Abstract
Introduction
The prevalence of chronic kidney disease (CKD) is gradually increasing in the elderly population. At the same time, frailty has become one of the research hotspots in the field of geriatrics. Bibliometric analyses help to understand the direction of a field. Therefore, this study aimed to analyze the status and emerging trends of frailty in CKD patients.
Data and methods
The Web of Science Core Collection (WoSCC) database was screened for relevant literature published between 1 January 2000 and 31 December 2021. Next, publications were analyzed for information including authors, journals, cited references, citing journals, institutions, countries and regions, high-frequency keywords and co-citations using VOSviewer, Microsoft Excel, and R software.
Results
A total of 2223 articles were obtained, from which 613 relevant articles were selected based on title and abstract screening. There was an upward trend in the number of annual publications and Johansen KL was considered the most contributing author in the field. The Clinical Journal of the American Society of Nephrology was the most productive research journal. Johns Hopkins University is the most published organization. The United States is the global leader in the field and contributes the most to research. Research hotspots focus on epidemiological studies of frailty and frailty intervention.
Conclusions
This study presents a comprehensive bibliometric analysis of CKD and frailty research. Key findings highlight the current focus on early screening and assessment of frailty in CKD patients, as well as physical function interventions in frail patients.
1. Introduction
Chronic kidney disease (CKD) is characterized by a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2, albuminuria exceeding 30 mg over 24 h, or persistent kidney damage indicators such as hematuria or structural abnormalities like polycystic kidneys for over three months [Citation1]. According to the latest epidemiological studies, an estimated 843.6 million people are affected by CKD stages 1–5 worldwide [Citation2]. In a recent systematic review and meta-analysis of 100 studies, including 6,908,440 patients, the global prevalence of CKD stages 1–5 was estimated to be 13.4% [Citation3]. An international study on disease burdens indicated that from 1990 to 2017, the age-adjusted mortality rate for CKD surged by 41.5% (95% CI 35.2–46.5). Among a hierarchy of 133 diseases based on mortality rates, CKD emerged as the twelfth leading cause of death. Over the past two decades, CKD-related fatalities have escalated, and the afflicted global population is anticipated to further increase, highlighting a significant public health dilemma [Citation4].
First introduced in 1968 by O’Brien et al. [Citation5], frailty refers to excessive or inappropriate responses to negative events in older adults. Dr. Linda Fried and colleagues from Johns Hopkins University, in a 2001 community-based study of cardiovascular health in older adults, described five important manifestations of frailty: fatigue, muscle weakness, slow gait, unexplained weight loss, and physical inactivity [Citation6]. In the same year, Rockwood and Mitnitski in Canada proposed the frailty index (FI), which was based on the accumulation of age-related deficits, and considered frailty as a dangerous state resulting from the accumulation of health deficits [Citation7]. Subsequently, in 2004, the American Geriatrics Society clarified the definition of ‘frailty’: a nonspecific state of decreased stress resistance due to reduced physiological reserve [Citation8]. Frailty primarily manifests as decreased levels of physiological function in several organ systems, including neuromuscular, endocrine, metabolic, and immune systems [Citation9]. A systematic review of 61,000 community-based older adults from high-income countries noted that the weighted average estimate of frailty prevalence was 11% [Citation10]. Notably, this review also showed that the prevalence of frailty varied widely across studies (ranging between 4 and 59%) due to a lack of standardization of concepts or measures.
A recent cross-sectional single-center study of 148 participants (including 60 patients on maintenance hemodialysis (HD) and 88 patients with stage 3–4 CKD) showed that 71.6% of HD patients and 53.4% of CKD stage 3–4 patients had pre-frailty or frailty [Citation11]. It is important to note that pre-frailty or frailty is common even among younger HD patients, with 23.0% of HD patients being under 50 years of age, and the incidence rate increases with age, suggesting that the prevalence of frailty is relatively high in patients with different stages of CKD. Research has shown that frailty is not only an independent risk factor for increased hospitalization and adverse outcomes such as deaths in patients with CKD [Citation12], but also shares the same physiopathological basis as CKD, which can further accelerate the course of poor patient prognosis. The 2012 US and European expert consensus on geriatrics suggests that all older adults aged 70 years and above need to be screened for frailty, especially those with underlying diseases such as heart failure, tumor, renal failure, diabetes mellitus, and those requiring surgery, and that early prevention and clinical interventions should be provided for debilitated patients [Citation13].
