 87.8% and 12.2% respectively.
Conclusions
Over 10 years, the proportion of gram-negative bacterial peritonitis increased, with E. coli epidermidis being the most common pathogen. More effluent WCC on the first day, longer time required for the WCC to normalize, and higher level of CRP are more common for gram-negative bacterial peritonitis. Prognosis of gram-negative bacterial peritonitis is worse.
1. Introduction
Peritoneal dialysis-related peritonitis (PDRP) is a primary complication associated with peritoneal dialysis (PD) treatment and is a common cause of dialysis failure and patient death [Citation1]. Recently, the incidence of peritonitis has decreased significantly due to improvements in the peritoneal dialysis connection system, the accumulation of experience, and the emphasis on education [Citation2]. However, due to the widespread use of antibiotics, the pathogenic spectrum associated with PDRP and the development of antibiotic resistance has evolved in different regions [Citation3]. Studies have shown that the incidence of gram-positive bacterial peritonitis decreased significantly, from 0.26 to 0.12 episodes per patient-year, whereas the gram-negative bacterial peritonitis rate did not change [Citation4]. Some scholars [Citation5] have found that the incidence rate and proportion of gram-negative bacterial peritonitis have increased gradually in recent decades, and the relapse and recurrence rate for gram-negative bacterial peritonitis is higher than that for other pathogens, with severe clinical manifestations and poor prognosis. At present, few studies have examined gram-negative bacterial peritonitis in China. Therefore, we retrospectively analyzed the clinical data of PDRP patients from January 2009 to December 2018 and evaluated the PDRP-associated pathogens and antibiotic resistance to guide the rational use of antibiotics and improve the cure rate for PDRP.
2. Materials and methods
2.1. Case selection
All episodes of PD-related peritonitis that occurred at the peritoneal dialysis center of the Second Affiliated Hospital of Suzhou University, from 1 January 2009 to 31 December 2018, were reviewed. During the study period, 617 episodes of peritonitis were recorded, and all case records were reviewed. The vast majority of patients had received a Tenckhoff catheter and were being dialyzed using continuous ambulatory PD with lactate-buffered glucose dialysate in a twin-bag connection system (Baxter Healthcare, Guangzhou, China). Patient demographic information, clinical symptoms, the results of the most recent laboratory examinations before peritonitis, the microbiology and antimicrobial sensitivity results, therapeutic responses, and clinical outcomes were examined.
PD patients were divided into two groups based on the results of dialysate effluent cultures: gram-positive bacteria group and gram-negative bacteria group. The peritonitis rates associated with different pathogenic bacteria were calculated, and the changes in the incidence rate associated with different pathogenic bacteria were analyzed. The patients were further divided into two groups based on the timing of the peritonitis incident: the 2009–2013 group and the 2014–2018 group. Differences in the composition of the pathogenic bacteria spectrum and the sensitivity to commonly used antibiotics were compared between the two time periods.
2.2. Diagnosis and treatment of peritonitis
Peritonitis was diagnosed according to the International Society of Peritoneal Dialysis (ISPD) guidelines, established in 2016 [Citation6], requiring at least two of the following three indicators: (1) the presence of peritonitis symptoms and signs, such as abdominal pain and a cloudy dialysate effluent, either with or without fever; (2) a white blood cell count (WCC) in the dialysate effluent greater than 100 × 106/L, comprised of greater than 50% neutrophils; and (3) the identification of a pathogenic microorganism, by staining or culturing the dialysate effluent. Relapse infections, as defined by the ISPD guidelines, were counted as one single episode, whereas recurrent and repeat infections were counted as separate episodes. The management of PDRP at our center involved empirical, anti-infective treatments. Antimicrobial therapies utilized first-generation treatments of cephalosporin or vancomycin, combined with a third-generation cephalosporin treatment or aminoglycoside drugs, which were administered intraperitoneally. The antibiotics were adjusted according to the results of dialysate effluent cultures and drug sensitivity tests.
2.3. The definition of peritonitis prognosis
Peritonitis clinical outcomes were divided into cure and failure. A cure was defined as a WCC below 100 × 106/L in the dialysate effluent, and negative culture results after antibiotic treatment. Treatment failure included the conversion to permanent hemodialysis and peritonitis-related death. Death related to peritonitis was defined as the death of a patient due to peritonitis, during hospitalization for peritonitis, or within 4 weeks of peritonitis [Citation6].
