Abstract
Background Sepsis-induced acute kidney injury (S-AKI) is a common complication in critically ill patients. Therefore, reliable biomarkers for predicting S-AKI outcomes are necessary. Serum cell-free DNA (cfDNA) is a circulating extracellular DNA fragment used as a noninvasive screening tool for many diseases, including sepsis. This study aimed to investigate the prognostic value of cfDNA in S-AKI patients and its relationship with some other parameters.Methods A total of 89 S-AKI patients admitted to the intensive care unit (ICU) from June 2021 to December 2021 were enrolled in this study. The patients were categorized into the low cfDNA group (< 855 ng/ml) and high cfDNA group (≥ 855 ng/ml) and were followed up for three months. CfDNA was extracted from serum and quantified using Quant-iT PicoGreen dsDNA Reagent.Results Overall survival was significantly lower in the high cfDNA group than in the low cfDNA group (Log-Rank p = 0.012). Univariate Cox proportional hazard model showed that cfDNA was significantly associated with all-cause mortality (HR [hazard ratio] 2.505, 95% CI [95% confidence interval] 1.184–5.298, p = 0.016). Also, serum cfDNA was a significant risk factor for all-cause mortality after adjusting for covariates (HR 2.191, 95% CI 1.017–4.721, p = 0.045). Moreover, cfDNA was positively correlated with several baseline parameters, including serum creatine, aspartate aminotransferase, alanine aminotransferase, prothrombin time, and International Normalized Ratio.Conclusion High serum cfDNA level is associated with higher mortality among the S-AKI population, indicating that cfDNA is a valuable biomarker for S-AKI prognosis.
Introduction
Sepsis is a life-threatening medical emergency associated with organ dysfunction caused by a dysregulated immune response to infection [Citation1]. Sepsis is the leading cause of death in intensive care units (ICU) worldwide [Citation2]. Sepsis-induced acute kidney injury (S-AKI) is a common, life-threatening complication associated with increased serum creatinine or decreased urinary output in the presence of sepsis [Citation3,Citation4]. About 40%–50% of patients with sepsis or septic shock develop S-AKI since the kidney is one of the earliest injured organs during sepsis [Citation4–6], thus increasing in-hospital mortality by 2.5 to 4 folds [Citation6]. The progression of S-AKI may be reversible, thus improving the short- and long-term survival of patients with sepsis [Citation7]. Notably, the recovered S-AKI patients may still be at risk of recurrent kidney injury with worse clinical outcomes and further develop into chronic kidney injury [Citation8,Citation9]. Therefore, novel biomarkers for predicting S-AKI outcomes are needed.
Serum cell-free DNA (cfDNA) is a circulating extracellular DNA fragment induced by cell necrosis, apoptosis, pyroptosis, and NETosis (a program for the formation of neutrophil extracellular traps). CfDNA is also derived from actively released DNA and exogenous sources [Citation10]. CfDNA is a noninvasive screening tool for many diseases, such as sepsis [Citation11–13], autoimmune rheumatic diseases [Citation13], trauma [Citation14], cancer [Citation15], and cardio-cerebrovascular disease [Citation16,Citation17]. Notably, a study showed that cfDNA level is associated with poor outcomes in critically ill patients. The study also showed that cfDNA level is increased in AKI patients undergoing continuous renal replacement therapy (CRRT) [Citation12]. However, it is unknown whether cfDNA can predict S-AKI prognosis.
In this study, we investigated the prognostic value of cfDNA in patients with S-AKI, aiming to explore a new biomarker for predicting the outcome of S-AKI.
Materials and methods
Study design and patient characteristics
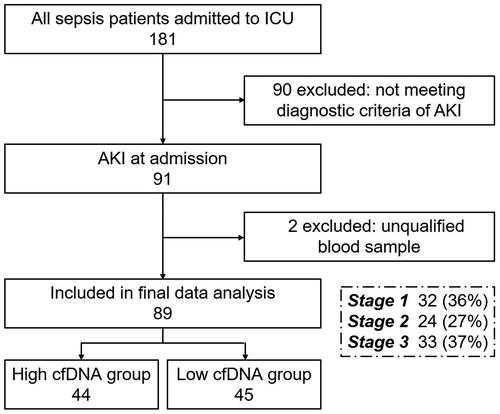
A total of 89 consecutive patients diagnosed with S-AKI at admission to the intensive care unit (ICU) of Zhongshan Hospital, Fudan University, Shanghai, from June 2021 to December 2021, were enrolled in this study (). These patients were diagnosed with S-AKI at admission to the ICU, and blood samples were collected immediately after admission. Sepsis was defined based on The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [Citation1], while AKI was defined based on KDIGO criteria [Citation18]. S-AKI was defined as the simultaneous presence of both sepsis and AKI according to the Sepsis-3 and KDIGO criteria and clinical judgment. Among S-AKI patients, 36% had stage I, 27% had stage II, and 37% had stage III, and 40% of them experienced septic shock (). The study was performed following the Declaration of Helsinki and was approved by the Ethical Committee of Zhongshan Hospital, Fudan University (B2022-172R). All-cause death was the endpoint.