The undeniable nexus between frailty and CKD has spurred significant scholarly attention, resulting in an influx of related publications. For example, Lorenz et al. wrote a narrative review describing the current state of research on frailty in patients with CKD [Citation14]. In addition, there have been some researchers who have explored the prevalence of frailty [Citation15] and the effectiveness of interventions [Citation16] in patients with CKD through systematic review; however, none of them have performed visual analyses. As the volume of literature expands, navigating the most recent advancements becomes a formidable challenge for researchers. While systematic reviews are beneficial, they often struggle to handle vast datasets or showcase time-bound research evolution. Thus, discerning future research trajectories is imperative. Bibliometrics, a concept introduced by Pritchard in 1969 [Citation17] and later enriched with infographics by Van Raan in 2004 [Citation18], emerges as a more potent tool than traditional narrative reviews. With advanced software, scholars can rapidly understand the nuances and emerging trends in specific fields [Citation19]. Particularly in medical research areas, notably postoperative cognitive dysfunction [Citation20] and COVID-19 [Citation21], bibliometric analysis provides both quantitative and qualitative insights. By pinpointing origin countries and contributing institutions and charting geographic distribution, it offers readers visually insightful results, lucidly delineating a field’s knowledge architecture and focal points, thus informing prospective research and policy decisions. While there have been bibliometric explorations concerning frailty and cardiovascular diseases [Citation22], the intersection of CKD and frailty remains underexplored.
Therefore, this study aimed to systematically investigate the status, hotspots, and emerging frontiers of global research on frailty in CKD patients over the past 20 years. Our study subjects described in this article are CRF patients, also known as stage 3–5 non-dialysis CKD patients defined by the KDIGO organization in the 2012 guidelines [Citation23] or ESKD/ESRD patients [Citation24] with frailty syndrome. Analyses addressing this theme will help to illustrate new directions for future research.
2. Materials and methods
2.1. Data source and search strategy
Web of Science Core Collection (WoSCC) is one of the most comprehensive and authoritative database platforms for scholarly information worldwide, with over 10,000 high-quality journals, and is the most commonly used database in previous bibliometric studies [Citation25,Citation26]. The database contains abstracts and other relevant data, such as citations and research collaboration information, to facilitate bibliometric analysis. In addition, it provides data in file formats that conform to the requirements of bibliometric software analysis.
We conducted a comprehensive search of the literature for the period 2000–2021 in the Science Citation Index Expanded (SCI-Expanded) through the WoSCC database on 22 November 2022, as a way to avoid bias due to database updates.
Our search strategy was as follows: TS = (chronic kidney disease) OR TS = (chronic renal failure) OR TS = (end-stage renal disease) OR TS = (chronic renal insufficiency) OR TS = (dialysis) OR TS = (peritoneal dialysis) OR TS = (hemodialysis) OR TS = (kidney transplantation) OR TS = (renal transplantation) AND TS = (Frail*) OR TS = (weakness) OR TS = (fatigue) OR TS = (feebleness) OR TS = (debility). The period was set to 2000–2021, the language was English, and the document type was Article.
2.2. Inclusion and exclusion criteria
A total of 2223 publications were retrieved, of which 1610 invalid records were excluded, including nonacademic and ineffective articles such as news reports, essays, editorials, case reports, conference summaries, and non-English literature, as well as duplicate papers. Finally, 613 valid publications were selected for analysis. After data validation and normalization, online publications, including complete records and cited references, were exported in plain text format and included in the study. Journal information, including impact factor (IF) and category quartiles (Q1–Q4), was collected from the 2022 Journal Citation Reports.
2.3. Data analysis and visualization
In this study, statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria) and Microsoft Excel 2016 (Redmond, WA). Visual analysis was performed using R software and VOSviewer.
Microsoft Excel was primarily used to collect and analyze WoSCC data to create histograms and construct regression models to predict the growth trend of publications.
VOSviewer is a Java-based freeware developed by van Eck and Waltman. It has powerful graphical processing capabilities and is suitable for large-scale handling of data [Citation27]. It is widely used for bibliographic visualizations, especially for the visual analysis of complex co-citation networks, such as relationships between coauthors, highly cited references, and keyword co-occurrence analysis [Citation28]. In this study, the software was used for visual analysis of country/author/institution collaboration networks, journal co-citation analysis, and keyword co-citation analysis, where the size of the node indicated the number of publications, the thickness of the line indicated the strength of the linkage, and the color of the node showed the different clusters or times.
The Bibliometrix R package was used to summarize the number of publications and citations for bibliometric analysis and determine the cumulative annual occurrence of popular keywords/terms and national publications for collaborative analysis [Citation29].
3. Results
3.1. Analysis of annual publications
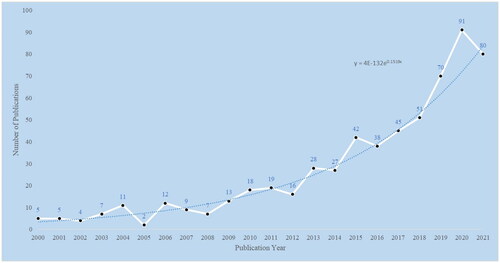
After literature screening, 613 publications on frailty and CKD were included in the final analysis, and the number and trend distribution of annual publications are shown in .
