Abstract
Chronic kidney disease (CKD) is a major complication of diabetes mellitus (DM). Inflammation is an essential component in the process of CKD progression in patients with DM. Diet is a significant determinant of systemic inflammation levels. However, the association between the dietary inflammatory index (DII) and CKD in individuals with DM remains largely unknown; therefore, the aim of this study was to explore whether the DII is linked to the prevalence of CKD in patients with DM. The research method was as follows: first, data from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2018 were obtained. There were 7,974 participants in our study. These individuals were then classified into three groups according to DII tertiles (T1-T3), with each group consisting of 2,658 participants. Logistic regression analysis was employed to examine whether there was a connection between the DII and CKD. We observed a significant association between the DII and the prevalence of CKD in individuals with DM. After full adjustment for age, sex, ethnicity, smoking, drinking, body mass index (BMI), triglyceride (TG), total cholesterol (TC), metabolic equivalents (METs), energy intake, hypoglycemic medications, hypertension, and cardiovascular disease (CVD), the group with a higher DII had a greater frequency of CKD (T2 group: OR: 1.40; 95% CI: 1.10–1.76; p = 0.006; T3 group: OR: 1.67; 95% CI: 1.29–2.17; p < 0.001). The implementation of an anti-inflammatory diet could serve as an intervention strategy for patients with DM to prevent the onset of CKD.
Introduction
Chronic kidney disease (CKD) is the main complication of diabetes (DM) [Citation1]. Greater than 30% of individuals with DM have CKD [Citation2]. CKD not only leads to a decrease in the quality of life of patients with DM [Citation3] but also increases the risk of mortality [Citation4]. Early prevention of CKD is of paramount importance in patients with DM. At present, the prevention of diabetic nephropathy (DN) is largely focused on avoiding hyperglycemia and reducing the activity of the renin–angiotensin system [Citation5]. Nevertheless, despite increasing the quality of medical care, the prevalence of DN continues to rise [Citation6]. Therefore, it is essential to explore additional measures for preventing CKD.
Multiple research findings have demonstrated that the progression of CKD in patients with DM is facilitated by inflammation [Citation7]. Various inflammatory indicators, including tumor necrosis factor-alpha (TNF-α) [Citation8], C-reactive protein (CRP) [Citation9], and interleukin (IL)-6 [Citation10], have been implicated in the development of DN. Hence, managing systemic inflammation could serve as a viable strategy for minimizing the likelihood of developing CKD in patients with DM [Citation11].
Diet is one of the modifiable lifestyle factors of DM patients and is strongly correlated with their level of systemic inflammation. The amount of energy and saturated fatty acids (SFAs) in foods can contribute to increased levels of inflammatory markers in people with DM [Citation12,Citation13]. However, the intake of individual nutrients is not representative of a patient’s overall dietary inflammatory potential. To tackle this issue, previous research developed the dietary inflammatory index (DII) by accounting for the anti-inflammatory and proinflammatory attributes of different nutrients in the diet to assess the overall dietary inflammatory potential of individuals [Citation14]. Specifically, a DII > 0 indicates a higher potential for dietary inflammation, i.e., a proinflammatory diet.
Although inflammation helps to increase the risk of CKD in people with DM [Citation8–10], previous studies have only linked inflammatory markers to CKD and have not directly discussed the effects of proinflammatory diets on CKD in patients with DM. Our hypothesis was that a proinflammatory diet would increase the prevalence of CKD in people with DM; therefore, the objective of our study was to explore whether the DII was linked to the prevalence of CKD in patients with DM.
Methods
Source of data and population
The research utilized data from the National Health and Nutrition Examination Survey (NHANES). The National Center for Health Statistics (NCHS) supervised the survey. This survey was conducted on a nationwide scale and involves the selection of 5,000 individuals each year, with corresponding weights assigned to the data.