Table 1. Baseline demographic, clinical, and biochemical characteristics.
Demographic data included age and gender. Elderly population was defined as over 65 years. The SOFA (Sequential Organ Failure Assessment) [Citation19] of each patient was estimated and recorded at admission. Clinical characteristics were also recorded. Biochemical parameters were measured in the laboratory via standard methods.
Measurements of serum cell-free DNA concentration
Serum samples were obtained immediately after S-AKI diagnosis and stored at −80 °C for further use. Serum cell-free DNA concentration was quantified using Quant-iT PicoGreen dsDNA Reagent (#P7581, Molecular Probes, Eugene, OR, USA) following the manufacturer’s instructions, as previously described [Citation20,Citation21], BioTek Epoch2 (BioTek Instrument, USA) was used to detect fluorescence at filter settings of 485 nm (excitation) and 538 nm (emission).
Statistical analyses
The sample size was estimated based on a previous study showing a 4.5-fold increase in mortality risk in sepsis patients in cfDNA high group compared with those in cfDNA low group (40.7% vs. 7.4%) [Citation22]. A total of 76 patients were needed to achieve 90% power and significance level (alpha) of 0.05 based on these parameters and the cohort sample size formula, and thus the present sample size (n = 89) met the minimum requirements for statistical capacity.
The association between serum cfDNA and all-cause death was evaluated using the Kaplan-Meier method and the Cox proportional hazard model. Hierarchical selection procedure was employed to adjust confounding risk factors. The criterion for Multivariate Cox Hazard model selection was determined as p < 0.05 in the univariate Cox proportional hazard model, and several factors correlated with other factors were excluded. Pearson’s correlation coefficient is used to test the correlation between cfDNA concentration and some of the other baseline parameters.
Data were expressed as mean ± SD, median (interquartile range), or frequency, where appropriate. An Independent sample t-test was used to compare two groups of normal data. A two-tailed p < 0.05 was considered statistically significant. SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for all data analyses.
Results
Baseline characteristics of the study population
The baseline characteristics of the patients are listed in , including demographic and clinical characteristics, as well as subsequent intervention (including application of ventilation, vasopressors, and CRRT). A total of 62 of 89 S-AKI patients were males, of which 66.3% were elderly population. The median SOFA score at admission was 7 (IQR 5–12). The patients were categorized into two groups based on median serum cfDNA concentration: The low cfDNA group (cfDNA < 855 ng/ml) and the high cfDNA group (cfDNA ≥ 855 ng/ml). Patients in the high cfDNA group had lower serum platelet levels and higher serum hsCRP (high sensitive C-reactive protein) levels than patients in the low cfDNA group. However, the other characteristics were not significantly different between the two groups ().
Association between baseline serum cell-free DNA level and all-cause mortality
A total of 32 patients died during the median follow-up of three months. Kaplan-Meier analysis found that overall survival was significantly lower in the high cfDNA group than in the low cfDNA group (Log-Rank p = 0.012) (). Univariate Cox proportional hazard model showed that cfDNA was significantly associated with all-cause mortality (HR [hazard ratio] 2.505, 95%vCI [95% confidence interval] 1.184–5.298, p = 0.016) (). Other variables including SOFA (sequential organ failure assessment), diabetes, neutrophil%, PT (prothrombin time), INR (international normalized ratio), and the application of ventilation and vasopressors were also significantly associated with all-cause mortality (). Notably, serum cfDNA was a significant risk factor for all-cause mortality even after adjusting for other risk factors (HR 2.191, 95% CI 1.017–4.721, p = 0.045) (). Multivariate Cox hazard model also showed that the percentage of neutrophils was a significant risk factor for all-cause mortality (HR 0.976, 95% CI 0.958–0.995, p = 0.011), as well as the application of vasopressors (HR 3.170, 95% CI 1.230–8.171, p = 0.017).
Figure 2. Kaplan-Meier survival Curves of overall survival in patients with sepsis-associated acute kidney injury.