2.4. Statistical analysis
Statistical analysis was performed using SPSS 22.0. Continuous variables that conformed to normal distributions were expressed as the mean ± standard deviation, whereas categorical variables with normal distributions were expressed as numbers and percentages. An independent-sample t-test was used to compare the measurement data between groups; the Chi-square test was used to compare the constituent ratios of the pathogenic bacteria spectra and drug sensitivity rates between different time periods. Poisson regression was used to test the incidence rate of peritonitis. Multivariate logistic regression analysis was used to screen the influencing factors associated with the incidence of different bacterial types associated with peritonitis, from which the value of ORs was obtained. For all comparisons, p < .05 was significant.
3. Results
3.1. Peritoneal dialysis-related peritonitis caused by gram-negative bacteria rates over ten years
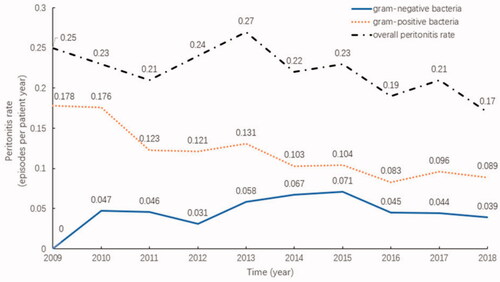
From 2009 to 2018, 898 PD patients were admitted to our center, all of whom were permanent residents of Suzhou. During this period, 677 episodes of peritonitis occurred in 344 PD patients. Among of them, 241(70.06%) experienced just 1 episode of peritonitis, 57(16.57%) had 2 episodes and 46(13.37%) had 3 or more episodes. The overall incidence of peritonitis decreased from 0.25 episodes per patient-year in 2009 to 0.17 episodes per patient-year in 2018, revealing a general downward trend (p = .088). The highest peritonitis rate was 0.27 episodes per patient-year, which was recorded in 2013, and the lowest was 0.17 episodes per patient-year in 2018. The infection rate associated with gram-positive bacteria significantly decreased (p = .006), and the infection rate associated with gram-negative bacteria did not change significantly (p = .288), as shown in .
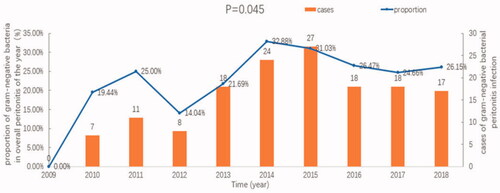
The percentage of gram-negative bacterial peritonitis among all peritonitis cases from 2009 to 2018 was analyzed. The results showed that the proportion of gram-negative bacteria increased over the 10-year study period (p = .045), as shown in .
3.2. Population characteristics of gram-negative bacterial peritonitis
From 1 January 2009 to 31 December 2018, 148 episodes of peritonitis occurred among the 109 patients in the gram-negative bacteria group, which including 53 men (48.62%) and 56 women (51.37%), with an average age of 60.98 ± 14.11 years. The primary diseases resulting in the necessity of dialysis included: chronic glomerulonephritis, in 54 cases (49.54%); diabetic nephropathy, in 19 cases (17.43%); hypertensive nephropathy, in 17 cases (15.59%); polycystic kidney, in 7 cases (6.42%); lupus nephritis, in 3 cases (2.75%); vasculitis nephropathy, in 2 cases (1.83%); and unknown nephropathy, in 7 cases (6.42%).
3.3. Bacterial spectrum and changes of gram-negative bacterial peritonitis
In the 148 cases of gram-negative bacterial peritonitis, the most common pathogens were Escherichia coli, in 57 cases (38.51%), followed by Klebsiella pneumoniae, in 29 cases (19.59%), and Enterobacter cloacae, in 15 cases (10.14%).
Comparing the compositions of pathogenic bacteria associated with gram-negative bacterial peritonitis between the 2009–2013 and 2014–2018 groups indicated that the numbers of gram-negative bacterial peritonitis, particularly E. coli-induced peritonitis, significantly increased in the 2014–2018 group compared with those in the 2009–2013 group. Compared with the proportions of other gram-negative bacteria, the proportion of E. coli in the 2014–2018 group was significantly larger (p = .039), as shown in .
