Abstract
Background
Pediatric nephrotic syndrome (NS) requires routine proteinuria monitoring, which is costly and affects patients’ quality of life. The gold-standard 24-h urine protein (UP) measurement is challenging in children, and first-morning urine collection requires specific conditions, making it difficult in outpatient settings. Studies have reported comparability of second or random morning urine sample to the first-morning specimen. This study aimed to compare outcomes of random morning proteinuria measurements to 24-h UP and the roles of the urinary protein creatinine ratio (UPCR) and dipstick tests in pediatric NS, based on International Pediatric Nephrology Association (IPNA) 2022 Guidelines.
Method
Twenty-four-hour and morning urine samples were collected from 92 pediatric NS patients. These were subjected to automated analyses for 24-h UP, UPCR, and semi-automated dipstick analysis. A blinded doctor performed manual dipstick analysis.
Results
UPCR had a stronger correlation with 24-h UP than with automated and manual urine dipstick tests. UPCR had the highest sensitivity and specificity for predicting no remission/relapse and high sensitivity but low specificity for complete remission. The optimal UPCR cutoff for remission was 0.44 mg/mg and for no remission/relapse was 2.08 mg/mg. Automated and manual dipstick tests demonstrated limited sensitivity but high specificity and similar AUC values for remission/relapse.
Conclusion
UPCR was sensitive and specific for diagnosing no remission/relapse and sensitive but not specific for detecting remission. Manual and automated urine dipstick tests were comparable for remission and no remission/relapse detection. This study supports the IPNA 2022 Guidelines, as 2 mg/mg was the optimal UPCR cutoff for no remission/relapse, while for remission the optimal cutoff was 0.4 mg/mg.
1. Introduction
The average incidence of pediatric nephrotic syndrome (NS) is 1–17 per 100,000, varying by ethnicity and region [Citation1]. Over 85% of cases of childhood NS respond to steroid therapy, while 10–15% remain unresponsive or develop steroid resistance [Citation2–4]. NS, particularly steroid-resistant NS, can progress into end-stage kidney disease (ESKD) in an average of 6–191 months [Citation5], contributing to as many as 10%–28% of the primary diagnoses of ESKD [Citation6–13]. NS requires routine monitoring, primarily of proteinuria. In the United States, pediatric NS has a median annual cost of $140 (IQR $40–$1000) for laboratory testing [Citation14]. Low self-confidence, distress, and frequent absences from school for hospital visits due to pediatric NS lower the quality of life of 94% of patients, resulting in lower social and educational attainment compared with healthy children [Citation15,Citation16].
Pediatric NS is diagnosed by nephrotic-range proteinuria, measured by first-morning, 24-h, or dipstick proteinuria [Citation17,Citation18]. The 24-h urine protein (24-h UP) method is the gold standard, but it is cumbersome in the outpatient setting and impractical in children, particularly when they are not toilet trained [Citation19,Citation20]. Yang et al. (2017) excluded approximately 30% of pediatric 24-h UP samples from their analysis due to inadequate urine collection [Citation21]. Second to 24-h UP, first-morning urine collection is preferred due to lack of biological variation [Citation22]. However, this collection method requires specific conditions, such as 4–8 hours of sleep to ensure the absence of hydration and physical activity [Citation22]. To prevent protein degradation, storage of urine samples at 2–8 °C is recommended (or at room temperature for 2–4 h after collection) [Citation23,Citation24]. Therefore, it is difficult to obtain the first-morning urine in an outpatient setting [Citation25]. Several studies have reported that second-morning urine samples taken at 8–10 AM are comparable to 24-h and first-morning urine samples [Citation24,Citation26]. Position statements from Kidney Disease Improving Global Outcomes (KDIGO), National Kidney Foundation, National Institute for Clinical Excellence, and Caring for Australians with Renal Impairments suggest that although first-morning urine is preferred, random urine collection may be used if first-morning collection is inconvenient (Table S1) [Citation27–30].
Random spot urine measurements, such as spot urinary protein creatinine ratio (UPCR) and urine dipstick tests, are semiquantitative methods widely used as initial screening tools for proteinuria due to their low cost, wide availability, and efficiency [Citation31–35]. Several studies have reported conflicting correlations between 24-h UP and random spot urine measurements. Studies have shown strong correlations between urine dipstick test values and 24-h UP excretion in patients with nephropathy (r = 0.75) [Citation36], as well as between UPCR and cumulative 24-h UP in children (r = 0.801, p < 0.001) [Citation21] and in adults (r = 0.98, p < 0.05) [Citation37], due to the stability of urinary creatinine and protein excretion rates throughout the day [Citation38]. One study reported only a moderate correlation between UPCR and 24-h UP in children (r = 0.67) and in adults (r = 0.60) [Citation39]. Therefore, whether UPCR is an equivalent predictor of kidney outcomes and a reliable replacement for 24-h UP remains unclear [Citation40].