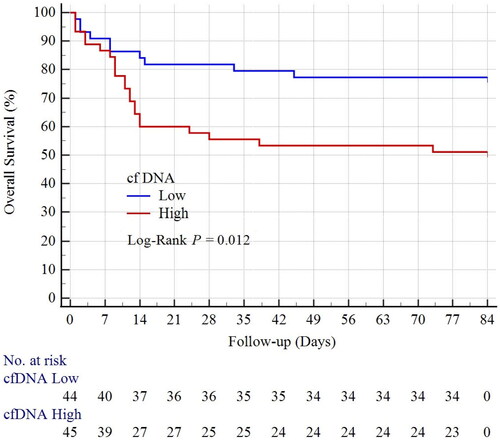
Table 2. Univariate Cox proportional hazard model identifying individual factors related to patient survival.
Table 3. Multivariate Cox Hazard ratios for all-cause mortality.
The relationship between cfDNA concentration and other baseline parameters
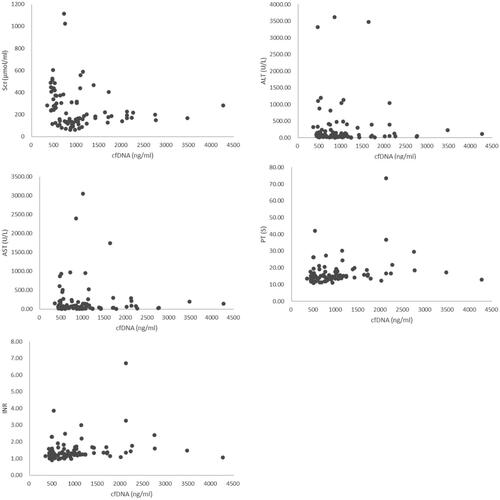
Organ dysfunction is a major characteristic of sepsis. Herein, the relationship between cfDNA concentration and several organ dysfunction parameters, including cTnT (heart), PaO2/FiO2 (lung), SCr (serum creatine, kidney), BUN (blood urea nitrogen, kidney), AST (aspartate aminotransferase, liver) and ALT (alanine aminotransferase, liver) were analyzed. Sepsis is also characterized by coagulation abnormality, and thus the correlation between cfDNA concentration and coagulation parameters, such as platelet, PT, TT, APTT, INR, and fibrinogen, was evaluated. cfDNA was positively correlated with several baseline parameters, including SCr (Pearson’s r = 0.221, p = 0.038), AST (Pearson’s r = 0.347, p = 0.001), ALT (Pearson’s r = 0.315, p = 0.003), PT (Pearson’s r = 0.235, p = 0.027), and INR (Pearson’s r = 0.213, p = 0.045) ( and ).
Table 4. The correlation between cfDNA concentration and other baseline parameters.
Discussion
High levels of circulating cfDNA were first reported in systemic lupus erythematosus [Citation23], then in cancer [Citation24], and plasma or serum of patients with many disease states. Sepsis patients have high cfDNA concentrations, especially in S-AKI patients [Citation12]. CfDNA is a prognostic marker in septic patients [Citation12] and patients in ICU [Citation25,Citation26]. However, it is unknown whether cfDNA can predict S-AKI prognosis. In this study, high serum cfDNA level was associated with higher mortality among the S-AKI population.
CfDNA’s source and functions could differ under different physiological and pathological conditions. It can be induced by cell necrosis, apoptosis, pyroptosis, and NETosis, as well as derived from actively released DNA and exogenous sources [Citation10]. In our study, we did not explore the underlying mechanism through basic research. Herein, we could only provide our hypothesis of the underlying mechanism based on previous studies and our data. We assume a possible resource of cfDNA in the S-AKI population is NETosis. NETosis is a rapid process limited to certain hematopoietic cell types, involving nuclear disintegration and cell death, leading to the extrusion of neutrophil extracellular traps (NETs). NET formation significantly increases cfDNA levels in sepsis patients [Citation27]. Sepsis induces neutrophil accumulation, which adheres to vascular endothelium in collaboration with platelets, thus forming NETs [Citation28]. NETs prevent microorganisms from spreading through binding and ensure a high local concentration of antimicrobial agents, thus eradicating foreign pathogens during infection [Citation29]. However, NETs may persist for several days during sepsis, thus becoming pathogenic by damaging epithelial and endothelial cells [Citation30,Citation31]. Therefore, NETs may induce direct cell damage in the kidney, or cause occlusion of the vasculature in the kidney, thus aggravating S-AKI and resulting in worse outcomes. Also, NETs promote vaso-thrombosis by providing a stimulus and scaffold for thrombus formation [Citation32–35]. In sepsis, activation of the coagulation system is common and can lead to disseminated intravascular coagulation (DIC), which is a thromboinflammatory response that strongly affects patient outcomes [Citation36]. NET-induced coagulation activation may also initiate DIC [Citation35]. In this study, serum cfDNA level was positively correlated with PT and INR, indicating that NETs may be related to sepsis-induced DIC. This finding indicates that cfDNA can predict S-AKI outcomes.