Table 1. Bacterial composition associated with peritonitis, according to study groups [n (%)].
3.4. Antimicrobial sensitivity and resistance of gram-negative bacterial peritonitis
Among the various antimicrobials used, the gram-negative bacteria group showed the highest sensitivities to meropenem, amikacin, imipenem, cefoperazone/sulbactam, piperacillin/sulbactam, with sensitivity rates of 98.5 7%, 97.84%, 97.12%, 90.91%, and 86.86%, respectively. Compared with the 2009–2013 group, the antimicrobial sensitivity of gram-negative bacteria to cefotaxime decreased, and overall antimicrobial resistance increased in the 2014–2018 group (p = .017). No significant changes were observed for the response to other commonly used antibiotics. These results are shown in .
Table 2. Antimicrobial sensitivity and resistance against commonly used antibiotics in cases of gram-negative bacterial peritonitis.
3.5. Peritoneal dialysis-related peritonitis caused by Escherichia coli
3.5.1. Population characteristics
E. coli was the most common causative organism associated with gram-negative peritonitis in PD patients. A total of 57 episodes of E. coli-associated peritonitis occurred, including 3 cases that were extended-spectrum beta-lactamase (ESBL) positive, which accounted for 5.26% of E. coli peritonitis. E. coli-associated peritonitis was recorded in 41 patients, including 19 men (46.34%) and 22 women (53.66%), with an average age of 59.75 ± 15.40 years. The demographic and clinical characteristics of this group can be found in .
Table 3. The demographic and clinical characteristics of patients diagnosed with E. coli peritonitis.
3.5.2. The incidence of E. coli-associated peritonitis each year
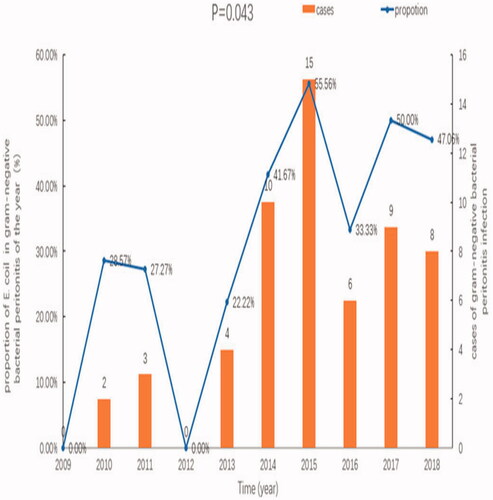
The incidence rates of E. coli-associated peritonitis for 2009–2018 (episodes per patient-year) were 0, 0.014, 0.013, 0, 0.013, 0.028, 0.039, 0.015, 0.022, and 0.017, respectively. An upward trend was observed, but this was not significant (p = .611). The proportions of E. coli-associated peritonitis among all cases of gram-negative bacterial peritonitis were 0%, 28.57%, 27.27%, 0%, 22.22%, 41.67%, 55.56%, 33.33%, 50.00%, and 47.06%, respectively for each of the 10 years, suggesting that the proportions of E. coli-associated peritonitis increased gradually (p = .043), as shown in .
3.5.3. Antimicrobial sensitivity and resistance in E. coli-associated peritonitis
E. coli-associated peritonitis was sensitive to imipenem, meropenem, amikacin, cefoperazone/sulbactam, and piperacillin/tazobactam, with sensitivities of 100%, 100%, 100%, 92.84%, and 89.26%, respectively. No significant changes in antimicrobial sensitivity or resistance for E. coli in response to commonly used antibiotics was observed between the 2009–2013 and the 2014–2018 groups, as shown in .
Table 4. Antimicrobial sensitivity and resistance in response to commonly used antibiotics in E. coli-associated peritonitis.
3.5.4. E. coli-associated peritonitis outcomes
During 2009–2018, among the 57 recorded episodes of E. coli-associated peritonitis, 47 episodes were cured, with a total cure rate of 82.46%. Treatment failure outcomes included the conversion to permanent hemodialysis and death associated with peritonitis. A total of 10 episodes ended in failure, including 7 patients who experienced permanent hemodialysis transfer and 3 deaths. Comparing the prognosis between the 2009–2013 and the 2014–2018 groups revealed no significant difference between groups (p = .151), as shown in .