To our knowledge, the current study is the first to test the role of spot UPCR and manual and automated dipstick values in diagnosing new cases, remission, and no remission/relapse of pediatric NS, based on the International Pediatric Nephrology Association (IPNA) 2022 and KDIGO 2021 Guidelines. Most studies assessing urine dipstick use in kidney disease have focused on albuminuria in adults with diabetes and/or chronic kidney disease. The diagnostic value of random dipstick proteinuria compared to UPCR and 24-h UP in children with established NS diagnoses was first reported in 1990 [Citation41]. However, the UPCR proteinuria cutoff then specified was lower than the current standard of nephrotic-range proteinuria, recommended by KDIGO in 2012 (> 1.0 g/g vs. ≥ 2.0 g/g, respectively) [Citation41,Citation42]. IPNA and KDIGO defined cutoffs of 0.2 mg/mg for complete remission and 2.0 mg/mg for relapse in pediatric NS, based on expert consensus [Citation17–19]. Consensus opinions were developed based on uncontrolled series of children or uncontrolled trials in adults [Citation19]. One pediatric study assessing the proteinuria cutoff included a broad diagnostic criterion, classifying the proteinuria as tubulointerstitial or glomerular, but the cutoff was not exclusively for NS [Citation43].
2. Material and methods
2.1. Ethics approval
The Research Ethics Committee of the Faculty of Medicine Universitas Indonesia, Cipto Mangunkusumo Hospital approved this study (number KET-1187/UN2.F1/ETIK/PPM.00.02/2020). Written informed consent was obtained from participants’ legal guardians or, when appropriate, the participants. The study followed the Helsinki Declaration.
2.2. Study subjects
Data were obtained from children aged 3–18 years admitted to the pediatric nephrology outpatient clinic or pediatric ward of the Cipto Mangunkusumo Hospital from 1 January 2021 to 31 December 2021. Ninety-two NS patients were included. They had received an initial diagnosis, had relapsed, and had received treatment but had not achieved complete remission. NS was diagnosed based on the IPNA guidelines () [Citation17]. Only patients with eGFR ≥ 60 mL/min per 1.73 m2 were included. Participants who had severe malnutrition or could not complete the 24-h UP test were excluded.
Table 1. Definitions related to nephrotic syndrome in children based on IPNA 2022 Guidelines [Citation2].
2.3. Urine tests
Participants were asked to provide 24-h urine samples by discarding their first-morning urine and then collecting each subsequent urination through the first-morning urination of the next day. Next, a morning urine sample, collected before 9 AM, was submitted for protein (mg/dL) and creatinine (mg/dL) estimation and dipstick tests (see limitations in the Discussion section). The 24-h UP, UPCR, and dipstick results were compared. UPCR and 24-h UP were analyzed via the ARCHITECT c8000 automated analyzer machine (Abbott USA) using the Jaffe enzymatic and colorimetric methods, respectively. The Siemens CLINITEK Advantus® urine chemistry analyzer performed automated urine analysis. Manual urine dipstick tests, which used Verify™ urinalysis reagent strips, were visually interpreted by a blinded doctor as negative, trace, 1+, 2+, or 3+ ().
2.4. Statistical analysis
Parameters were investigated using STATA 17.0. Data are expressed as median with interquartile range (IQR). Nonparametric analysis was performed, as the Kolmogorov–Smirnov test indicated non-normally distributed data. p < 0.05 was considered statistically significant. Cutoffs were determined by selecting the value yielding the highest Youden index, calculated as sensitivity-(1-specificity). Correlations between 24-h UP and the UPCR and dipstick test results were calculated as Spearman’s correlation coefficient. Agreement between automated and manual urinary dipstick tests was measured using the Kappa (κ) statistic. Our sample size had 92.5% power to evaluate the specificity of UPCR in diagnosing NS with no remission/relapse.
3. Results
3.1. Patient characteristics
Ninety-two pediatric NS patients participated. Their median age was 10.04 years (IQR 6.54–12.96). Most participants were male (63.04%), and 81.52% were diagnosed with steroid-resistant NS (SRNS). The rest were steroid-dependent NS (SDNS, 14.13%), in primary remission (2.17%) or had only received an initial diagnosis (2.17%) ().
Table 2. Nephrotic syndrome patient characteristics.
3.2. Urinary protein creatinine ratio, automated dipstick, and manual dipstick tests
Spearman’s correlation coefficients are reported in . UPCR demonstrated a stronger correlation with 24-h UP (r = 0.83, p < 0.001) than automated (r = 0.79, p < 0.001) and manual urine dipsticks (r = 0.78, p < 0.001). The sensitivity, specificity, PPV, and NPV of the three proteinuria measurements for identifying relapse and remission were calculated ( and ). UPCR had the highest sensitivity (95.24%) and specificity (91.55%) for identifying no remission/relapse, while dipstick tests had the highest specificity for identifying complete remission.
Table 3. Correlation coefficients between morning spot proteinuria measurements and 24-h urine protein.
Table 4. Sensitivity, specificity, and predictive values of morning spot proteinuria assessments to identify no remission/relapse based on IPNA 2022 Guidelines.
Table 5. Sensitivity, specificity, and predictive values of morning spot proteinuria assessments to identify complete remission based on IPNA 2022 Guidelines.
3.3. Validity of the urinary protein creatinine ratio
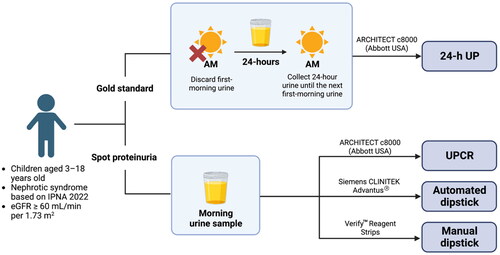
The ability to identify optimal cutoff values of UPCR for complete remission and no remission/relapse was analyzed by comparing the highest Youden indices and the areas under the receiver operating characteristic (ROC) curves (AUCs) against the IPNA standard. The optimal UPCR cutoff for identifying no remission/relapse was 2.08 mg/mg (sensitivity = 95.24%, specificity = 91.55%, AUC = 0.93), similar to the 2 mg/mg recommended by the IPNA (). For complete remission, the optimal UPCR cutoff was 0.44 mg/mg, which differed from the recommended value of 0.2 mg/mg ( and S2). At the 0.44 mg/mg cutoff, sensitivity, specificity, and AUC were 77.97%, 87.88%, and 0.83, respectively, compared with the IPNA recommendations ( and S2, ).