We assume another possible resource of cfDNA in our study is cell damage. Phagocytosis-mediated DNA release from dead or dying cells at the infection site is another key source of cfDNA during sepsis [Citation10]. Sepsis is associated with life-threatening organ dysfunction [Citation1], where phagocytes digest or do not digest apoptotic or necrotic cells and discharge the cleaved DNA fragments, causing phagocyte damage and release of their DNA into blood, resulting in cfDNA release [Citation27]. And previous study suggests that cfDNA can be considered a direct marker of cell apoptosis since caspase-3 is correlated to cfDNA concentrations in septic patients [Citation12]. Therefore, higher cfDNA levels may suggest severe organ dysfunction. In this study, cfDNA level was associated with SCr (renal function), AST, and ALT (liver function). The association between serum cfDNA level and higher mortality in the S-AKI population may also be due to more active phagocytosis and severe kidney injury.
Our result shows two other risk factors for S-AKI mortality, which are a lower percentage of neutrophils and the application of vasopressors. Sepsis is characterized by an initial hyperinflammatory phase, followed by a protracted immunosuppressive phase [Citation37]. Lower percentage of neutrophils may indicate immunosuppressive circumstances, probably due to sepsis, use of immunosuppressive drugs, or other comorbidities, such as tumors and autoimmune diseases. Neutropenic sepsis is associated with a higher-grade organ dysfunction and higher mortality than non-neutropenic sepsis [Citation38], consistent with this study. As for the application of vasopressors, a probable explanation is that patients with severe conditions are more tend to be treated with vasopressors, but still with higher mortality due to critical status.
Nevertheless, this study has some limitations. First, the study had a relatively small sample size. Moreover, cfDNA was measured only at admission rather than during or after the therapeutic process. Also, a causal relationship between cfDNA and other parameters (organ dysfunction and coagulopathy parameters) could not be established. Therefore, the mechanism of the association between cell-free DNA and all-cause mortality of S-AKI cannot be sufficiently explained. Besides, the study design does not allow us to make conclusions about causality. Therefore, a prospective study with a larger population and more frequent cfDNA measurement is necessary to validate the role of serum cfDNA level as a predictive marker for S-AKI prognosis. Although cfDNA may be an effective biomarker, the underlying mechanism is unclear. Therefore, further studies should assess whether high cfDNA level represents severe sepsis, poorer microcirculatory function, disruption of the coagulation system, or worse multi-organ function.
Conclusion
In this study, high serum cfDNA level was associated with higher mortality among the S-AKI population, indicating that cfDNA can be a valuable biomarker of S-AKI prognosis. Also, cfDNA was positively associated with SCr (renal dysfunction), AST, ALT (liver dysfunction), PT, and INR (coagulopathy), providing a hypothesis of the mechanism. This study provides a basis for larger studies that may confirm the prognostic value of cfDNA in the S-AKI population and clarify the underlying mechanism. Nonetheless, identification of the pathophysiological effects of cfDNA in S-AKI may enhance monitoring therapeutic modalities.
Authors’ contributors
All authors listed made a substantial, direct, and intellectual contribution to the work and approved the final manuscript for publication.
Disclosure statement of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):1–9. doi: 10.1001/jama.2016.0287.
- Mayr VD, Dunser MW, Greil V, et al. Causes of death and determinants of outcome in critically ill patients. Crit Care. 2006;10(6):R154. doi: 10.1186/cc5086.
- Kuwabara S, Goggins E, Okusa MD. The pathophysiology of sepsis-associated AKI. Clin J Am Soc Nephrol. 2022;17(7):1050–1069. doi: 10.2215/CJN.00850122.
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813.
- Kellum JA, Chawla LS, Keener C, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193(3):281–287. doi: 10.1164/rccm.201505-0995OC.
- Fiorentino M, Tohme FA, Wang S, et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLOS One. 2018;13(6):e0198269. doi: 10.1371/journal.pone.0198269.