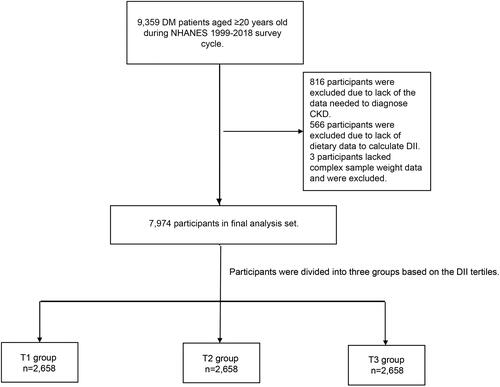
Between 1999 and 2018, the NHANES survey included 9,359 individuals who were at least 20 years old and had been diagnosed with DM. A total of 816 patients were excluded due to a lack of data, including the randomized urinary albumin-to-creatinine ratio (uACR) and estimated glomerular filtration rate (eGFR), which were calculated by CKD Epidemiology Collaboration (CKD-EPI 2009) equations and were needed to diagnose CKD. A total of 566 patients were excluded due to a lack of nutrient data needed to calculate the DII, and three patients were excluded due to a lack of diet-related sampling weight data. Finally, the analysis encompassed a cohort of 7,974 patients with DM ().
Definition of DM
NHANES officials asked participants if they had been told they had type-2 diabetes (T2D) by a medical professional, and those who answered affirmatively were considered to have T2D. In addition, individuals were also considered to have T2D if they met one of the following requirements: (1) glycosylated hemoglobin, type A1C (HbA1c) > 6.5%; (2) fasting glucose ≥ 7.0 mmol/L; (3) random blood glucose ≥ 11.1 mmol/L; 4. 2-h Oral Glucose Tolerance Test (OGTT) blood glucose ≥ 11.1 mmol/L; or 5. use of diabetes medication or insulin [Citation15]. Hypoglycemic drugs included insulin and oral hypoglycemic drugs (including acarbose, glimepiride, glipizide, linagliptin, metformin, sitagliptin, nateglinide, pioglitazone, repaglinide, rosiglitazone and sitagliptin).
Primary outcome
The primary outcome was CKD. Patients who met one of the following conditions were classified as having CKD: (1) eGFR < 60 mL/min/1.73 m2; or (2) uACR > 30 mg/g [Citation16].
Calculation of the DII
In our study, a total of 28 nutrients were utilized to calculate the DII. The nutrients included cholesterol, SFAs, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), n-3 fatty acids, n-6 fatty acids, niacin, retinol, thiamin, riboflavin, pyridoxine, cobalamin, ascorbic acid, vitamin D, tocopherol, iron, magnesium, zinc, selenium, folic acid, and beta-carotene. In addition, carbohydrates, protein, total fat, alcohol, fiber, caffeine and energy are also included in the DII calculation. The provided equations from a previous study were employed to compute the DII [Citation14]. The nutrient intake information was calculated by the NHANES professionals through the participants’ dietary recall over a 24-h period. The NHANES publishes detailed intake levels on its website (https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/DR1TOT_C.htm#Component_Description).
Definition of confounding variables
Participants reported their age, sex, ethnicity, and body mass index (BMI). Smoking and drinking habits were also self-reported by the patients. The calculation of metabolic equivalent (MET) values from patients’ self-reported weekly physical activity time followed a methodology consistent with previous research [Citation17]. Participants who self-reported having high blood pressure or were taking blood pressure medications or whose systolic or diastolic blood pressure measured by NHANES staff was greater than the threshold (140 mmHg or 90 mmHg) were considered to have hypertension. Patients who self-reported being diagnosed with cardiovascular disease (CVD) by their physician were defined as having CVD in our study.