As shown in , there is an upward trend with slight fluctuations in the number of annual publications in this field, with a maximum of 91 articles published in 2020. The total number of citations for these publications is 18,247, with an average of 28.8 citations per publication and an H-index of 9.8. The H-index, proposed by Hirsch [Citation30], is a hybrid quantitative index used to assess the quantity and level of a researcher’s scholarly output. The higher a researcher’s H-index, the more significant the impact of their articles.
3.2. Analysis of most productive countries/regions
and show the top 10 countries/regions in terms of the number of publications per year, indicating rapid growth in research paper count. The United States is the most productive country, with 167 (27.8%) publications, followed by China, the United Kingdom, and Japan. A collaborative network map of countries was constructed using VOSviewer (). The boundaries between countries show the intensity of collaboration. The United States is the center of cooperation in this field, with the broadest range of partnerships involving several countries, followed by Australia, Denmark, Sweden, and Germany. In contrast, China, South Korea, and Japan have fewer collaborations. Overall, most collaborations are confined mainly to Europe, the United States, and Australia. This indicates that cooperation between East Asia and other countries needs to be further strengthened.
Figure 2. (A) Annual number of publications by countries. (B) World map showing the contribution of each country based on research paper count.
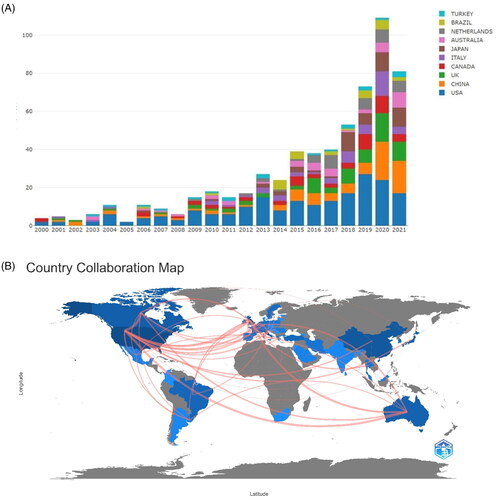
Table 1. Top 10 productive countries/regions based on the number of publications.
3.3. Analysis of most productive institutions
shows the top 10 most productive institutions in the research field. Six of these institutions are in the United States, one in Italy, one in the Netherlands, and two in Taiwan. Specifically, Johns Hopkins University is the most active institution, ranking first with 61 articles, followed by the University of California, San Francisco.
Figure 3. (A) Most relevant affiliations. (B) Visualization map of organizations’ collaborations based on CiteSpace.
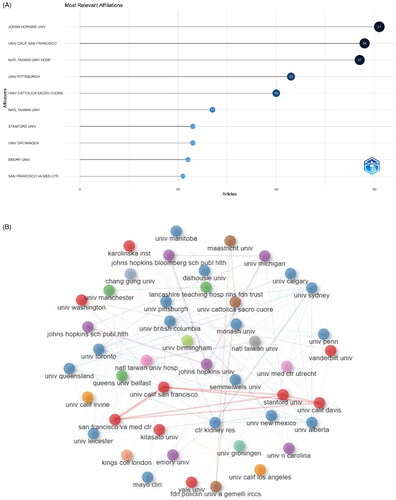
highlights the close and complex collaborative relationships among different institutions. It can be seen that inter-institutional collaborations are scattered among high-income countries in Oceania, North America, and Europe. Collaborations centered on the University of California, San Francisco, Stanford University, and the University of California, Davis are the most frequent, indicating the strong influence of these institutions in this field. All the institutions are universities, except San Francisco Veterans Affairs Medical Center and Division of Nephrology, which are hospitals.
3.4. Analysis of higher-impact journals
A total of 197 academic journals have published papers on frailty in the last two decades. Supplementary Figure 4 shows the top 10 journals in terms of the number of publications. The Clinical Journal of the American Society of Nephrology published the most number of articles (38 articles), followed by BMC Nephrology (30 articles), and American Journal of Kidney Diseases (24 articles). According to the 2022 Journal Citation Reports, four of the top 10 academic journals belong to Q1, with the American Journal of Kidney Diseases having the highest IF (11.072), followed by the Clinical Journal of the American Society of Nephrology (10.614), and Nephrology Dialysis Transplantation (7.186). Regarding the research areas of interest, nearly 60% of the journals deal with topics of urology and nephrology. As for publication regions, five journals are from the United States, two are from the United Kingdom, and the others are from Australia, the Netherlands, and Switzerland. Notably, most of these active journals are published in Europe and North America.