- Harris DG, Koo G, McCrone MP, et al. Recurrent kidney injury in critically ill surgical patients is common and associated with worse outcomes. J Trauma Acute Care Surg. 2014;76(6):1397–1401. doi: 10.1097/TA.0000000000000241.
- Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC.
- Aucamp J, Bronkhorst AJ, Badenhorst C, et al. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev Camb Philos Soc. 2018;93(3):1649–1683. doi: 10.1111/brv.12413.
- Saukkonen K, Lakkisto P, Pettila V, et al. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin Chem. 2008;54(6):1000–1007. doi: 10.1373/clinchem.2007.101030.
- Clementi A, Virzi GM, Brocca A, et al. The role of cell-free plasma DNA in critically ill patients with sepsis. Blood Purif. 2016;41(1–3):34–40. doi: 10.1159/000440975.
- Duvvuri B, Lood C. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. Front Immunol. 2019;10:502. doi: 10.3389/fimmu.2019.00502.
- Gogenur M, Burcharth J, Gogenur I. The role of total cell-free DNA in predicting outcomes among trauma patients in the intensive care unit: a systematic review. Crit Care. 2017;21(1):14. doi: 10.1186/s13054-016-1578-9.
- Wu TL, Zhang D, Chia JH, et al. Cell-free DNA: measurement in various carcinomas and establishment of normal reference range. Clin Chim Acta. 2002;321(1–2):77–87. doi: 10.1016/s0009-8981(02)00091-8.
- Brusca SB, Elinoff JM, Zou Y, et al. Plasma cell-free DNA predicts survival and maps specific sources of injury in pulmonary arterial hypertension. Circulation. 2022;146(14):1033–1045. doi: 10.1161/CIRCULATIONAHA.121.056719.
- Polina IA, Ilatovskaya DV, DeLeon-Pennell KY. Cell free DNA as a diagnostic and prognostic marker for cardiovascular diseases. Clin Chim Acta. 2020;503:145–150. doi: 10.1016/j.cca.2020.01.013.
- Kidney Disease: improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751.
- Arai Y, Yamashita K, Mizugishi K, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(12):1683–1689. doi: 10.1016/j.bbmt.2013.09.005.
- Qi Y, Uchida T, Yamamoto M, et al. Perioperative elevation in cell-free DNA levels in patients undergoing cardiac surgery: possible contribution of neutrophil extracellular traps to perioperative renal dysfunction. Anesthesiol Res Pract. 2016;2016:2794364. doi: 10.1155/2016/2794364.
- Avriel A, Paryente WM, Almog Y, et al. Admission cell free DNA levels predict 28-day mortality in patients with severe sepsis in intensive care. PLOS One. 2014;9(6):e100514. doi: 10.1371/journal.pone.0100514.
- Tan EM, Schur PH, Carr RI, et al. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966;45(11):1732–1740. doi: 10.1172/JCI105479.
- Leon SA, Shapiro B, Sklaroff DM, et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37(3):646–650.
- Saukkonen K, Lakkisto P, Varpula M, et al. Association of cell-free plasma DNA with hospital mortality and organ dysfunction in intensive care unit patients. Intensive Care Med. 2007;33(9):1624–1627. doi: 10.1007/s00134-007-0686-z.
- Wijeratne S, Butt A, Burns S, et al. Cell-free plasma DNA as a prognostic marker in intensive treatment unit patients. Ann N Y Acad Sci. 2004;1022(1):232–238. doi: 10.1196/annals.1318.036.
- Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol. 2012;144(1):32–40. doi: 10.1016/j.clim.2012.04.006.
- Denning NL, Aziz M, Gurien SD, et al. DAMPs and NETs in sepsis. Front Immunol. 2019;10:2536. doi: 10.3389/fimmu.2019.02536.
- Branzk N, Lubojemska A, Hardison SE, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15(11):1017–1025. doi: 10.1038/ni.2987.
- Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLOS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366.
- Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450.
- Brill A, Fuchs TA, Chauhan AK, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117(4):1400–1407. doi: 10.1182/blood-2010-05-287623.
- Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107.
- Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110.
- Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–896. doi: 10.1038/nm.2184.
- Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578.
- Hotchkiss RS, Sherwood ER. Immunology. Getting sepsis therapy right. Science. 2015;347(6227):1201–1202. doi: 10.1126/science.aaa8334.
- Na SJ, Oh DK, Park S, et al. Clinical characteristics and outcomes of neutropenic sepsis: a multicenter cohort study. Shock. 2022;57(5):659–665. doi: 10.1097/SHK.0000000000001907.