Laboratory measurements
Blood glucose, HBA1c, creatinine (Cr), CRP, triglyceride (TG), and total cholesterol (TC) were collected by NHANES staff and sent to the laboratory for determination. Similarly, urine samples were collected and measured by professional technicians in the NHANES. The NHANES released a detailed collection process and instruments and experimental methods, and the specific process can be queried on the following web site (cdc.gov/nchs/data/nhanes/2009-2010/manuals/lab.pdf).
Grouping method
The DII tertile was calculated, and the patients were stratified into 3 groups according to the calculated value: the T1 group (DII lower than 1.1), T2 group (DII between 1.1 and 2.7), and T3 group (DII equal to or higher than 2.7).
Statistical analyses
As per the NHANES guidelines, individuals were assigned appropriate sampling weights based on the provided equation, and all statistical calculations were performed considering these weights. We utilized the mean (standard error) to characterize continuous variables, while counts and percentages (weighted) were employed to depict categorical variables. The comparison of baseline differences between groups in continuous and categorical variables was conducted using analysis of variance (ANOVA) and χ2 tests, respectively.
Sampling weighted logistic regression analysis was employed to assess whether the DII was linked to the prevalence of CKD. Model 1 represented the raw data without any adjustments. Model 2 was adjusted for age, sex, and ethnicity as adjusting factors. Model 3 was a fully adjusted model that adjusted for age, sex, ethnicity, cigarette smoking status, alcohol consumption, BMI, TG level, TC level, total METs, energy intake, hypoglycemic medications, hypertension and CVD. Restricted cubic splines (RCS) were used to visualize the association between the DII and CKD. Furthermore, we conducted stratified analyses by considering factors such as age, sex, usage of hypoglycemic drugs, and hypertension. Additionally, a multiplicative interaction model was employed to examine potential interactions between the DII and the aforementioned stratified variables. To ensure the robustness of the validation results, sensitivity analysis was conducted over a 10-year time span. In addition, participants were regrouped based on the quartiles and quintiles of DII and whether the DII was greater than 0.
Data analysis was performed with R Studio (version 4.2.2). A p value <0.05 was considered indicative of statistical significance.
Results
Characteristics at baseline
Among the 7,974 patients involved in the study, the average age was 59.5 (0.3) years old, with 3,843 (48.9%) being female. The entire population was then classified into tertiles according to their DII value, resulting in three groups of 2,658 patients each. The group with a higher DII had a higher BMI and was less likely to drink alcohol. With respect to laboratory test results, patients with a higher DII had a lower eGFR and higher TC, CRP and TG levels. In addition, a higher prevalence of hypertension and CVD was observed in the group with a higher DII. The T3 group had the lowest proportion of hypoglycemic medication use. There was no significant difference in age, blood glucose, HbA1c, uACR, or cigarette smoking status at baseline. provides further information regarding the baseline characteristics of the participants in the study.
Table 1. Baseline study population characteristics (weighted).
Association between the DII and CKD
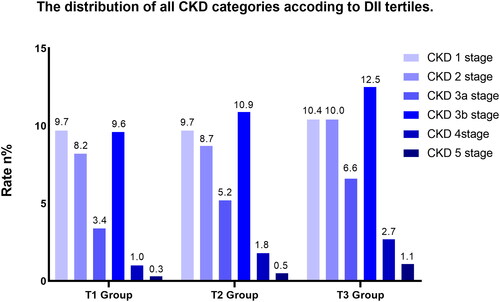
The distribution of all CKD categories is shown in . In univariate weighted logistic regression analysis, a positive correlation was observed between the DII and the frequency of CKD in patients with DM (odds ratio (OR): 1.14; 95% confidence interval (CI): 1.10–1.18; p < 0.001). A higher frequency of CKD was observed in the group with a higher DII (T2 groups: OR: 1.24; 95% CI: 1.04–1.47; p = 0.015; T3 groups: OR: 1.62; 95% CI: 1.37–1.91; p < 0.001). After full adjustment for potential confounders (as shown in ), the positive association between the DII and the frequency of CKD remained unchanged (OR: 1.15; 95% CI: 1.09–1.22; p < 0.001). The ORs of the T2 (OR: 1.40; 95% CI: 1.10–1.76; p = 0.006) and T3 (OR: 1.67; 95% CI: 1.29–2.17; p < 0.001) groups were still higher than that of the T1 group ().