Table 5. Comparison of outcomes between the two groups.
3.6. Risk factors of gram-negative bacterial peritonitis
The patients were divided into gram-negative and gram-positive bacteria groups according to the dialysate effluent culture results. The mean age of the patients in the gram-negative bacteria group was significantly higher than that in the gram-positive bacteria group (p = .014). The mean dialysis time for the gram-negative bacteria group was shorter than that for the gram-positive bacteria group, but the difference was not significant (p = .064).The proportions of patients with fever (p < .001), abdominal pain (p = .001), and muddy dialysate effluent (p = .001) in the gram-negative bacteria group were significantly higher than those in the gram-positive bacteria group.No significant differences were observed in hemoglobin levels, the nutrition index, electrolyte levels, or other indicators between the gram-negative and gram-positive bacteria groups (p > .05). The serum creatinine level of the gram-negative bacteria group was lower (p = .006) than that in the gram-positive bacteria group, but the effluent WCC on the first day of peritonitis was higher, the time required for the WCC to normalize was longer, and the level of C-reactive protein (CRP) was higher in the gram-negative bacteria group (p < .05) than in the gram-positive bacteria group, as shown in .
Table 6. Comparison of characteristics between the two groups.
The gram-negative and gram-positive bacteria were treated as dependent variables, and the differences between the two types of peritonitis were treated as independent variables for the multivariate logistic regression analysis. The results showed that effluent WCC on the first day (OR: 1.374;95%CI: 1.248–1.563; p < .001), the time required for the WCC to normalize (OR: 1.100;95%CI: 1.037–1.189; p = .003), and CRP levels (OR: 1.038;95%CI: 1.026–1.042; p < .001) were related to the types of pathogens. The possibility of gram-negative bacteria increased by 10% with each day of delay in the recovery of effluent WCC to normalize, as shown in .
Table 7. Logistic multivariate regression analysis for different types of bacterial peritonitis.
3.7. Outcome analysis of gram-negative bacterial peritonitis
A total of 455 episodes of peritonitis were recorded in this study, including 148 episodes of gram-negative bacterial peritonitis and 307 episodes of gram-positive bacterial peritonitis. Treatment failure outcomes included the conversion to permanent hemodialysis and death associated with peritonitis. The prognosis of peritonitis was compared between the gram-negative and gram-positive bacteria groups, which showed that 18 episodes ended in treatment failure in the gram-negative bacteria group, which represented a significantly higher proportion (12.2%; p = .036) than that in the gram-positive bacteria group, including 8 patients transferred to permanent hemodialysis and 10 deaths ().
Table 8. Clinical outcomes of peritonitis, according to group [Case (%)].
Further analysis of the number of cured, transferred to hemodialysis and dead patients in the non-E. coli gram-negative peritonitis group and the E. coli peritonitis group showed that there was a significant difference in the prognosis between the two groups in 10 years (p = .013), as shown in .
Table 9. Comparison of prognosis between non-E. coli gram-negative bacteria group and E. coli bacteria group [cases (%)].
4. Discussion
This is a confirmatory study, which found that the average incidence of gram-negative bacterial peritonitis decreased, but the proportion increased over time. E. coli was the most common pathogen of the gram-positive organisms. Except for cefotaxime, the antimicrobial sensitivity of gram-negative bacteria to other antibiotics did not change. This study is the first to find that more effluent WCC on the first day, longer time required for the WCC to normalize, and higher level of CRP were more common for gram-negative bacterial peritonitis. Furthermore, we analyze the prognosis of E. coli peritonitis and non-E. coli gram-negative peritonitis. The results show that the prognosis of non-E. coli peritonitis was better.
In our study, we found that the overall incidence of peritonitis at our center decreased, from 0.25 episodes per patient-year in 2009 to 0.17 episodes per patient-year in 2018, which was far lower than the 0.50 episodes per patient-year reported by the 2016 ISPD guidelines for peritonitis [Citation6]. These low rates may be due to the continuously strengthened management measures that have been implemented at our center. A prospective study on the incidence and outcome of peritonitis in seven countries found that higher automated PD use, used antibiotics at catheter insertion and PD training duration of 6 or more days could significantly reduce the incidence of peritonitis [Citation7].