3.5. Analysis of productive authors
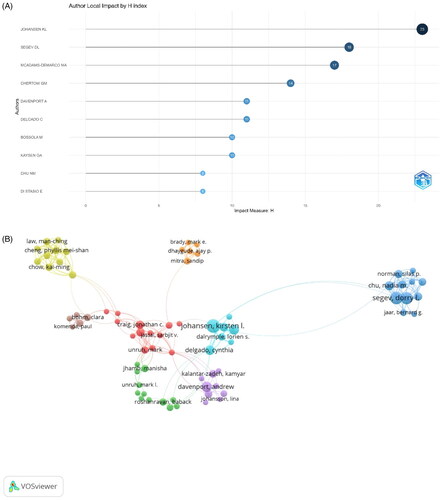
Among the 10 most prolific authors with maximum papers on frailty in CKD patients (), Johansen KL from the University of California, San Francisco, is ranked first, followed by Segev DL and McAdams-DeMarco MA. Johansen KL is also the author with the highest H-index. McAdams-DeMarco MA, Segev DL, and Chu NM are from the same institution, Johns Hopkins University School of Medicine. Notably, eight of the top 10 authors are from US institutions, and the other two are from Italy and France. A visualization of the authors’ coauthorship analysis was generated using VOSviewer (). Johansen KL had the largest number of collaborations, indicating his strong influence and dominance in the field.
3.6. Analysis of most cited documents
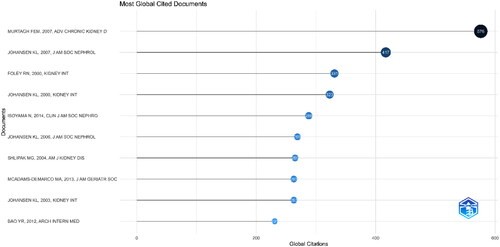
and Supplementary Table 2 show details of the top 10 most cited papers, with citations ranging from 231 to 576. Nine of these are original articles, and one is a systematic review. Three of these articles were published in the Journal of the American Society of Nephrology (IF = 18.998), three in Kidney International (IF = 14.978), two in the American Journal of Kidney Diseases (IF = 11.072), and one in the Clinical Journal of the American Society of Nephrology (IF = 10.614). The article by Murtagh et al. in Advances in Chronic Kidney Disease (IF = 4.305) ranked first with 576 citations [Citation31]. It was followed by Johansen et al.’s article titled ‘Significance of frailty among dialysis patients’ in the Journal of the American Society of Nephrology (IF = 18.998; 417 citations) [Citation12]. Foley et al.’s article in Kidney International (IF = 14.978) was at the third position with 331 citations [Citation32]. Most of these articles focused on the epidemiological investigation of frailty.
3.7. Analysis of co-cited references
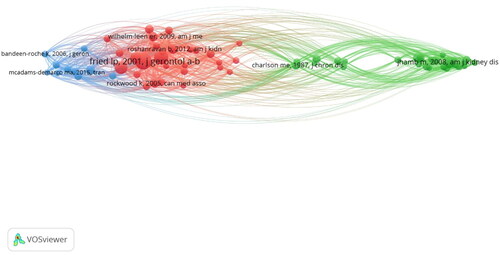
Fried LP from Columbia University was the most cited author (), with a paper proposing the Fried frailty phenotype (FP), containing five criteria, allowing frailty to be quantified and further studied. In the same year, Professor Lockwood K proposed the FI to assess the health status of older adults. FI includes 92 variables of symptoms, abnormal laboratory values, disease classification, and disability. Compared to FP, FI is more comprehensive. This is because FI contains more items, thus allowing a more comprehensive prediction of poor outcomes of frailty. Overall, both authors have contributed to the assessment of frailty.
3.8. Analysis of keywords and burst keywords
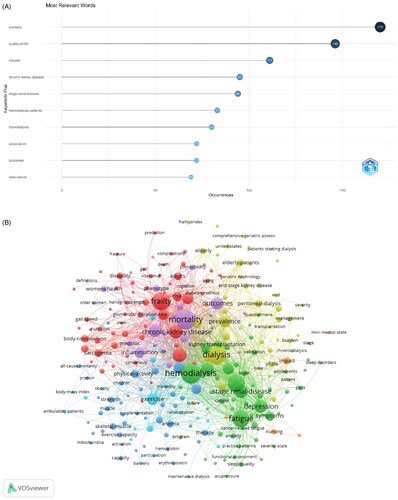
A keyword profile accurately summarizes an article’s topic and can be used for searching and cataloging. It can be easily deduced that the more frequently a keyword appears in a certain period, the more likely it represents the cutting-edge research direction in the field. According to , excluding ‘chronic kidney disease,’ ‘hemodialysis patients,’ and ‘hemodialysis,’ the keywords that appear more frequently include ‘mortality,’ ‘quality of life,’ ‘outcomes,’ and ‘older adults,’ and most of these terms are related to poor outcomes of frailty.
Figure 7. (A) Keywords related to the research topic. (B) Visualization map of popular keywords. (C) Co-occurrence view of the keywords. (D) Map showing thematic trends over time.