Table 2. The association between the DII and CKD in patients with DM (weighted).
Restricted cubic spline regression
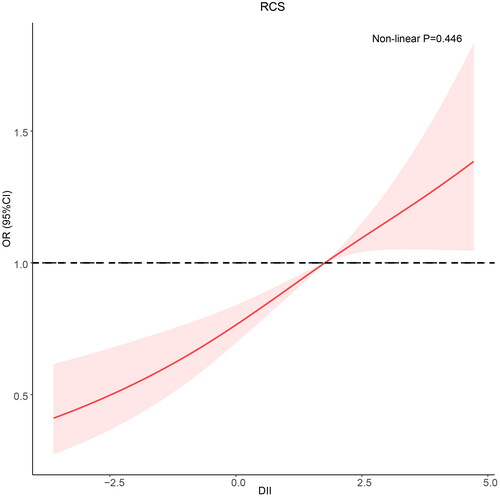
In RCS, a linear relationship between the DII and CKD was observed in DM patients (nonlinear p = 0.446). As the DII increased, so did the frequency of CKD in patients with DM ().
Analysis based on subgroups
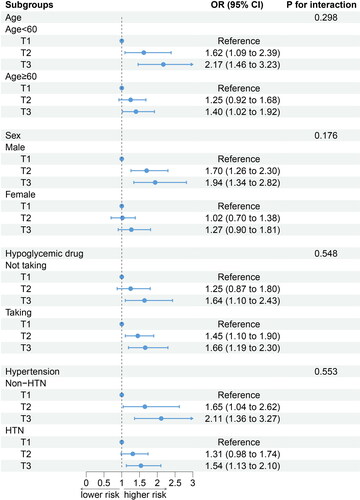
The relationship between the DII and CKD remained consistent when the participants were categorized according to age, sex, hypoglycemic medications, and hypertension. Additionally, there were no observed interactions between the stratified variables and the DII ().
Sensitivity analysis
After sensitivity analysis based on whether the DII was greater than 0 or the quartile or quintile of the DII, the results remained robust after regrouping participants (Supplementary Table 1). In addition, after grouping participants based on the time they participated in the survey, no differences were observed in the analysis results between 1999–2008 and 2009–2018 (Supplementary Table 2).
Discussion
This cross-sectional study included 7,974 people with DM, weighted to be representative of 33,315,777 people in the United States. Our research findings showed that a higher DII was linked to an increased frequency of CKD, which suggests that a proinflammatory diet may contribute to the development of CKD in patients with DM. After stratifying according to age, sex, hypoglycemic drug usage, and hypertension, the DII was still linked to the frequency of CKD, and no significant interaction between stratified variables and the DII was observed.
As one of the main complications of DM, CKD accounts for an enormous medical burden worldwide [Citation18]. At present, early prevention of CKD in patients with DM is mainly focused on controlling the blood glucose level and regulating the activity of the renin–angiotensin system [Citation5]. Despite advances in medical technology, the prevalence of DN continues to rise. The projected number of individuals suffering from CKD associated with DM is estimated to exceed 150 million worldwide by 2035 [Citation19]. Therefore, new approaches are urgently needed to prevent DN.
Diet is an important way to prevent DN. In a prospective study from Iran, the dietary approach to stop hypertension (DASH) was shown to delay the progression of DN in women [Citation20]. Additionally, al-Jaydi and colleagues proposed that the Mediterranean dietary pattern was linked to the incidence and progression of DN [Citation21]. These findings further highlight the importance of dietary patterns in preventing CKD in patients with DM.