The incidence of overall peritonitis and gram-positive bacterial peritonitis decreased during the 10-year study period, but gram-positive organisms still had the predominant position. The proportion of gram-negative bacterial peritonitis among total peritonitis cases increased year by year. This is basically consistent with the results of Hwang et al. [Citation8]. The number of gram-negative bacterial peritonitis episodes in the 2014–2018 group was significantly higher than that in the 2009–2013 group, which is consistent with the conclusion that gram-negative bacterial peritonitis has demonstrated an increasing trend, which has been reported by several countries and regions [Citation9]. Over the past 10 years, gram-negative bacterial peritonitis accounted for 20%–30% of total peritonitis cases. At the peritoneal dialysis center of the First Affiliated Hospital of Sun Yat-Sen University from 2001 to 2005, gram-negative bacterial infections accounted for 35.5% of the cultured bacteria [Citation10]. These findings indicated that gram-negative bacteria have increasingly become an important causal pathogen for peritonitis.
Some studies have reported that among all gram-negative bacteria, Enterobacteriaceae is the most common bacteria associated with PDRP [Citation10]. According to our central data, the incidence of E. coli-associated peritonitis was 38.51%, which was the largest proportion of gram-negative peritonitis at our center. K. pneumoniae and E. cloacae, which belong to Enterobacteriaceae, were the second and the third most common, respectively. Compared with the 2009–2013 group, the number of E. coli-associated peritonitis episodes significantly increased in the 2014–2018 group, and some studies have reported that E. coli accounted for more than 50% of all gram-negative bacterial peritonitis cases [Citation11,Citation12]. Research data from Taiwan showed that Enterobacteriaceae bacterial peritonitis accounted for 12% of peritonitis cases at one center from 1995 to 2004. The most common bacteria in this study was reported to be E. coli, which accounted for 53% of Enterobacteriaceae bacterial peritonitis [Citation13]. One study suggested that this high proportion is due to peritoneal lesions in peritoneal dialysis patients [Citation14]. This study also found that the proportion of P. aeruginosa increased significantly from 2014 to 2018, with marginal significance. Other studies have also reported [Citation15] that P. aeruginosa is the main pathogenic bacteria of export infection and tunnel infection. Ozisik [Citation14] also confirmed that catheter-related infection is a risk factor for peritonitis. Therefore, the increase of peritonitis associated with P. aeruginosa in our center during the most recent five years of the study period may be related to these types of infections; however, these findings should be confirmed in future studies.
It is very important to determine the choice of empirical antibiotic treatments. A study in Australia found that the use of antibiotics directly affects the prognosis of peritonitis [Citation16]. Once the PD effluent Gram stain or culture and sensitivity results are available, antibiotic therapy can be adjusted accordingly [Citation17]. For gram-negative bacterial peritonitis, the initial treatment plan adopted by our center is the use of third-generation cephalosporins or aminoglycoside antibiotics, according to the recommendations found in the 2016 ISPD guidelines. Gram-negative bacteria are highly sensitive to meropenem, amikacin, imipenem, cefoperazone/sulbactam, and piperacillin/sulbactam. The use of antibiotics at the same time will inevitably lead to the problem of antibiotic resistance, which has seriously threatened the global public health system [Citation18]. We have done relevant research and found that compared with the 2009–2013 group, the resistance of gram-negative bacteria to cefotaxime increased significantly, and the sensitivity decreased significantly in the 2014–2018 group. But for the other third-generation of cephalosporins, ceftazidime, and cefoperazone/sulbactam, the antibiotic resistance also increased, to varying degrees. The results reported by Kitter et al. [Citation19] were similar to those found at our center, with a gradual increase in the resistance of gram-negative bacteria against third-generation cephalosporins and a decrease in the sensitivity to ceftazidime, from 100% to 84%. Certain organisms, particularly gram-negative organisms, undergo genetic mutations when challenged with antibiotics, which apply a selection pressure for beta-lactamase-producing mutants [Citation20], allowing the organism to become resistant to cephalosporins. This study also showed that the sensitivity and drug resistance of gram-negative bacteria to amikacin did not change significantly. The study by McGuire [Citation2] supported our view that gentamicin did not increase drug resistance due to the widespread use of empirical treatment. In this study, we found that the treatment with carbapenems against gram-negative bacteria demonstrated a sustained level of effectiveness over the 10-year study period. Studies performed by Leung et al. [Citation21] showed that the efficacy of carbapenems for the treatment of PDRP was equivalent to that for cefazolin or ceftazidime combined with netilmicin; however, the exact efficacy of carbapenems and whether the use of this treatment is associated with a risk of flora imbalance and double infection requires confirmation in more prospective studies with larger sample sizes. Therefore, carbapenems are not recommended as part of the initial treatment. Barrett [Citation3] reported that the sensitivity of Enterobacteriaceae to gentamicin, ceftazidime, ofloxacin, imipenem, and cefepime did not change, which was similar to the results of the present study.