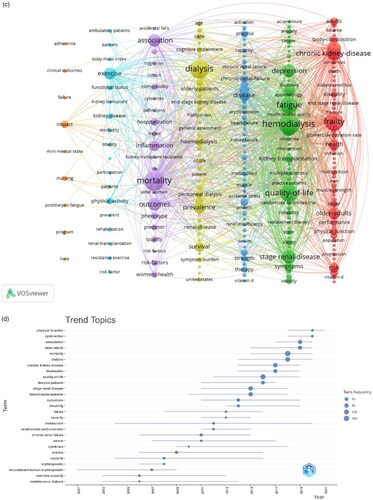
Looking at the connections between keywords, we can further understand the segmented research directions (see for details). The popular keywords can be grouped into four research hotspots. Glomerular filtration rate, risk, and diabetes mellitus shown in red are closely related to the theme word ‘frailty,’ which focuses on the mechanism of debilitation. The topic ‘hemodialysis’ in green is related to fatigue, depression, and ESRD, and focuses on the common symptoms of dialysis patients. The keywords in purple are related to prognosis, including mortality, outcomes, and predictor. The blue keywords are related to intervention treatment, including physical activity, exercise, and therapy. presents a visualization of keywords that appeared more than five times in the frailty literature; the node size is proportional to the frequency of keywords, and the thick line between two nodes is related to their co-occurrence frequency. The more closely associated keywords were divided into six groups #0 exercise intervention, #1 epidemiology, #2 frailty assessment, #3 complications of CKD, #4 common symptoms in dialysis patients, and #5 prognosis of frailty.
provides a graphical representation of the thematic trends. In addition to the words ‘association’ and ‘older adults,’ which have no practical meaning, the trending words with increased research focus include ‘physical function,’ ‘dysfunction,’ and ‘mortality.’ Similarly, the trending themes for each period can also be analyzed. The size of the circles in the figure represents the proportion of each keyword based on region.
According to Supplementary Figure 8E, the initial focus of research in this field was on the epidemiology and mechanisms of frailty; however, as the field evolved, the research focus is now on physical interventions for frailty in CKD patients.
4. Discussion
This study represented the first attempt to comprehensively analyze the current state of research, emerging frontiers, and hotspots related to frailty in CKD patients. Our approach involved a bibliometric analysis of relevant studies spanning from 2000 to 2021, coupled with the use of visualization software to quantitatively demonstrate the distribution of collaborations among authors, institutions, and countries. Additionally, we utilized co-citation reference and keyword analyses to showcase the evolution of research hotspots. By presenting this information, we enable scholars to gain a rapid, systematic, and intuitive understanding of an unfamiliar field, which can prove invaluable in shaping future research.
4.1. General information about research
A total of 613 studies were analyzed to identify research trends in the field of CKD and frailty. Over the past two decades, the number of papers published each year has shown a gradual upward trend, signifying the substantial academic attention garnered by CKD and frailty research. This observed increase can be attributed to demographic shifts in the global population’s age structure, the rising prevalence of CKD, and the growing emphasis on enhancing the quality of patient care. In recent years, there has been a decline in the amount of research literature in this area. This dip can be plausibly attributed to a potential shift in researchers’ focus away from this specific area. Furthermore, the European Working Group on Sarcopenia in Older People (EWGSOP) has underscored the intricate connection between frailty and sarcopenia, noting that a majority of frail older individuals exhibit manifestations of sarcopenia. Conversely, some older individuals diagnosed with sarcopenia also display characteristics of frailty [Citation33]. There is a strong conceptual correlation and overlap between frailty and sarcopenia, particularly in the physical domain. Notably, sarcopenia itself serves as a significant indicator of frailty [Citation34]. Therefore, another plausible explanation for the decrease in research output may be that researchers have shifted their focus to other aspects of sarcopenia or frailty rather than frailty itself. It is also plausible that the database might have included incomplete or insufficient literature, contributing to this observation.
Regarding the nations producing the most research papers on the topic, over half of the top 10 institutions were from the United States, with close collaborative relationships with other countries, while the remaining institutions were from Europe, Oceania, and East Asia. This could be associated with the level of medical care and economic conditions in diverse regions, with developing countries/regions having lower medical expenditure compared to developed countries. Multidisciplinary integration through various institutions collaboration leads to superior quality academic results, and reinforcing transnational collaboration could aid Asian countries in enhancing the quality of their research. In addition, we noted that nearly all research studies were conducted by universities, with fewer hospitals involved.
Concerning the journals publishing literature on the topic, nearly 60% of the journals focus on the domains of urology and nephrology, according to the Journal Citation Reports 2022. Among the top 10 prolific journals, the American Journal of Kidney Diseases (11.072) had the highest IF, followed by the Clinical Journal of the American Society of Nephrology (10.614), and Nephrology Dialysis Transplantation (7.186), indicating their high standing and authority in the field. It is important to note that fewer studies originated from East Asia with less impact, suggesting that Asian countries should bolster transnational cooperation and information exchange to increase their academic influence in the field. Additionally, 30% of the journals belong to the Q1 category of Journal Citation Reports.