Dietary components, such as SFAs, trans fats, and cholesterol, all contribute to the increase in markers of systemic inflammation [Citation12,Citation13,Citation22]. In patients with DM, inflammatory markers have demonstrated their capability to serve as stand-alone risk factors for the onset and advancement of CKD [Citation8–10,Citation23]. The DII ranges from −5 to 5, with the former indicating the most anti-inflammatory diets and the latter representing highly proinflammatory diets. Numerous investigations have verified the association between an increased DII and increased markers of systemic inflammation [Citation24]. Nevertheless, there is limited research that has established a direct correlation between the DII and the frequency of CKD in patients with DM.
A previous study of Iranian adults explored the relationship between the DII and CKD [Citation25] and found that the DII was linked to the risk of CKD. In addition, another study in the United States involving DII and CKD came to the same conclusion [Citation26]. However, as a high-risk group for CKD [Citation27], patients with DM were not discussed in their study. Diet is an important and modifiable lifestyle for DM patients [Citation28]; thus, it is necessary to directly discuss the relationship between diet and CKD in patients with DM.
According to our results, the DII was linked to the prevalence of CKD in patients with DM. The RCS showed that the frequency of CKD in DM patients increased along with the DII. Following the stratified analysis that accounted for age, sex, usage of hypoglycemic medications, and presence of hypertension, no substantial alterations were observed in the findings. Although there are differences in the pathogenesis of type-1 diabetes (T1D) and T2D [Citation29], we have not conducted further sensitivity analysis according to the type of DM because there are no data on the diagnosis of T1D provided in the NHANES database. However, previous studies have pointed out that inflammation plays a significant role in the development of DN in both T1D and T2D patients [Citation30–32]. Therefore, the DII may be applicable to both T1D and T2D patients, but as a hypothesis, this needs to be confirmed by further experimental studies. Notably, although no interaction between the DII and hypertension was observed in the multiplicative interaction model, in patients with DM, the OR values were lower in the subgroup with hypertension than in the subgroup without hypertension. This may be because patients with hypertension need to take blood pressure medications compared to patients without hypertension. Some blood pressure medications reduce blood pressure while also having anti-inflammatory effects [Citation33–35], which may reduce the harm caused by a proinflammatory diet.
The DII is a comprehensive tool for assessing dietary inflammation potential. A low DII indicates a high potential for an anti-inflammatory diet, and a high DII indicates a high potential for a proinflammatory diet. Several parameters contained in the DII, such as vitamin E, n-3 fatty acids, and MUFAs, are major anti-inflammatory components of the diet [Citation36]. Vitamin E may alleviate the progression of DN by reducing autophagy [Citation37]. n-3 fatty acids and MUFAs have also been shown to improve outcomes in patients with DM by reducing inflammation and endothelial dysfunction and improving the control of dyslipidaemia [Citation38]. In addition, proinflammatory parameters in the DII, including cholesterol and SFAs, are important factors that promote the occurrence and development of CKD in DM patients [Citation39]. A study by Kessler et al. [Citation40] reported that a high cholesterol intake increases CRP and worsens kidney function. A prospective study in Finland confirmed that SFA consumption accelerated the progression of CKD in DM patients by increasing the level of inflammation in diabetic patients [Citation41]. Based on this evidence, we believe that it is feasible to link the DII with CKD in DM patients. Adopting the DII to assess the dietary inflammatory potential of patients with DM, as well as adopting appropriate dietary interventions, may help prevent the development of CKD in patients with DM.
As mentioned above, current measures to prevent DN are mainly focused on blood glucose control and renin–angiotensin system activity [Citation5]. Interestingly, in our study, after the participants were grouped according to the DII, no statistically significant disparities were observed in blood glucose levels and HbA1c across the groups. This discovery implies that consumption of a diet that reduces inflammation could reduce the likelihood of developing CKD while having no impact on blood glucose levels. We were unable to verify whether a proinflammatory diet affected renin–angiotensin system activity because there were no biomarkers for renin–angiotensin system activity in our data. However, based on the results of our study, we speculate that an anti-inflammatory diet could serve as an alternative and efficient strategy for the prevention of CKD in addition to controlling blood glucose and regulating the activity of the renin–angiotensin system in patients with DM. To validate our hypothesis, it is necessary for future research to include additional randomized controlled studies.