This study analyzed and compared the general characteristics, clinical manifestations, and laboratory indicators of patients with gram-negative and gram-positive bacterial peritonitis. The results showed that the patients with gram-negative bacterial peritonitis were older because gram-negative bacterial peritonitis was typically an enterogenous infection, and the incidence of gastrointestinal diseases was higher in older patients with peritoneal dialysis, including constipation, mesenteric ischemia, diverticulosis, and malignant tumor [Citation22]. The proportions of fever, abdominal pain, and peritoneal dialysis fluid turbidity were significantly higher in the gram-negative bacteria group than in the gram-positive bacteria group. Foreign reports [Citation23] also confirmed that the initial clinical manifestations of gram-negative bacterial peritonitis are very serious, with diarrhea and abdominal pain reported as the most common manifestations. Comparing the factors influencing peritonitis between the two groups revealed that the effluent WCC on the first day of peritonitis was higher, and the time required for the WCC normalization was longer in the gram-negative bacteria group than in the gram-positive bacteria group. The number of effluent WCC directly reflects the severity of peritonitis [Citation24]. The strong virulence of gram- negative bacteria leads to severe peritonitis [Citation25]. Therefore, the number of white blood cells in peritoneal dialysis fluid is more. Generally, the peak of effluent WCC is supposed to occur on day 1 of peritonitis [Citation26]. So the higher of the effluent WCC on the first day means the more likely the occurrence of gram-negative bacterial peritonitis. Xu Rong et al. [Citation26] also found that the change trend of effluent WCC was related to the type of peritonitis, because the cytokine immune response was delayed when gram-negative bacteria caused abdominal infection. We speculate that the above reasons lead to the prolongation of the time required for the white blood cell count of peritoneal dialysis fluid to return to normal. Therefore, the duration of leukocyte rise in peritoneal dialysis fluid has a certain predictive value for judging whether it is gram-negative bacterial peritonitis. CRP is an acute-phase protein produced by the body during an acute inflammatory reaction and represents a reliable and accurate marker of an inflammatory reaction in vivo. We also found that the level of CRP in the gram-negative bacteria group was significantly higher than that in the gram-positive bacteria group. Troidle et al. [Citation27] also noted that the elevation of CRP was most striking in patients with gram-negative peritonitis. This shows that CRP has a certain correlation with gram-negative peritonitis. High level of CRP is helpful to predict the occurrence of gram-negative peritonitis.