The top three authors with the most papers and highest H-index in the field are Johansen KL, Segev DL, and McAdams-DeMarco MA. Johansen KL has published 28 papers to date on CKD patients with frailty. His 2007 paper titled ‘Significance of frailty among dialysis patients’ is the second most cited in the field and includes 2275 adults in a prospective study, which revealed that two-thirds of the participants met the criteria for frailty. Moreover, frailty was independently associated with a higher risk of death and the combined outcome of death or hospitalization. This discovery has significantly increased scholarly interest in CKD patients with frailty.
4.2. Current status of research
4.2.1. Definition and assessment of frailty
The precise definition of frailty remains a controversial issue. Accurately diagnosing frailty and understanding its various manifestations present significant clinical challenges [Citation35]. In addition, researchers have developed unique scales for assessing frailty, often based on their specific areas of expertise, guidelines, and areas of concern. This variability has resulted in a diverse array of interpretations of frailty in the research community [Citation36]. A systematic review of existing tools for assessing frailty [Citation37] brought attention to the absence of a universally accepted gold standard [Citation38]. Consequently, many HD units refrain from routine frailty screening due to the lack of consensus regarding the most appropriate assessment tool [Citation39]. It is worth noting that frailty tools may not be interchangeable in their settings of application, so careful consideration of the key indicators to be assessed is essential when measuring frailty. Furthermore, the application of frailty scales and their items requires consideration of their limitations in terms of time, resources constraints, and their applicability to specific populations. For example, the application of certain items, such as ergometer-based assessment of gait speed, may not always be feasible in primary care settings. Moreover, the reliability of results must be taken into account when using frailty scales, particularly when working with specific population groups such as individuals with disabilities [Citation40].
4.2.2. Epidemiological studies of frailty in CKD
The first cluster of keywords, denoted as #1, encompasses epidemiological investigations related to frailty, which includes terms such as mortality, outcomes, predictors, associations, and risk factors. From 2015 to 2020, frailty became an increasingly prominent focus of study, particularly concerning dialysis patients. The NHANES study revealed a substantial correlation between the declining estimated glomerular filtration rate (eGFR) and an increased risk of frailty. More precisely, individuals with stages 1, 2, or 3a of CKD faced double the risk of being classified as debilitated compared to those without CKD, while individuals with CKD stage 3b or more experienced an almost sixfold elevated risk compared to those without CKD [Citation41]. Subsequent research has elucidated the hierarchical relationship between the prevalence of frailty and the severity of renal disease. In particular, the prevalence of frailty in patients with eGFR levels ≥60, 45–59, 30–44, and <30 mL/min/1.73 m2 was 8.0%, 10.8%, 18.0%, and 32.8%, respectively [Citation42].
Up to this point, research on patients with non-dialysis-dependent chronic kidney disease (NDD-CKD) has remained relatively scarce. Only three studies have examined the prevalence of frailty in patients with CKD stage 3–4. However, due to the diversity within the study populations, the reported prevalence rates varied, ranging from 53.4% to 64.7% [Citation11]. In the meantime, prospective studies have unequivocally demonstrated that a FP is linked to an increased risk of requiring dialysis treatment (2.5-fold; 95% CI, 1.4–4.4) and higher mortality (after adjusting for factors like age, sex, body mass index, and comorbidities) [Citation43]. Patients with end-stage renal disease (ESRD) exhibit a higher prevalence of frailty compared to patients with NDD-CKD [Citation14]. Meta-analyses have indicated that the prevalence of frailty in dialysis patients ranges from 14% to 73% [Citation15]. A Korean study employing a self-report questionnaire for frailty screening found no significant difference in frailty scores among patients receiving different dialysis modalities, with HD patients scoring a total frailty score (mean ± SE) of 1.89 ± 0.04, while peritoneal dialysis (PD) patients scored 1.96 ± 0.08 (p = .433). In a single-center observational cohort study, 22% of participants chose to discontinue maintenance HD due to frailty, ranking as the second most common reason. This study also underscored the inadequacy of palliative and end-of-life care for ESRD patients [Citation44].
Incorporating screening for frailty into candidate eligibility assessment is an internationally recognized priority for kidney transplantation (KT) [Citation45]. The current study highlights the frequent occurrence of frailty in KT candidates and recipients as a factor that may contribute to poor outcomes [Citation46]. Frailty in KT patients has been associated with post-KT complications, such as prolonged hospitalization and mortality [Citation47]. Therefore, early intervention for frailty in KT candidates and recipients is imperative to enhance their health and quality of life and to mitigate perioperative complications.
