Abstract
The relationship between serum insulin-like growth factor-1 (IGF-1) levels and anemia in patients undergoing maintenance hemodialysis (MHD) remains unclear. This cross-sectional study included patients who underwent MHD treatment for >3 months at our dialysis center in March 2021. Demographic and clinical data were recorded. Blood samples were collected before the hemodialysis sessions, and general serum biochemical parameters, routine blood markers, and serum IGF-1 levels were measured. Patients were divided into a group without anemia (hemoglobin ≥110 g/L) and a group with anemia (hemoglobin <110 g/L), and multivariable linear and binary logistic regression analyses were performed to study the relationship between the levels of serum IGF-1 and anemia. A total of 165 patients (male/female = 99:66) with MHD were enrolled in the study, with a median age of 66.0 (58.0, 75.0) years and a median dialysis vintage of 27.0 (12.0, 55.0) months. The mean hemoglobin level was 96.38 ± 16.72 g/L, and 126 patients had anemia (76.4%). Compared to patients without anemia, patients with anemia had lower serum IGF-1 and triglyceride levels and higher intravenous iron supplementation on dialysis (all p < 0.05). After adjusting for confounding factors in different models, the nine-model multivariate binary logistic regression analyses also confirmed that lower serum IGF-1 levels and serum IGF-1 < 197.03 ng/ml were both independently associated with anemia in patients undergoing MHD. However, further multicenter studies with larger sample sizes are required to confirm these findings.
Graphical Abstract
Introduction
Anemia is a common complication in patients undergoing maintenance hemodialysis (MHD) and is associated with an increased risk of cardiovascular events and all-cause mortality [Citation1]. In 2016, the Dialysis Outcomes and Practice Patterns Study in China reported that the proportion of patients undergoing MHD with hemoglobin levels less than 90 g/L was 21%, compared to 10% and 3% in North America and Japan, respectively [Citation2]. Although iron and erythropoietin treatments have been widely used in recent years, the current situation still warrants greater attention and further improvements, and other factors contributing to anemia in patients undergoing MHD deserve to be explored.
Studies have shown that in nondiabetic adults [Citation3] and older adult populations [Citation4], higher levels of serum insulin-like growth factor (IGF-1) are independently associated with higher hemoglobin levels. It has also been reported that IGF-1 may play an important role in regulating erythropoiesis independent of erythropoietin levels in patients with erythrocytosis undergoing MHD [Citation5]. However, few clinical studies have focused on the association between serum IGF-1 and hemoglobin levels in patients undergoing MHD. Thus, this cross-sectional study investigated the relationship between serum IGF-1 and hemoglobin levels in patients undergoing MHD.
Materials and Methods
Population
This single-center cross-sectional study included patients who underwent MHD at the Guangzhou Red Cross Hospital Hemodialysis Center in March 2021. The inclusion criteria were as follows: (1) dialysis vintage ≥3 months, three times a week, 4 h per visit; (2) age 18 years or older; and (3) informed consent. The exclusion criteria were as follows: (1) a history of continuous ambulatory peritoneal dialysis or renal transplantation before MHD; (2) a history of gastrointestinal bleeding or other acute blood loss within 6 months; (3) a history of blood transfusion within the past 3 months; (4) a history of trauma or surgery within the past 3 months; (5) severe infection, heart failure, or malignancy; and (6) refusal to participate in this study. This study was reviewed and approved by the Ethics Committee of Guangzhou Red Cross Hospital, Jinan University (ID: 2021–202-02).
Demographic, clinical, and laboratory data
The demographic and clinical data of these patients were extracted from the data management system for hemodialysis (Hope®, software) developed by our group [Citation6,Citation7]. Venous blood samples were collected before the hemodialysis session and sent to the clinical laboratory at our hospital within 2 h. The laboratory parameters were as follows: Kt/v, serum insulin, serum IGF-1, blood urea nitrogen (BUN), serum creatinine, serum phosphorus, serum parathyroid hormone, serum vitamin B12, serum folic acid, serum high-density lipoprotein cholesterol, serum low-density lipoprotein cholesterol, serum total cholesterol, serum triglycerides, serum ferritin, serum transferrin, serum albumin, hemoglobin, serum prealbumin, serum interleukin-6, and serum high-sensitivity C-reactive protein. Drug prescriptions in the past 3 months were also recorded, including the dosages of recombinant human erythropoietin (rHuEPO) (U/kg/week) and iron supplementation (mg/kg/week).