Figure 2. The receiver operator characteristics curve showed that the optimal cutoff urinary protein creatinine ratio for no remission/relapse was 2.08 mg/mg with an AUC value of 0.93. AUC: area under the curve; CI: confidence interval; ROC: receiver operator characteristics; UPCR: urinary protein creatinine ratio
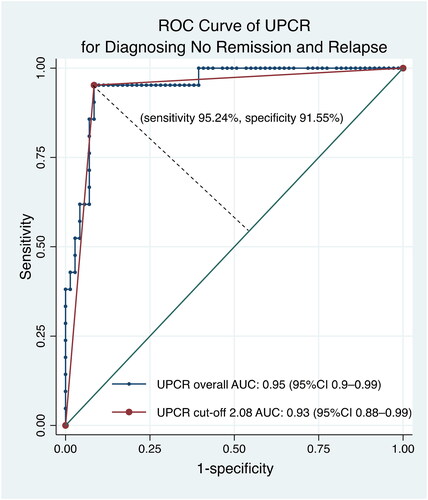
Figure 3. The receiver operator characteristics curve showed the optimal cutoff of urinary protein creatinine ratio for complete remission was 0.44 mg/mg with an AUC value of 0.83. AUC: area under the curve; CI: confidence interval; ROC: receiver operator characteristics; UPCR: urinary protein creatinine ratio
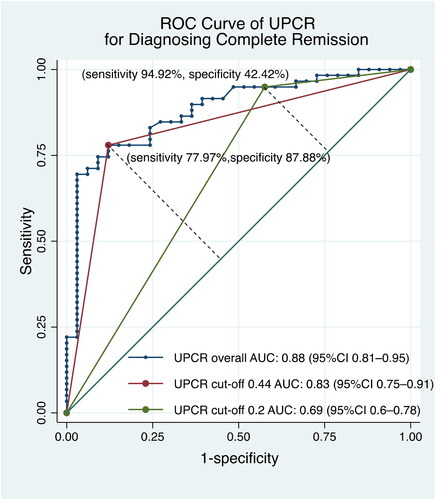
Table 6. Sensitivity, specificity, and predictive values of UPCR cutoff based on IPNA 2022 Guidelines and the value in our study for diagnosing complete remission.
3.4. Validity of automated and manual dipstick tests
The diagnostic abilities of urinary dipstick tests were analyzed to find optimal cutoffs for identifying complete remission and no remission/relapse (). The only dipstick cutoff that was compatible with the IPNA recommendation was the manual dipstick result for identifying complete remission, which was indicated by a result of negative or trace (Tables S4 and S5). The manual dipstick test had a slightly higher AUC for identifying no remission/relapse than the automated dipstick test (). The automated dipstick test had a slightly higher AUC for identifying complete remission (). Manual and automated dipstick readings showed moderate agreement (k = 0.53, p < 0.001).
Figure 4. The receiver operator characteristics curve showed that the manual dipstick had higher AUC value of 0.93 compared to the automated dipstick for identifying no remission/relapse, k = 0.53 (p < 0.001). AUC: area under the curve; CI: confidence interval; ROC: receiver operator characteristics
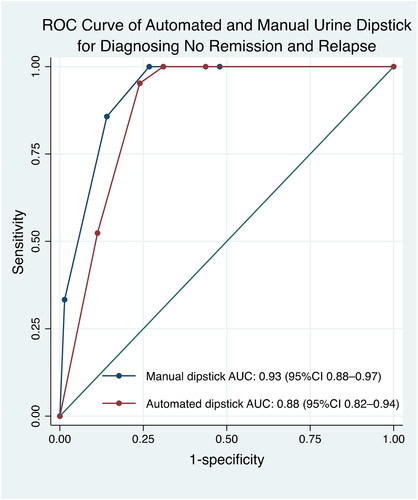
Figure 5. The receiver operator characteristics curve showed that the automated dipstick had higher AUC value of 0.85 compared to the manual dipstick for identifying complete remission, k = 0.53 (p < 0.001). AUC: area under the curve; CI: confidence interval; ROC: receiver operator characteristics
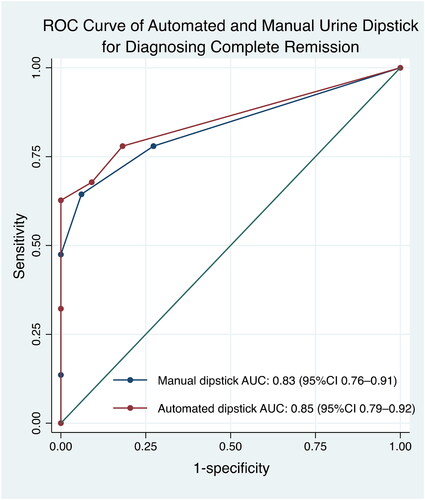
Table 7. The cutoff points of automated and manual dipstick tests with the best combination of sensitivity and specificity in predicting complete remission and no remission/relapse using IPNA 2022 Guidelines as proteinuria gold standard.