We found that the prognosis of gram-negative bacterial peritonitis is poor. Wei-Hung et al. [Citation28] also found that patients with gram-negative bacterial peritonitis had higher risks of hospitalization, extubation, permanent high-risk hemodialysis metastasis, and death. The poor prognosis of gram-negative bacterial peritonitis may be associated with contact contamination, outlet infection, or the cross-wall migration of constipation, colitis, bacteria, or abdominal cavity infections. The exact etiology of gram-negative bacterial peritonitis remains unclear. Some studies [Citation29] suggested that the prognosis of peritonitis caused by gram-negative bacteria, such as E. coli, may be worse due to biofilm production [Citation30]. The virulence of E. coli is more severe than previously reported, resulting in a worse prognosis among PD patients with E. coli-associated peritonitis. Since the 1980s, the incidence rate of E. coli-associated peritonitis caused by ESBL strains increased [Citation31]. ESBL is a β-lactamases, capable of hydrolyzing β-lactam rings, conveying resistance to β-lactam antibiotics, such as penicillins, cephalosporins, and monobactams [Citation32]. Plasmids that encode ESBL typically also carry genes that convey resistance to other antibiotics, such as aminoglycosides, which makes the choice of antibiotics that can be used to treat ESBL-producing microorganisms extremely limited, resulting in severe infection outcomes. Yip et al. [Citation31] found that compared with ESBL-negative E. coli-associated peritonitis, the treatment failure rate and mortality rate associated with peritonitis caused by ESBL-producing E. coli were significantly increased. No significant difference was observed in the prognosis of E. coli-associated peritonitis at our center before and after the 5-year mark, which is likely associated with the small number of ESBL-producing E. coli identified at our center, and the sensitivity of E. coli to third-generation cephalosporins or aminoglycoside antibiotics did not change significantly over the 10-year study period, resulting in better clinical treatment effects.
This study still features some limitations. This study was a single-center, retrospective study with relatively small sample size. Moreover, drug sensitivity was related to long-term medication habits at our center; therefore, the results may not be applicable to other centers. In the follow-up study, we will perform a multi-center, large-sample, prospective study to verify our findings. If we can further confirm the clinical characteristics and influential factors that drive gram-negative bacterial peritonitis, such a study would likely have increased representative and clinical value.
In conclusion, the incidence of gram-negative bacterial peritonitis has not decreased but shows an upward trend. Gram-negative bacterial peritonitis is more severe and has a worse prognosis than gram-positive bacterial peritonitis. Therefore, we must further strengthen preventive measures designed to reduce infection risk. We hope to evaluate causative organisms and antibiotic resistance and determine the independent risk factors that promote gram-negative bacterial peritonitis to design effective strategies for the prevention and treatment of peritonitis.
Ethical approval
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University. Ethic committee’s Approval Number is JD-LK-2020-024-01. Due to the retrospective nature of the study, informed written consent was waived. All patients’ information was maintained confidential, and data were analyzed anonymously. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge the peritoneal dialysis team of the Second Affiliated Hospital of Suzhou University for their contributions (doctors, database administrators, nurses, and patients) and their provision of information and maintaining the databases for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ballinger AE, Palmer SC, Wiggins KJ, et al. Treatment for peritoneal dialysis-associated peritonitis. Cochrane Database Syst Rev. 2014;26(4):CD005284.
- McGuire AL, Carson CF, Inglis TJ, et al. Effects of a statewide protocol for the management of peritoneal dialysis-related peritonitis on microbial profiles and antimicrobial susceptibilities: a retrospective five-year review. Perit Dial Int. 2015;35(7):722–728.
- Barretti P, Pereira D, Brasil Maria A, et al. Evolution of gram-negative bacilli susceptibility in peritoneal dialysis-related peritonitis in Brazil: a single center’s experience over nine years. Perit Dial Int. 2009;29(2):230–233.
- Gadola L, Poggi C, Dominguez P, et al. Risk factors and prevention of peritoneal dialysis-related peritonitis. Perit Dial Int. 2019;39(2):119–125.
- Feng X, Yang X, Yi C, et al. Escherichia coli Peritonitis in peritoneal dialysis: the prevalence, antibiotic resistance and clinical outcomes in a South China dialysis center. Perit Dial Int. 2014;34(3):308–316.
- Li PK, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508.
- Perl J, Fuller DS, Bieber BA, et al. Peritoneal dialysis-related infection rates and outcomes: results from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am J Kidney Dis. 2020;76(1):42–53.
- Hwang TY, Kim MG, Oh SW, et al. Pathogens of peritoneal dialysis peritonitis: trends from a single-center experience over 15 years. Kidney Res Clin Pract. 2020;39(2):221–227.
- Huang ST, Chuang YW, Cheng CH, et al. Evolution of microbiological trends and treatment outcomes in peritoneal dialysis-related peritonitis. Clin Nephrol. 2011;75(05):416–425.
- Yang X, Yu X. Clinical prevention and treatment strategy of gram-negative bacterial peritonitis. J Nephrol Dialy Transplant. 2008;6(17):534–535.