Serum IGF-1 levels were measured by high-performance liquid chromatography–mass spectrometry (Thermo Q, Thermo Fisher, Massachusetts, US). According to the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [Citation8], patients were divided into a group with anemia (hemoglobin < 110 g/L) and a group without anemia (hemoglobin ≥ 110 g/L).
The devices used for hemodialysis treatment were a Braun Dialog+ (B. Braun Co., Ltd., Melsungen, Germany) and a REXEED-15L high-throughput polysulfone membrane dialyzer (Asahi Kasei Corp., Tokyo, Japan) with a membrane area of 1.5 m2, dialysis blood flow rate of 200–300 mL/min, dialysis fluid flow rate of 500 mL/min, and dialysis duration of 4 h.
Statistical methods
Descriptive statistics include the mean ± standard deviation for continuous variables with a normal distribution, the median (25–75% interquartile range) for data with a skewed distribution, and percentages for categorical variables. Differences between the two groups were tested using the t test, nonparametric test, or chi-square test, as appropriate. Pearson’s or Spearman’s analyses were used to explore the correlation between clinical parameters and serum IGF-1 levels. Univariate and multivariate binary logistic regression or multiple linear regression analyses were used to investigate the relationship between serum IGF-1 levels and anemia in patients undergoing MHD. Data were analyzed using SPSS (version 26.0; IBM Corp, Armonk, NY, USA) statistical software, and a p values <0.05 was considered to indicate statistical significance.
Results
Characteristics of the study population
According to the inclusion and exclusion criteria, a total of 165 (male: female = 99:66) patients undergoing MHD were included in the study, with a median age of 66.0 (58.0, 75.0) years and a median dialysis vintage of 27.0 (12.0, 55.0) months, and 92 of them had diabetes (55.8%). The sample selection flowchart is presented in . The mean hemoglobin level was 96.38 ± 16.72 g/L, and 126 (76.4%) patients had anemia.
Comparison between groups
Compared with patients without anemia, patients with anemia had higher levels of intravenous iron supplementation (p = 0.047) and lower levels of serum IGF-1 (p = 0.007) and serum triglycerides (p = 0.008) (). Grouped by the mean serum IGF-1 level, the study participants were divided into two groups: a high IGF-1 group (IGF-1 ≥ 197.03 ng/ml) and a low IGF-1 group (IGF-1 < 197.03 ng/ml). Compared to the high IGF-1 group, the low IGF-1 group had a lower blood hemoglobin level (101.00 ± 15.71 vs. 92.98 ± 16.71 g/L, p = 0.002) ().
Figure 2. Comparison of hemoglobin levels between groups according to median serum IGF-1 in patients undergoing MHD. *p < 0.05. Hb: hemoglobin; IGF-1: insulin-like growth factor-1.

Table 1. Clinical characteristics of the study population before and after stratification into groups by anemia.
Associations between IGF-1 and anemia in patients undergoing MHD
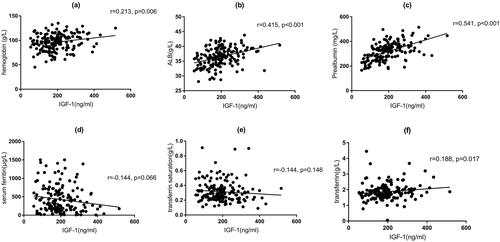
In the correlation analysis of IGF-1 with clinical parameters, the results of Spearman’s analysis () showed that IGF-1 was positively correlated with hemoglobin (r = 0.213, p = 0.006), serum albumin (r = 0.415, p < 0.001), serum prealbumin (r = 0.541, p < 0.001), and transferrin (r = 0.188, p = 0.017) ().
Figure 3. Correlation analysis between levels of hemoglobin, serum albumin, serum prealbumin, serum ferritin, transferrin saturation, serum transferrin, and levels of serum IGF-1. ALB: albumin; IGF-1: insulin-like growth factor-1.

Table 2. Correlation analysis of IGF-1 with clinical parameters.