3.5. Validity of UPCR, automated dipstick, and manual dipstick in Steroid-Resistant nephrotic syndrome
We analyzed the performance of morning spot proteinuria measurements specifically in SRNS, which made up 81.52% of cases in our study (). The sensitivity, specificity, PPV, and NPV of the three proteinuria measurements to identify relapse and remission are presented in . UPCR had the highest sensitivity (95.24%) and specificity (94.44%) for identifying no remission/relapse, while dipstick tests had the highest specificity for identifying complete remission.
Table 8. Steroid-resistant nephrotic syndrome patient characteristics.
Table 9. Sensitivity, specificity, and predictive values of morning spot proteinuria assessments to identify relapse and complete remission based on IPNA 2022 Guidelines.
4. Discussion
Our participants were predominantly male, consistent with studies in which 57–65.87% of pediatric NS patients were male () [Citation44–46]. The participants had a median age of 10.04 years, whereas previous pediatric NS studies have reported median ages of 4.5–6.9 years [Citation44–46]. Most children in our study were well nourished; we excluded children with malnutrition because creatinine levels are generally lower in malnutrition [Citation47], which could skew the UPCR value. Our study samples had a median urine specific gravity of 1.020 (IQR 1.010–1.025), displaying reliable proteinuria findings since dilute urine may give false-negative results for proteinuria [Citation19,Citation48]. Most cases in our study were SRNS, while other reports suggest that only 10–15% of pediatric NS cases are SRNS [Citation3]. As a national referral hospital in Indonesia, we receive referral cases from other centers following initial steroid treatment failures. Therefore, the high proportion of SRNS cases and older patient age reflects that most of our cases are atypical, difficult-to-treat NS [Citation49,Citation50].
UPCR has shown a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 92%, 78%, 95%, and 70%, respectively, for diagnosing pediatric NS [Citation31]. In pediatric patients with fever and NS, automated urine dipstick test values of 2+ have shown values of 60%, 89%, 43%, and 94%, respectively, for detecting non-nephrotic proteinuria [Citation51], while values of 3+/4+ have shown values of 90%, 91%, 96%, and 77% for detecting nephrotic-range proteinuria [Citation51].
In our study, UPCR was strongly correlated with 24-h UP (). A high correlation between UPCR and 24-h UP is evident in other nephropathies. In pediatric patients with proteinuria due to IgA vasculitis-associated nephritis (IgAVN), lupus nephritis, or primary NS, UPCR and 24-h UP were strongly correlated (r = 0.869) [Citation52]. In adults with IgAN, UPCR and 24-h UP were also strongly correlated (r = 0.847, p < 0.001) [Citation40], as well as in hepatocellular carcinoma patients with lenvatinib-associated proteinuria (r = 0.86) [Citation53]. A study investigating amyloid light-chain amyloidosis reported a moderate correlation between UPCR and 24-h UP in patients with proteinuria levels of 500–3,000 mg/day (r = 0.57) or > 3000 mg/day (r = 0.62) but a strong correlation in lower levels of proteinuria (< 500 mg/day, r = 0.75) [Citation54]. Different correlation strengths with different proteinuria levels have been demonstrated in pediatric glomerulonephritis, with a stronger correlation in non-nephrotic-range proteinuria (r = 0.806) and a moderate correlation in nephrotic-range proteinuria (r = 0.586) [Citation52]. The stronger UPCR and 24-h UP correlation in the current study may be caused by the lower range of proteinuria in our participants.
UPCR was highly sensitive and specific for identifying a lack of remission/relapse (). Zhai et al. reported that UPCR had a sensitivity of 89.9% and specificity of 92.2% for diagnosing nephrotic-range proteinuria [Citation52]. We analyzed the optimal diagnostic boundary to determine remission or relapse using ROC curves ( and ). The cutoff point of UPCR for no remission/relapse was 2.08 mg/mg. Huang et al. reported a similar optimal cutoff of 2.09 mg/mg for pediatric nephrotic-range proteinuria [Citation55]. Our study only involved pediatric NS, which is considered to be characterized by selective albuminuria, while Huang et al. included participants with selective albuminuria due to NS (58.6%) and nonselective albuminuria, such as IgAVN (38.2%), IgAN (1.8%), and lupus nephritis (1.4%) [Citation55,Citation56]. Nevertheless, both cutoffs were in line with IPNA guidelines. Lane et al. reported a higher optimal cutoff of 2.35 mg/mg for nephrotic-range proteinuria in adult patients in renal and hypertension clinics [Citation57].
To diagnose complete remission, the IPNA guidelines recommend a UPCR cutoff of < 0.2 mg/mg, corresponding to the recommendations of the National Kidney Foundation and American College of Rheumatology (ACR) 2006, which proposed a standard diagnostic boundary for complete remission of kidney diseases [Citation17,Citation19,Citation58]. In children with nephropathies, Huang et al. reported an optimal cutoff of 0.18 mg/mg [Citation55]. However, our study concluded that the recommended cutoff of 0.2 mg/mg, although having high sensitivity, had low specificity. UPCR performed best at a cutoff of 0.44 mg/mg (, ). Although the optimal cutoff in our study was higher than the recommendation, a higher cutoff for complete remission might be plausible. Prior research investigating the reference intervals for proteinuria in healthy children has reported upper limits for UPCR above 0.2 mg/mg [Citation59]. In healthy girls and boys, the UPCR values ranged from 0.04–0.34 mg/mg (upper limit 90% CI 0.21–0.43) and 0.03–0.26 mg/mg (upper limit 90% CI 0.11–0.38), respectively [Citation59].