Advantages and limitations
In our research, we employed a sample that represented the entire nation, weighted to be representative of 33,315,777 patients with DM in the United States. Compared to previous studies, we more fully adjusted for many risk factors for developing CKD in patients with DM. This finding makes our results reliable. Our study was subject to certain limitations. Initially, due to the cross-sectional design of our study, it was not feasible to establish a cause-and-effect connection between the DII and CKD. Second, the calculation of DII only incorporated 28 nutrients; nonetheless, previous research has demonstrated that the predictive capability of DII remains consistent regardless of whether it is calculated using a limited set of 28 nutrients or a larger set of 45 nutrients [Citation42]. Third, our data were taken from the NHANES, which used a complex sampling design, and all statistical analyses should use complex sampling and weighting. Currently, there is no ROC curve code for complex sampling and weighting in R, so we could not perform the ROC test; however, we used RCS to investigate the association between the DII and the prevalence of CKD, and our results still have reasonable reliability. Additionally, due to the lack of relevant data, we could not calculate the energy-adjusted-DII (E-DII), so we adjusted for energy consumption in the regression model. Finally, although variables that were expected to affect the results were included in the regression model as much as possible, the impact of age, sex and other confounding variables cannot be completely eliminated by the adjustment of the regression model alone. Further randomized clinical trials are needed to confirm this result in the future.
Conclusions
The DII was linked to the prevalence of CKD in patients with DM. The frequency of CKD increased with increasing DII. Proinflammatory diets may increase the frequency of CKD in people with DM. The implementation of an anti-inflammatory diet could serve as an intervention strategy for diabetic patients to prevent the onset of CKD. Additional research is necessary to validate this idea.
Ethics approval and consent to participate
Informed consent was obtained from every participant, and therefore, there was no need for any ethical approval in this study. The NCHS ethics review board approved the NHANES protocol. All procedures were performed in accordance with the relevant guidelines and regulations.
Author contributions
YL designed the research and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; CHG conducted the analysis and wrote the first draft of the paper; and YL, SCW, HQL, MW, and FZW revised the manuscript.
Supplemental Material
Download PDF (127.5 KB)Supplemental Material
Download PDF (134 KB)Disclosure statement
All authors (Chunhua Guo, Yong Lin, Senchao Wu, Huaqing Li, Meng Wu, and Fuzhen Wang) report no conflicts of interest.
Availability of data and materials
All data are available as publicly accessible datasets through the NHANES, and they can be accessed through the following link: https://wwwn.cdc.gov/nchs/nhanes/.
Additional information
Funding
References
- Yokoyama K, Nakashima A, Urashima M, et al. Interactions between serum vitamin D levels and vitamin D receptor gene FokI polymorphisms for renal function in patients with type 2 diabetes. PLoS One. 2012;7(12):1. doi:10.1371/journal.pone.0051171.
- Fox KM, Yee J, Cong Z, et al. Transfusion burden in non-dialysis chronic kidney disease patients with persistent anemia treated in routine clinical practice: a retrospective observational study. BMC Nephrol. 2012;13(1):5. doi:10.1186/1471-2369-13-5.
- Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1(1):15018. doi:10.1038/nrdp.2015.70.
- Kong APS, Yang X, Luk A, et al. Hypoglycaemia, chronic kidney disease and death in type 2 diabetes: the Hong Kong diabetes registry. BMC Endocr Disord. 2014;14(1):48. doi:10.1186/1472-6823-14-48.