The results of the univariate binary logistic regression analyses are shown in . We observed that lower levels of serum IGF-1 (odds ratio [OR] = 0.99, 95% confidence interval [CI] = 0.99–1.00, p = 0.010), serum IGF-1 < 197.03 ng/mL (OR = 0.36, 95% CI = 0.17–0.75) p = 0.007), and serum triglycerides (OR = 0.77, 95% CI = 0.59–0.99, p = 0.044) () were related to anemia. After adjustment for age, sex, dialysis vintage, diabetes, serum high-sensitivity C-reactive protein, iron supplementation dosage, rHuEPO dosage, serum triglycerides, serum albumin and prealbumin, the nine models’ multiple linear regression analyses showed that without the inclusion of serum albumin or prealbumin, higher levels of serum IGF-1 and serum IGF-1 ≥ 197.03 ng/ml were independently associated with higher hemoglobin levels. However, when serum albumin or prealbumin was included in the model, only serum IGF-1 ≥ 197.03 ng/ml was independently associated with higher hemoglobin levels (). After adjusting for the same confounders mentioned above in different models, the nine-model multivariate binary logistic regression analyses also confirmed that lower serum IGF-1 levels and serum IGF-1 < 197.03 ng/ml were both independently associated with anemia in patients undergoing MHD ().
Table 3. Univariate binary logistic regression between clinical parameters and anemia.
Table 4. Results of multiple linear regression models relating indicators of insulin growth factor-1 (independent variables) with hemoglobin (dependent variable).
Table 5. Results of multivariate binary logistic regression analyses of anemia (dependent variable) according to related IGF-1 indicators (independent variables).
Discussion
Our study showed that after adjusting for confounding factors, a low serum IGF-1 level was independently associated with anemia in patients undergoing MHD in different multivariable regression models, and positive correlations were observed between IGF-1 levels and albumin, prealbumin, and transferrin levels in this population.
As an active protein polypeptide, IGF-1 is produced by various cells, including those of the liver, kidney, and spleen. Many different cell types in the body express IGF-1 receptors. IGF-1 is synthesized in various tissues and acts in an autocrine or paracrine manner to promote cell growth or division. Studies have shown that patients with chronic kidney disease (CKD) and anemia have lower serum IGF-1 levels [Citation9].
However, the underlying factors associated with these findings are complex. First, IGF-1 promotes erythropoiesis both in vitro and in vivo [Citation10–12] and stimulates the proliferation and differentiation of late primitive erythroid progenitors and/or early erythroid progenitors, although the specific mechanism is not clear. Shimon et al. [Citation13] found that the specific knockdown of megakaryocyte and platelet C-type lectin-like receptor 2 (CLEC-2) decreased IGF-1 concentrations in the serum and extracellular fluid of bone marrow, with an increase in apoptosis of bone marrow erythroid cells, which increased the risk of anemia. In this study, pretreatment with IGF-1 receptor inhibitors increased the rate of apoptosis and reduced erythroblast proliferation in vitro. Therefore, the authors suggest that IGF-1 secretion from podoplanin (PDPN)-expressing stromal cells by CLEC-2 stimulation positively regulates erythroblasts.
Second, IGF-1 regulates several physiological processes, including cell proliferation and migration, cell growth, angiogenesis, and apoptosis, by activating the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase/Akt signaling pathways [Citation14]. Terrence et al. [Citation15] reported that mesenchymal stromal cells that were genetically engineered to overexpress IGF-1 enhanced the effects of cell-based gene therapy for renal failure-induced anemia treated with IGF-1 and that IGF-1-mediated proliferation might play a role in this process. Third, IGF-1 synergistically induced the expression of Bcl-xL in the BaF3 pro-B-cell line, which is essential for the inhibition of apoptosis and promotion of erythropoiesis [Citation16]. Additionally, IGF-1 reduces the expression of inflammatory molecules and decreases the levels of C-reactive protein and fibrinogen [Citation17]. Moreover, inflammatory status is an important factor for anemia in patients undergoing MHD [Citation18]. A decrease in circulating IGF-1 may lead to an increase in the concentration of inflammatory proteins, which may affect erythropoietin secretion and erythropoiesis [Citation19]. Furthermore, it has been suggested that decreased IGF-1 levels may be responsible for reduced erythropoietin activity and iron metabolism [Citation20]. It can be inferred that IGF-1 may exert a proerythropoietic effect through a variety of potential mechanisms.