The automated and manual dipstick tests demonstrated similar correlations with 24-h UP (). However, both dipstick test values showed a lower correlation than that between UPCR and 24-h UP. Automated and manual dipstick tests demonstrated limited sensitivity but relatively high specificity () and similar AUC values for identifying remission or relapse ( and ). Similarly, Gai et al. (2006) compared 24-h UP, UPCR, and automated dipstick tests in adults with nephropathy and reported a stronger correlation between UPCR and 24-h UP (r = 0.82) than dipstick and 24-h UP (r = 0.75) [Citation36]. The sensitivity, specificity, and AUC values for the automated dipstick test (49.2%, 93.8%, and 0.778, respectively) were also lower than those for UPCR (91.4%, 75%, and 0.840, respectively) [Citation36]. Furthermore, the automated dipstick test failed to identify pathological proteinuria (≥ 500 mg/day) in 31.6% of the study’s participants [Citation36]. An earlier study investigating pediatric NS reported a sensitivity of 70% and specificity of 68% for dipstick tests in identifying nephrotic-range proteinuria, which is a higher sensitivity but lower specificity than reported in our study [Citation41]. This previous study also reported that the dipstick test performed worse than UPCR, resulting in more proteinuria misclassification [Citation41]. These results are due to automated dipstick tests being semiquantitative assays, while UPCR is purely quantitative. Therefore, dipstick values are less precise and have a lower correlation with 24-h UP. The green hues visible in a dipstick test occur due to a chemical reaction; therefore, false-negative results may occur in significantly diluted urine, while false-positive results may occur in very alkaline or contaminated urine [Citation19,Citation48].
We analyzed the dipstick tests’ optimal cutoffs for identifying remission and relapse (, and 7). The automated dipstick test performed best at a cutoff of 1+ for complete remission and 2+ for no remission/relapse. These thresholds differed from the IPNA-recommended cutoff points of negative or trace results for complete remission and 3+ for no remission/relapse. For the manual dipstick test, the result of the trace performed best for identifying complete remission, consistent with the IPNA guidelines. For no remission/relapse, the cutoff was 1+, which was different from the IPNA recommendation of 3 + [Citation17].
A prior study reported that automated dipstick values were unreliable for estimating 24-h UP, being poor predictors of daily urinary protein excretion in adult renal and hypertension clinic patients [Citation48]. A meta-analysis assessing proteinuria in adults with kidney disease and pregnant females reported that an automated dipstick cutoff of 1+ had a sensitivity of 67–100% and specificity of 36–98% for detecting 24-h UP > 300 mg/day [Citation60]. Another study investigating proteinuria in older adults found that the optimal automated dipstick cutoff for UPCR ≥ 0.2 mg/mg was trace (sensitivity = 90.9%, specificity = 87.2%) [Citation32]. Unfortunately, we could not find a study that assessed urine dipstick test cutoff values using the IPNA definition of proteinuria in pediatric NS for equal comparison. We also could not find studies assessing manual dipstick tests for diagnosing pediatric NS. Therefore, we could not compare our results with those of previous studies.
Our study has several strengths. Previously published studies assessing proteinuria in children investigated proteinuria for different causes, such as fever, glomerulonephritis, and other nephropathies. Abitbol et al. (2006) suggested that proteinuria and albuminuria profiles are both essential to guide the management of proteinuric diseases in children, including NS [Citation43]. In our study, we investigated only proteinuria in pediatric NS. We compared the validity of urinary automated and manual dipstick tests with the currently accepted gold-standard test, 24-h UP. The manual dipstick test is a practical and helpful screening method for home proteinuria monitoring [Citation60]. However, previous studies have reported that dipstick readings are unreliable for therapeutic decisions, which should be based on a more precise quantitative measurement, such as first-morning UPCR or 24-h UP [Citation61]. Moreover, manual dipstick tests are associated with possible errors due to the need for manual interpretation, which could be impacted by the reading time. For example, a false positive could appear if a dipstick is submerged in urine for too long [Citation62]. Therefore, further studies assessing the validity of manual dipstick tests for at-home NS monitoring are essential.
Our study has several limitations. It was done at a single referral center with a high proportion of SRNS cases and older pediatric patients, so the results might represent the difficult-to-treat pediatric NS population more than the general pediatric NS population. The identification of relapse and remission is ideally based on dipstick tests over three consecutive days [Citation17], but our samples were collected at a single point. IPNA also recommends using first-morning urine for diagnosing remission. However, we used morning urine samples, not necessarily the first, because of sample transportation considerations. The children voided their first-morning urine at home, generally before 7 AM; it is usual for children in Indonesia to awaken and void their first-morning urine between 5 and 6 AM. We wanted to use fresh urine samples, voided a maximum of one hour before the test, for the dipstick tests. It is generally challenging for patients living far away to reach the hospital in adequate time to suit the ideal urine collection and storage method [Citation23]. For this reason, we used morning urine voided before 9 AM for our UPCR and dipstick samples. This may have an impact on our findings. However, although first-morning urine has the best correlation to 24-h urine collection [Citation62,Citation63], second-morning urine taken at 8–10 AM also presents acceptable results, showing comparable analytes, including protein, to first-morning urine [Citation23,Citation26].