- Tang W-H, Yu T-H, Lee H-L, et al. Interactive effects of intrinsic capacity and obesity on the KDIGO chronic kidney disease risk classification in older patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2023;15(1):1. doi:10.1186/s13098-022-00975-x.
- Cassimatis M, Kavanagh DJ, Hills AP, et al. The OnTrack diabetes web-based program for type 2 diabetes and dysphoria self-management: a randomized controlled trial protocol. JMIR Res Protoc. 2015;4(3):e97. doi:10.2196/resprot.2813.
- Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–9. doi:10.1042/CS20120198.
- Yang H, Xie T, Li D, et al. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol Metab. 2019;23:24–36. doi:10.1016/j.molmet.2019.02.007.
- Tang PM-K, Zhang Y-Y, Hung JS-C, et al. DPP4/CD32b/NF-κB circuit: a novel druggable target for inhibiting CRP-driven diabetic nephropathy. Mol Ther. 2021;29(1):365–375. doi:10.1016/j.ymthe.2020.08.017.
- Yaribeygi H, Atkin SL, Sahebkar A. Interleukin-18 and diabetic nephropathy: a review. J Cell Physiol. 2019;234(5):5674–5682. doi:10.1002/jcp.27427.
- Jonasson L, Guldbrand H, Lundberg AK, et al. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med. 2014;46(3):182–187. doi:10.3109/07853890.2014.894286.
- Das UN. Is there a role for bioactive lipids in the pathobiology of diabetes mellitus? Front Endocrinol. 2017;8:182. doi:10.3389/fendo.2017.00182.
- Roche HM. Dietary modulation of energy homoeostasis and metabolic-inflammation. Proc Nutr Soc. 2019;78(3):313–318. doi:10.1017/S0029665118002872.
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi:10.1017/S1368980013002115.
- Qiu Z, Chen X, Geng T, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. 2022;45(6):1453–1461. doi:10.2337/dc21-2371.
- KDIGO. Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276.
- Saint-Maurice PF, Graubard BI, Troiano RP, et al. Estimated number of deaths prevented through increased physical activity among US adults. JAMA Intern Med. 2022;182(3):349–352. doi:10.1001/jamainternmed.2021.7755.
- Debele GR, Hajure M, Wolde HF, et al. Incidence and predictors of chronic kidney disease among diabetes mellitus patients: a retrospective follow-up study at a tertiary Health-Care setting of Ethiopia. Diabetes Metab Syndr Obes. 2021;14:4381–4390. doi:10.2147/DMSO.S335572.
- Chiu Y-L, Tsai W-C, Hung R-W, et al. Emergence of T cell immunosenescence in diabetic chronic kidney disease. Immun Ageing. 2020;17(1):31. doi:10.1186/s12979-020-00200-1.
- Soltani S, Hashemi R, Heshmat R, et al. Association of dietary approaches to stop hypertension eating style and risk of sarcopenia. Sci Rep. 2020;10(1):19339. doi:10.1038/s41598-020-76452-0.
- Jayedi A, Mirzaei K, Rashidy-Pour A, et al. Dietary approaches to stop hypertension, mediterranean dietary pattern, and diabetic nephropathy in women with type 2 diabetes: a case-control study. Clin Nutr ESPEN. 2019;33:164–170. doi:10.1016/j.clnesp.2019.05.021.
- Noori N, Dukkipati R, Kovesdy CP, et al. Dietary omega-3 fatty acid, ratio of omega-6 to omega-3 intake, inflammation, and survival in long-term hemodialysis patients. Am J Kidney Dis. 2011;58(2):248–256. doi:10.1053/j.ajkd.2011.03.017.
- Wan H, Wang Y, Fang S, et al. Associations between the neutrophil-to-LYMPHOCYTE ratio and diabetic complications in adults with diabetes: a cross-sectional study. J Diabetes Res. 2020;2020:6219545–6219549. doi:10.1155/2020/6219545.