In patients newly requiring hemodialysis, low serum IGF-1 levels are correlated with body composition as well as mineral and bone metabolism markers and predict an increased risk of mortality. Serum IGF-1 is a nutritional status marker in patients with end-stage renal disease (ESRD) and is negatively correlated with the subjective global assessment (SGA) nutritional status in patients undergoing hemodialysis [Citation21]. Our findings demonstrate a positive correlation between serum albumin, prealbumin, and IGF-1 levels, indicating that the impact of IGF-1 on nutrition may also play a role in anemia. However, the specific mechanisms underlying this association require further investigation.
Intervention studies have shown that in malnourished patients undergoing dialysis [Citation22] and older adults [Citation23], short-term treatment with recombinant human growth hormone (GH) could increase hemoglobin levels and thus improve anemia. By regulating the amount of IGF-1 synthesized and released by the liver or by modulating the plasma concentrations of IGF-1-binding proteins, GH can affect the amount of free IGF-1 bound to receptors on the cell surface, which reflects the bioavailability of IGF-1. Disturbances in GH and IGF-1 bioavailability may lead to renal anemia [Citation24]. Kim et al. found that the levels of free IGF-1 and its bioactivity in patients undergoing MHD were approximately half of those observed in healthy participants. During dialysis, the bioactivity of IGF-1 decreases by >50%. Insulin-like growth factor-binding proteins (IGFBPs) are important regulators of IGF-1 action. Reduced renal clearance leads to the accumulation of intact and partially degraded IGFBPs in serum, resulting in the downregulation of free IGF-1 levels and activity [Citation25]. In summary, IGF-1 may be an important factor in the regulation of erythropoiesis in patients with CKD. Although during our literature search, we found that most studies on the relationship between serum IGF-1 and hemoglobin in patients with CKD or on dialysis were published in the late 20th and early 21st centuries, no study has yet to show that exogenous IGF-1 preparations can improve anemia in this population.
This study has several limitations. First, it had a cross-sectional design; thus, a causal relationship between serum IGF-1 levels and anemia could not be determined. Second, this was a single-center study with a small sample size; therefore, it had limited statistical power and possible unknown biases. Third, the researchers measured hemoglobin and IGF-1 only once and did not perform repeated measurements at different time points; therefore, the association between IGF-1 and hemoglobin levels needs to be further confirmed.
Conclusion
In summary, we found that low serum IGF-1 levels were independently associated with anemia and lower hemoglobin levels in patients undergoing MHD. Thus, the erythropoietic function of IGF-1 may be an associated factor. However, prospective studies with large sample sizes and multiple centers need to be conducted. Furthermore, serum IGF-1 and hemoglobin levels should be repeatedly measured to confirm the associations between the two to provide a theoretical basis for prospective and interventional studies on the treatment of IGF-1 in patients undergoing MHD with anemia.
Author contributions
The authors’ contributions are as follows: Shilin Xu contributed to the study design and drafting of the manuscript; Jun Ren contributed to the data collection and analysis; Yun Liu was involved in manuscript revision and data analysis; Yuping Yao and Chunjie Jiang participated in the data collection and summary; Danping Qin Xiaoshi Zhong, Yan Liu, and Rongshao Tan participated in the review and revision of the manuscript; and Wenxuan Chen contributed to the revision of important contents and the final approval of the manuscript.
Acknowledgements
The authors are indebted to all nephrologists and nurses in the Nephrology Department of Guangzhou Red Cross Hospital, Jinan University, for their excellent management of hemodialysis patients. We thank the patients and staff involved in this cross-sectional study. We especially thank Shilin Xu, B.S. Nurs, as all the laboratory data in the study were derived from the electronic management system for the blood purification center (Hope®, software) that he developed, which can import the laboratory test results according to the patient IDs included in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28(1):1–8.
- Zuo L, Wang M, Hou F, et al. Anemia management in the China dialysis outcomes and practice patterns study. Blood Purif. 2016;42(1):33–43.
- Succurro E, Arturi F, Caruso V, et al. Low insulin-like growth factor-1 levels are associated with anaemia in adult non-diabetic subjects. Thromb Haemost. 2011;105(2):365–370.
- De Vita F, Maggio M, Lauretani F, et al. Insulin-like growth factor-1 and anemia in older subjects: the inchianti study. Endocr Pract. 2015;21(11):1211–1218.