Our sample size had less than 80% power to evaluate the sensitivity of urine dipstick tests ( and ). Future studies should use a multicenter approach with a greater sample size to represent the general pediatric NS population. We recommend involving parents or caregivers to determine the validity of manual dipstick test implementation for home monitoring of pediatric NS. A prospective study using a proteinuria selectivity index can be useful to predict outcomes in pediatric NS and provide more information than quantitative spot and 24-h proteinuria measurements. The proteinuria selectivity index provides information about the degree of tubulointerstitial and glomerular damage and thus can predict functional and clinical outcomes, treatment response, and disease progression [Citation65,Citation66]. In this study, we could not apply any changes in patient management based on our proteinuria test results. Therefore, a prospective study using our UPCR and dipstick cutoffs and changing the disease management based on the disease status category (remission or relapse) will be beneficial to test the worth of the new cutoffs.
5. Conclusions
In our study, UPCR was more sensitive and specific in identifying no remission/relapse in pediatric NS than the automated and manual dipstick tests. UPCR was more sensitive than urinary dipstick tests for identifying complete remission. The optimal UPCR cutoffs for identifying complete remission and no remission/relapse in pediatric NS were 0.4 and 2.0 mg/mg, respectively. Urine dipstick is a highly specific method for identifying remission. The manual dipstick test performed comparably to the automated test. These could be used interchangeably to detect remission or no remission/relapse and could be beneficial for home monitoring.
Supplemental Material
Download PDF (234.9 KB)Acknowledgements
We would like to express our gratitude to Professor Taralan Tambunan, Professor Partini Pudjiastuti Trihono, Professor Sudung Oloan Pardede, Eka Laksmi Hidayati, MD, and Henny Adriani Puspitasari, MD, for their care of the patients at the Department of Child Health, Cipto Mangunkusumo Hospital. We are also thankful to Darmawan Budi Setyanto, MD, Mulya Rahma Karyanti, MD, and Dina Muktiarti, MD, for their feedback on the development of this manuscript and to Ghafur Rasyid Arifin, MD and Wani Riselia Sirait, MD, for their assistance in data collection. This work was previously presented as a scientific poster at the 55th Annual Meeting European Society for Paediatric Nephrology Congress 2023 in Vilnius, Lithuania.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data analyzed or generated are included in this article and the Supplementary Materials. Further inquiries regarding data can be directed to the corresponding author.
Additional information
Funding
References
- Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:1. doi: 10.3389/fped.2016.00039.
- Trautmann A, Schnaidt S, Lipska-Ziętkiewicz BS, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28(10):3055–11. doi: 10.1681/ASN.2016101121.
- Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet registry. Front Pediatr. 2018;6:200. doi: 10.3389/fped.2018.00200.
- Tarshish P, Tobin JN, Bernstein J, et al. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the international study of kidney disease in children. J Am Soc Nephrol. 1997;8(5):769–776. doi: 10.1681/ASN.V85769.
- Paik KH, Lee BH, Cho HY, et al. Primary focal segmental glomerular sclerosis in children: clinical course and prognosis. Pediatr Nephrol. 2007;22(3):389–395. doi: 10.1007/s00467-006-0301-5.
- Ambarsari CG, Hidayati EL, Trihono PP, et al. Experience of the first 6 years of pediatric kidney transplantation in Indonesia: a multicenter retrospective study. Pediatr Transplant. 2020;24(8):e13812. doi: 10.1111/petr.13812.
- Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12(3):133–146. doi: 10.1038/nrneph.2015.205.
- Wente-Schulz S, Aksenova M, Awan A, et al. Aetiology, course and treatment of acute tubulointerstitial nephritis in paediatric patients: a cross-sectional web-based survey. BMJ Open. 2021;11(5):e047059. doi: 10.1136/bmjopen-2020-047059.
- Ambarsari CG, Hidayati EL, Mushahar L, et al. Dressing versus non-dressing technique for long-term exit-site care in children on continuous ambulatory peritoneal dialysis: a single-center retrospective cohort study. Med J Indones. 2020;29(3):290–297. doi: 10.13181/mji.oa.204171.
- Palupi-Baroto R, Hermawan K, Murni IK, et al. High fibroblast growth factor 23 as a biomarker for severe cardiac impairment in children with chronic kidney disease: a single tertiary center study. Int J Nephrol Renovasc Dis. 2021;14:165–171. doi: 10.2147/IJNRD.S304143.
- Santoso DN, Sinuraya FAG, Ambarsari CG. Distal renal tubular acidosis presenting with an acute hypokalemic paralysis in an older child with severe vesicoureteral reflux and syringomyelia: a case report. BMC Nephrol. 2022;23(1):248. doi: 10.1186/s12882-022-02874-9.
- Ambarsari CG, Trihono PP, Kadaristiana A, et al. Low-dose maintenance intravenous iron therapy can prevent anemia in children with end-stage renal disease undergoing chronic hemodialysis. Int J Nephrol. 2020;2020:3067453–3067458. doi: 10.1155/2020/3067453.
- Ambarsari CG, Cho Y, Milanzi E, et al. Epidemiology and outcomes of children with kidney failure receiving kidney replacement therapy in Australia and New Zealand. Kidney Int Rep. 2023. doi: 10.1016/j.ekir.2023.07.006.