- Tandorost A, Kheirouri S, Moludi J, et al. Association of dietary inflammatory index (DII) with disease activity and inflammatory cytokines in the patients with rheumatoid arthritis. Int J Clin Pract. 2021;75(11):e14792. doi:10.1111/ijcp.14792.
- Moludi J, Fateh HL, Pasdar Y, et al. Association of dietary inflammatory index with chronic kidney disease and kidney stones in Iranian adults: a cross-sectional study within the Ravansar non-communicable diseases cohort. Front Nutr. 2022;9:955562. doi:10.3389/fnut.2022.955562.
- Mazidi M, Shivappa N, Wirth MD, et al. Greater dietary inflammatory index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. 2018;120(2):204–209. doi:10.1017/S0007114518001071.
- McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36(2):237–244. doi:10.2337/dc12-0706.
- Chen Z, Zuurmond MG, van der Schaft N, et al. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam study. Eur J Epidemiol. 2018;33(9):883–893. doi:10.1007/s10654-018-0414-8.
- Arneth B, Arneth R, Shams M. Metabolomics of type 1 and type 2 diabetes. Int J Mol Sci. 2019;20(10):2467. doi:10.3390/ijms20102467.
- Triñanes J, Salido E, Fernández J, et al. Type 1 diabetes increases the expression of proinflammatory cytokines and adhesion molecules in the artery wall of candidate patients for kidney transplantation. Diabetes Care. 2012;35(2):427–433. doi:10.2337/dc11-1665.
- Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23(3):516–524. doi:10.1681/ASN.2011060628.
- Poulsen CG, Rasmussen DGK, Genovese F, et al. Marker for kidney fibrosis is associated with inflammation and deterioration of kidney function in people with type 2 diabetes and microalbuminuria. PLoS One. 2023;18(3):e0283296. doi:10.1371/journal.pone.0283296.
- Samaha MM, Helal MG, El-Sherbiny M, et al. Indapamide increases IRS1 expression and modifies adiponectin/NLRP3/PPARγ crosstalk in type 2 diabetic rats. Antioxidants. 2022;11(4):691. doi:10.3390/antiox11040691.
- Kostakoglu U, Mercantepe T, Yilmaz HK, et al. The protective effects of perindopril against acute kidney damage caused by septic shock. Inflammation. 2021;44(1):148–159. doi:10.1007/s10753-020-01316-8.
- Koz C, Baysan O, Yokusoglu M, et al. The effects of perindopril on aortic elasticity and inflammatory markers in hypertensive patients. Med Sci Monitor. 2009;15(7):Pi41–45.
- Liu Z, Liu H, Deng Q, et al. Association between dietary inflammatory index and heart failure: results from NHANES (1999–2018). Front Cardiovasc Med. 2021;8:702489. doi:10.3389/fcvm.2021.702489.
- Zhao Y, Zhang W, Jia Q, et al. High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front Physiol. 2018;9:1939. doi:10.3389/fphys.2018.01939.
- Shapiro H, Theilla M, Attal-Singer J, et al. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat Rev Nephrol. 2011;7(2):110–121. doi:10.1038/nrneph.2010.156.
- Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009;24(11):3288–3296. doi:10.1093/ndt/gfp302.
- Kaesler N, Baid-Agrawal S, Grams S, et al. Low adherence to CKD-specific dietary recommendations associates with impaired kidney function, dyslipidemia, and inflammation. Eur J Clin Nutr. 2021;75(9):1389–1397. doi:10.1038/s41430-020-00849-3.
- Mäkinen V-P, Tynkkynen T, Soininen P, et al. Metabolic diversity of progressive kidney disease in 325 patients with type 1 diabetes (the FinnDiane study). J Proteome Res. 2012;11(3):1782–1790. doi:10.1021/pr201036j.
- Li A, Chen Y, Schuller AA, et al. Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol. 2021;48(7):907–918. doi:10.1111/jcpe.13472.