- Shih LY, Huang JY, Lee CT. Insulin-like growth factor I plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol. 1999;10(2):315–322.
- Liu Y, Hu J, Tang R, et al. Association between the blood manganese (Mn) and hemoglobin in patients undergoing maintenance hemodialysis. J Trace Elem Med Biol. 2022;71:126947.
- Liu Y, Wang L, Li S, et al. Associations between blood trace element levels and nutritional status in maintenance hemodialysis. J Ren Nutr. 2021;31(6):661–668.
- Kidney Disease: improving Global Outcomes (KDIGO) CKDMBD Update Work Group. KDIGO 2017 clinical practice guide- line update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD- MBD). Kidney Int Suppl. 2017;2011(suppl):1–59.
- Deicher R, Hörl WH. Hormonal adjuvants for the treatment of renal anaemia. Eur J Clin Invest. 2005;35(Suppl 3):75–84.
- Correa PN, Axelrad AA. Production of erythropoietic bursts by progenitor cells from adult human peripheral blood in an improved serum-free medium: role of insulinlike growth factor 1. Blood. 1991;78(11):2823–2833.
- Claustres M, Chatelain P, Sultan C. Insulin-like growth factor I stimulates human erythroid colony formation in vitro. J Clin Endocrinol Metab. 1987;65(1):78–82
- Miyagawa S, Kobayashi M, Konishi N, et al. Insulin and insulin-like growth factor I support the proliferation of erythroid progenitor cells in bone marrow through the sharing of receptors. Br J Haematol. 2000;109(3):555–562.
- Otake S, Sasaki T, Shirai T, et al. CLEC-2 stimulates IGF-1 secretion from podoplanin-positive stromal cells and positively regulates erythropoiesis in mice. J Thromb Haemost. 2021;19(6):1572–1584.
- Butler AA, Yakar S, Gewolb IH, et al. Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology. Comp Biochem Physiol B Biochem Mol Biol. 1998;121(1):19–26.
- Kucic T, Copland IB, Cuerquis J, et al. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295(2):F488–96.
- Suzuki J, Kaziro Y, Koide H. Synergistic action of R-Ras and IGF-1 on bcl-xL expression and caspase-3 inhibition in BaF3 cells: r -Ras and IGF-1 control distinct anti-apoptotic kinase pathways. FEBS Lett. 1998;437(1-2):112–116.
- Hribal ML, Procopio T, Petta S, et al. Insulin-like growth factor-I, inflammatory proteins, and fibrosis in subjects with nonalcoholic. J Clin Endocrinol Metab. 2013;98(2):E304–8.
- Coyne DW, Fleming R. Will targeting interleukin-6 in the anemia of CKD change our treatment paradigm? J Am Soc Nephrol. 2021;32(1):6–8.
- Palles C, Johnson N, Coupland B, et al. Identification of genetic variants that influence circulating IGF1 levels: a targeted search strategy. Hum Mol Genet. 2008;17(10):1457–1464.
- Akahane K, Tojo A, Urabe A, et al. Pure erythropoietic colony and burst formations in serum-free culture and their enhancement by insulin-like growth factor I. Exp Hematol. 1987;15(7):797–802.
- Jia T, Gama Axelsson T, Heimbürger O, et al. IGF-1 and survival in ESRD. Clin J Am Soc Nephrol. 2014;9(1):120–127.
- Iglesias P, Díez JJ, Fernández-Reyes MJ, et al. Recombinant human growth hormone therapy in malnourished dialysis patients: a randomized controlled study. Am J Kidney Dis. 1998;32(3):454–463.
- Chu LW, Lam KS, Tam SC, et al. A randomized controlled trial of low-dose recombinant human growth hormone in the treatment of malnourished elderly medical patients. J Clin Endocrinol Metab. 2001;86(5):1913–1920.
- Soliman AT, De Sanctis V, Yassin M, et al. Growth and growth hormone–insulin like growth Factor -I (GH-IGF-I) axis in chronic anemias. Acta Biomed. 2017;88(1):101–111.
- Kim DH, Kim TY, Kim SM, et al. IGF-1 is an independent risk factor for anemia in diabetic pre-dialysis patients. Korean J Intern Med. 2007;22(3):186–191.