- Simon CA, Salmon E, Desmond HE, et al. The health economic impact of nephrotic syndrome in the United States. Kidney360. 2022;3(6):1073–1079. doi: 10.34067/KID.0005072021.
- Li N, Hao J, Fu T, et al. Evaluating the quality of life of 231 children with primary nephrotic syndrome and assessing parental awareness of the disease. Front Pediatr. 2021;9:745444. doi: 10.3389/fped.2021.745444.
- Allam N, Bashar A, Eid R. Assessment of health-related quality of life in Sudanese children with nephrotic syndrome: a questionnaire-based study. Pan Afr Med J. 2022;43:154. doi: 10.11604/pamj.2022.43.154.34980.
- Trautmann A, Boyer O, Hodson E, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2023;38(3):877–919. doi: 10.1007/s00467-022-05739-3.
- Kidney Disease: improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021.
- Hogg RJ, Portman RJ, Milliner D, et al. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the national kidney foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics. 2000;105(6):1242–1249. doi: 10.1542/peds.105.6.1242.
- Leung AK, Wong AH. Proteinuria in children. Am Fam Physician. 2010;82(6):645–651.
- Yang EM, Yoon BA, Kim SW, et al. Clinical utility of spot urine protein-to-creatinine ratio modified by estimated daily creatinine excretion in children. Pediatr Nephrol. 2017;32(6):1045–1051. doi: 10.1007/s00467-017-3587-6.
- Witte EC, Lambers Heerspink HJ, de Zeeuw D, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20(2):436–443. doi: 10.1681/ASN.2008030292.
- Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009; 55(1):24–38. doi: 10.1373/clinchem.2008.106567.
- Veljkovic K, Rodríguez-Capote K, Bhayana V, et al. Assessment of a four hour delay for urine samples stored without preservatives at room temperature for urinalysis. Clin Biochem. 2012; 45(10-11):856–858. doi: 10.1016/j.clinbiochem.2012.04.010.
- Sontrop JM, Garg AX, Li L, et al. Consecutive first-morning urine samples to measure change in the albumin-to-creatinine ratio: a pilot study of a home urine collection protocol. Can J Kidney Health Dis. 2016; 3:3. doi: 10.1186/s40697-016-0095-8.
- Hofmann W, Guder WG. A diagnostic programme for quantitative analysis of proteinuria. J Clin Chem Clin Biochem. 1989; 27(9):589–600. doi: 10.1515/cclm.1989.27.9.589.
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005; 67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(2 Suppl 1):S1–S266.
- Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Urine protein as diagnostic test: performance characteristics of tests used in the initial evaluation of patients at risk of renal disease. Nephrology (Carlton). 2004; 9(Suppl 3):S8–S14. doi: 10.1111/j.1440-1797.2004.00312.x.
- National Collaborating Centre for Chronic Conditions (UK). Type 2 diabetes: national clinical guideline for management in primary and secondary care (update). London: royal College of Physicians (UK); 2008.
- Sukmawati M, Suarta K. Validity of protein-creatinine and protein-osmolality ratios in the estimation of massive proteinuria in children with nephrotic syndrome. PI. 2007;47(4):139–143. doi: 10.14238/pi47.4.2007.139-43.
- Lim D, Lee DY, Cho SH, et al. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res Clin Pract. 2014;33(4):199–203. doi: 10.1016/j.krcp.2014.10.003.
- Leung AK, Wong AH, Barg SS. Proteinuria in children: evaluation and differential diagnosis. Am Fam Physician. 2017;95(4):248–254.
- Mejia JR, Fernandez-Chinguel JE, Dolores-Maldonado G, et al. Diagnostic accuracy of urine dipstick testing for albumin-to-creatinine ratio and albuminuria: a systematic review and meta-analysis. Heliyon. 2021;7(11):e08253. doi: 10.1016/j.heliyon.2021.
- Ambarsari CG, Tambunan T, Pardede SO, et al. Role of dipstick albuminuria in progression of paediatric chronic kidney disease. J Pak Med Assoc. 2021;71(Suppl 2):S103–S106.
- Gai M, Motta D, Giunti S, et al. Comparison between 24-h proteinuria, urinary protein/creatinine ratio and dipstick test in patients with nephropathy: patterns of proteinuria in dipstick-negative patients. Scand J Clin Lab Invest. 2006;66(4):299–307. doi: 10.1080/00365510600608563.
- Patil P, Shah V, Shah B. Comparison of spot urine protein creatinine ratio with 24 hour urine protein for estimation of proteinuria. J Assoc Physicians India. 2014;62(5):406–410.
- Agarwal, Indira, Kirubakaran, Chellam, Markandeyulu, Selvakumar Quantitation of proteinuria by spot urine sampling. Indian J Clin Biochem. 2004;19(2):45–7. doi: 10.1007/BF02894256.
- Hogan MC, Reich HN, Nelson PJ, et al. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int. 2016;90(5):1080–1089. doi: 10.1016/j.kint.2016.06.020.
- Yu G, Cheng J, Li H, et al. Comparison of 24-h urine protein, urine albumin-to-creatinine ratio, and protein-to-creatinine ratio in IgA nephropathy. Front Med (Lausanne). 2022;9:809245. doi: 10.3389/fmed.2022.809245.