4.2.3. Frailty interventions
A large cross-sectional study conducted in Europe confirmed that physical inactivity is an independent risk factor for overall frailty [Citation48]. Common keywords in cluster #0 are exercise, physical activity, functional status, and rehabilitation, implying a focus on exercise interventions for frail patients. As CKD progresses and symptom burden increases [Citation49], physical activity tends to decrease. Among CKD patients, a striking feature is their tendency toward limited physical activity and an inherent tendency toward inactivity [Citation50]. An extensive multicenter cross-sectional observational study, which recruited the participation of 5656 volunteers in England, demonstrated low rates of physical activity (ranging from 6% to 34%) in a cohort of patients with CKD, a trend that increased significantly as the disease progressed [Citation50].
Numerous cross-sectional investigations have consistently shown a significant inverse relationship between GFR and parameters of exercise capacity, including maximal exercise capacity [Citation51], walking distance [Citation52], strength, balance, and fine motor skills [Citation53]. The Dialysis Outcomes and Practice Patterns Study (DOPPS) provides compelling evidence that patients engaging in weekly exercise exhibit a reduced risk of mortality in comparison to those who exercise less than once a week or remain entirely sedentary (hazard ratio = 0.73 [0.69–0.78]; p < .0001) [Citation54]. A deeper exploration into the specific exercise modalities unveils that a meta-analysis has demonstrated that clinical studies integrating aerobic exercise therapy with resistance training not only enhance exercise tolerance to a greater degree but also have the potential to improve overall fitness, in contrast to studies solely relying on aerobic exercise therapy [Citation55].
Apart from insufficient physical activity, malnutrition has garnered significant attention within the CKD patient population. Malnutrition stands as a pivotal factor contributing to muscle depletion and weakness in CKD patients [Citation56]. With the advancement of our understanding of the pathophysiological underpinnings, the concept of protein-energy wasting (PEW) was introduced. The International Society of Renal Nutrition and Metabolism (ISRNM) defines PEW as a condition present in CKD patients, characterized by diminished protein and energy reserves, which reflects the intricate pathophysiological mechanisms of malnutrition within the CKD population [Citation57]. The development and progression of this condition stem from a multitude of factors, including inflammation, losses induced by dialysis, insufficient nutrient intake, chronic acidosis, and other related variables [Citation58].
Therefore, in the context of frail CKD patients, it is critical to address the underlying etiological factors that contribute to PEW. These include uremia, chronic acidosis, comorbidities, and depression, as well as other associated diseases [Citation59]. The strategic restriction of dietary phosphate necessitates a nuanced approach, as the potential risks may outweigh the associated benefits, potentially leading to adverse outcomes such as malnutrition and protein-energy wastage. Consequently, the limitation of dietary phosphate mandates a well-informed and individualized decision-making process, with further research needed to validate the merits of phosphate restriction [Citation59]. Maintaining nutritional status should be prioritized over any other dietary restriction, as emphasized in the latest guidelines published by the European Renal Best Practice Group in 2016 [Citation60]. Nonetheless, the research field focusing on the effectiveness of nutritional interventions in mitigating frailty in CKD patients remains significantly underdeveloped. Furthermore, there is a distinct lack of high-level evidence for the potential benefits of nutritional interventions in the context of the frail elderly population [Citation61].
4.2.4. Quality of life on CKD patients
In cluster 3, the prevalence of common keywords such as disease, heart failure, metabolism, and anemia emphasized the complexity of CKD comorbidities. There has been a significant shift in perspective when confronting the intricate landscape of comorbidities commonly encountered in the elderly population, underscoring the inadequacy of the traditional disease-centric management model [Citation62]. This noticeable shift in perspective has been ongoing since 2004. The co-occurring manifestations of CKD and its associated complications have shown a strong correlation with heightened healthcare utilization, prolonged hospital stays, and increased mortality [Citation63]. For example, a cross-sectional study showed that 98.2% of patients with CKD had comorbidities and only 1.8% had no complications. In contrast, this stood at 48.2% for non-CKD patients [Citation63]. Cardiovascular risk increases as CKD progresses. 33.3–37.1% of patients with mild to moderate CKD and 39.9% of patients with advanced CKD develop cardiovascular complications [Citation64]. Several nontraditional risk factors, such as volume overload, anemia, proteinuria, abnormal calcium phosphate metabolism, inflammation, and oxidative stress, contribute to the development and progression of cardiovascular disease in patients with CKD [Citation65]. The 2017 Global Burden of Disease Study on Chronic Kidney Disease highlighted that nearly 7% of the cardiovascular disease burden is attributable to impaired kidney function. It is of utmost importance to recognize that cardiovascular disease is the leading cause of death in patients with CKD [Citation66,Citation67]. Given the multifaceted and intricate nature of complications in frail patients, interdisciplinary collaboration holds paramount importance in the realm of patient care [Citation68].