- Cho Y, Htay H, Johnson DW. Center effects and peritoneal dialysis-related peritonitis. Nephrol Dial Transplant. 2017;32(6):913–915.
- Hautem N, Morelle J, Sow A, et al. The NLRP3 inflammasome has a critical role in peritoneal dialysis-related peritonitis. J Am Soc Nephrol. 2017;28(7):2038–2052.
- Yang C-Y, Chen T-W, Lin Y-P, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int. 2008;28(4):361–370.
- Ozisik1 L, Nurhan Ozdemir F, Tanriover MD, et al. The changing trends of peritoneal dialysis related peritonitis and novel risk factors. Ren Fail. 2015;37(6):1027–1032.
- Wang H-H, Huang C-H, Kuo M-C, et al. Microbiology of peritoneal dialysis-related infection and factors of refractory peritoneal dialysis related peritonitis: a ten-year single-center study in Taiwan. J Microbiol Immunol Infect. 2019;52(5):752–759.
- Htay Htay Y, Cho EM, Pascoe , et al. Center effects and peritoneal dialysis peritonitis outcomes: analysis of a national registry. Am J Kidney Dis. 2018;71(6):814–821.
- Szeto C-C, Li PK-T. Peritoneal dialysis-associated peritonitis. Clin J Am Soc Nephrol. 2019;14(7):1100–1105.
- Ferri M, Ranucci E, Romagnoli P, et al. Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876.
- Kitterer D, Latus J, Pöhlmann C, et al. Microbiological surveillance of peritoneal dialysis associated peritonitis: antimicrobial susceptibility profiles of a referral center in Germany over 32 years. PLoS One. 2015;10(9):e0135969.
- Jacoby GA, Munoz–Price LS. The new beta-lactamases. N Engl J Med. 2005;352(4):380–391.
- Leung CB, Szeto CC, Chow KM, et al. Cefazolin plus ceftazidime versus imipenem/cilastatin monotherapy for treatment of CAPD peritonitis – a randomized controlled trial. Perit Dial Int. 2004;24(5):440–446.
- D'Souza AL. Ageing and the gut. Postgrad Med J. 2007;83(975):44–53.
- Zurowska A, Feneberg R, Warady BA, et al. Gram-negative peritonitis in children undergoing long-term peritoneal dialysis. Am J Kidney Dis. 2008;51(3):455–462.
- Krishnan M, Thodis E, Ikonomopoulos D, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int. 2002;22(5):573–581.
- Li Y, Gong N, Zhou H, et al. The pathogen spectrum and resistance in patients with peritoneal dialysis-associated peritonitis: a single-center, observational clinical study. Clin Nephrol. 2019;92(1):44–51.
- Xu R, Chen Luo YS, et al. Clincal characteristics and outcomes of peritoneal dialysis-related peritonitis with different trends of change in effluent white well count: a longitudinal study. Perit Dial Int. 2013;33(4):436–444.
- Troidle L, Kliger A, Gorban-Brennan N, et al. Course of C-reactive protein during continuous peritoneal dialysis-associated peritonitis. Nephrology. 2005;10(5):442–445.
- Wei-Hung L, Chin-Chung T, An-Bang W, et al. Clinical and microbiological characteristics of peritoneal dialysis-related peritonitis caused by Klebsiella pneumoniae in Southern Taiwan. J Microbiol Immunol Infect. 2015;48(3):276–283.
- Li PH, Cheng VCC, Yip T, et al. Epidemiology and clinical characteristics of Acinetobacter peritoneal dialysis-related peritonitis in Hong Kong – with a perspective on multi-drug and carbapenem resistance. Perit Dial Int. 2017;37(2):177–182.
- Dias RCB, Vieira MA, Moro AC, et al. Characterization of Escherichia coli obtained from patients undergoing peritoneal dialysis and diagnosed with peritonitis in a Brazilian centre. J Med Microbiol. 2019;68(9):1330–1340.
- Yip T, Tse KC, Lam MF, et al. Risk factors and outcomes of extended-spectrum beta-lactamase-producing E. coli peritonitis in CAPD patients. Perit Dial Int. 2006;26(2):191–197.
- Malloy AM, Campos JM. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J. 2011;30(12):1092–1093.