- Abitbol C, Zilleruelo G, Freundlich M, et al. Quantitation of proteinuria with urinary protein/creatinine ratios and random testing with dipsticks in nephrotic children. J Pediatr. 1990;116(2):243–247. doi: 10.1016/s0022-3476(05)82881-1.
- Kidney Disease: improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2(2):139–274.
- Abitbol CL, Chandar J, Onder AM, et al. Profiling proteinuria in pediatric patients. Pediatr Nephrol. 2006;21(7):995–1002. doi: 10.1007/s00467-006-0103-9.
- Parikh RV, Tan TC, Fan D, et al. Population-based identification and temporal trend of children with primary nephrotic syndrome: the kaiser permanente nephrotic syndrome study. PLoS One. 2021;16(10):e0257674. doi: 10.1371/journal.pone.0257674.
- Franke I, Aydin M, Llamas Lopez CE, et al. The incidence of the nephrotic syndrome in childhood in Germany. Clin Exp Nephrol. 2018;22(1):126–132. doi: 10.1007/s10157-017-1433-6.
- Sato M, Ishikura K, Ando T, et al. Prognosis and acute complications at the first onset of idiopathic nephrotic syndrome in children: a nationwide survey in Japan (JP-SHINE study). Nephrol Dial Transplant. 2021;36(3):475–481. doi: 10.1093/ndt/gfz185.
- Hari P, Bagga A, Mahajan P, et al. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatr Nephrol. 2007;22(10):1757–1761. doi: 10.1007/s00467-007-0535-x.
- Loghman-Adham M. Evaluating proteinuria in children. Am Fam Physician. 1999;59(3):540.
- Santín S, Tazón-Vega B, Silva I, et al. Clinical value of NPHS2 analysis in early- and adult-onset steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6(2):344–354. doi: 10.2215/CJN.03770410.
- Ambarsari CG, Saraswati M, Laudza GS. Rituximab, mycophenolic acid, and calcineurin inhibitors achieve long-term remission in pediatric focal segmental glomerulosclerosis with steroid-resistant and frequently relapsing nephrotic syndrome: a report of two cases. Case Rep Nephrol Dial. 2022;12(3):167–177. doi: 10.1159/000525776.
- Alaydrus C, Soenarto Y, Damanik M. Results of proteinuria measurement using semiquantitative dipstick in children with fever or nephrotic syndrome. PI. 2008;48(1):10–14. doi: 10.14238/pi48.1.2008.10-4.
- Zhai P, Huang Y, Yue S, et al. Diagnostic efficacy and influence factors of urinary protein/creatinine ratio replacing 24-h urine protein as an evaluator of proteinuria in children. Int Urol Nephrol. 2022;54(6):1409–1416. doi: 10.1007/s11255-021-03021-3.
- Evans TRJ, Kudo M, Finn RS, et al. Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer. 2019;121(3):218–221. doi: 10.1038/s41416-019-0506-6.
- Mendelson L, Sanchorawala V, Connors L, et al. Correlation between 24-hour urine protein and random urine protein-creatinine ratio in amyloid light-chain amyloidosis. Kidney Med. 2022;4(4):100427. doi: 10.1016/j.xkme.2022.100427.
- Huang Y, Yang X, Zhang Y, et al. Correlation of urine protein/creatinine ratios to 24-h urinary protein for quantitating proteinuria in children. Pediatr Nephrol. 2020;35(3):463–468. doi: 10.1007/s00467-019-04405-5.
- Tojo A. Mechanism underlying selective albuminuria in minimal change nephrotic syndrome. Int J Nephrol. 2019;2019:5859102–5859108. doi: 10.1155/2019/5859102.
- Lane C, Brown M, Dunsmuir W, et al. Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? Nephrology (Carlton). 2006;11(3):245–249. doi: 10.1111/j.1440-1797.2006.00564.x.
- Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American college of rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54(2):421–432. doi: 10.1002/art.21625.
- Slev PR, Bunker AM, Owen WE, et al. Pediatric reference intervals for random urine calcium, phosphorus and total protein. Pediatr Nephrol. 2010;25(9):1707–1710. doi: 10.1007/s00467-010-1544-8.
- Craig JC, Barratt A, Cumming R, et al. Feasibility study of the early detection and treatment of renal disease by mass screening. Intern Med J. 2002;32(1-2):6–14. doi: 10.1046/j.1445-5994.2002.00155.x.
- Trautmann A, Vivarelli M, Samuel S, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529–1561. doi: 10.1007/s00467-020-04519-1.
- Delanghe J, Speeckaert M. Preanalytical requirements of urinalysis. Biochem Med (Zagreb). 2014;24(1):89–104. doi: 10.11613/BM.2014.011.
- Ginsberg JM, Chang BS, Matarese RA, et al. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309(25):1543–1546. doi: 10.1056/NEJM198312223092503.
- Rodby RA. Timed urine collections for albumin and protein: "the king is dead, long live the king!. Am J Kidney Dis. 2016;68(6):836–838. doi: 10.1053/j.ajkd.2016.06.025.
- Nakamura J, Nagatoya K, Fujii N, et al. New selectivity index calculated using protein fraction as a substitute for the conventional selectivity index. Clin Exp Nephrol. 2019;23(10):1196–1201. doi: 10.1007/s10157-019-01753-2.
- Bazzi C, Petrini C, Rizza V, et al. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int. 2000;58(4):1732–1741. doi: 10.1046/j.1523-1755.2000.00334.x.