Cluster #4 pertains to a focus on the typical symptoms experienced by individuals undergoing maintenance HD. Patients afflicted with CKD routinely grapple with a pronounced burden of symptoms [Citation31,Citation69]. Within the construct of the FP, self-perceived fatigue assumes paramount importance and substantially contributes to the amelioration of HRQoL [Citation70]. As Traditional Chinese Medicine Expert Prof. Zhang Qi once mentioned, frailty in CKD resembles “deficiency syndrome” which is composed of symptoms like fatigue, loss of appetite, muscle weakness, reduced activity, etc. The identification of ‘fatigue’ serves as a pivotal harbinger for the early detection of frailty risk, conferring robust predictive potential for an array of adverse outcomes encompassing functional impairments, disabilities, mortality, and various other untoward consequences [Citation71]. Furthermore, the progressive intensification of symptom burden engenders a cascade of deleterious consequences, encompassing further deterioration in physical functioning, restriction of physical activity, and an exacerbation in unfavorable health outcomes [Citation72].
4.3. Frontiers about CKD with frailty
Early frailty is often regarded as a potential reversible condition [Citation73,Citation74]. Evaluating the prognosis of frailty can significantly enhance patient-centered care and decision-making for individuals with ESRD [Citation75,Citation76]. Keyword cluster #5 suggests a focus on prognostic studies of frailty. In this context, a dual approach must be adopted. First, gaining a profound understanding of the mechanisms underlying individual frailty becomes crucial. Second, primary healthcare providers must increase awareness about frailty and avoid misconceptions that categorize it as a normal part of the aging process. Hence, healthcare professionals should prioritize early screening of elderly individuals in the broader community, extending their focus to patients who have not yet undergone dialysis and those awaiting KT. Moreover, the establishment of a robust monitoring system, combined with ongoing follow-up and regular patient education, contributes to the effectiveness of the healthcare system in addressing the needs of this specific population [Citation77]. Additionally, it is crucial to acknowledge the multifaceted nature of vulnerability. Consequently, the development of effective management strategies necessitates a multidisciplinary approach, including areas such as nutrition, psychology, and rehabilitation [Citation78].
The clinical practice guidelines issued by the Kidney Disease: Improving Global Outcomes Organization (KDIGO) in 2012, as well as other relevant guidelines, endorse the use of increased physical activity levels as a therapeutic intervention [Citation23]. Unfortunately, these guidelines do not offer precise recommendations regarding the types, intensity, and quantity of exercise suitable for individuals with CKD. This deficiency still exists, mainly due to limitations caused by a lack of participants in existing research participants, poor research quality, and limited clinical applicability. Therefore, the optimal exercise plan for CKD patients remains uncertain [Citation79]. The implementation of exercise therapy for dialysis patients involves three crucial phases: comprehensive pre-exercise physical assessment, the judicious formulation of an appropriate exercise prescription, and the establishment of sustainable support strategies [Citation80]. Furthermore, dialysis patients are primarily concerned with alleviating fatigue and enhancing their overall quality of life in contrast to the potential advantages offered by exercise. Effective symptom management is, therefore, indispensable in facilitating patient engagement in physical activity [Citation81]. While there is a considerable body of clinical research on the impact of exercise on CKD patients, substantial heterogeneity exists due to variations in frailty assessments applied in these studies [Citation14]. Moreover, most investigations primarily focus on the relationship between exercise in CKD patients and functional recovery, with a noticeable shortage of studies using frailty as an outcome measure. Consequently, there is an urgent need for more comprehensive research to bridge these substantial gaps in our understanding.
The limitations of this study must be considered when analyzing the results. First, the heterogeneity of the definitions of frailty hindered the synthesis of the evidence. Second, we only retrieved data from the WoSCC database and did not include other databases, resulting in an incomplete literature collection. Third, this study included only English literature, which might have led to biased findings. In addition, there is no systematic standard for the parameter settings and analysis methods used in this study, which may lead to differences in the results. Also, software such as VOSviewer only supports the file format of the WoSCC database, which may lead to publication bias. Therefore, the design of this study needs further improvement.
5. Conclusions
An increasing number of scholars are beginning to pay attention to CKD patients with frailty. The United States and Europe play a dominant role and are the centers for conducting research. Johns Hopkins University has the most published papers, while Johansen KL has contributed the most to the field. This paper also summarizes the hotspots of CKD with frailty, focusing on epidemiological studies of frailty and mechanisms of frailty production, with the frontiers of early screening and assessment of frailty in CKD patients, physical function interventions for frail patients. To the best of our knowledge, this is the first study to conduct a comprehensive bibliometric analysis of publications related to frailty and CKD in the past 20 years, which is of great significance for future related research.
Supplemental Material
Download PDF (248 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1–16. doi: 10.1001/jama.2019.14745.
- Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–1805. doi: 10.1093/ndt/gfz174.
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLOS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765.
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7–11. doi: 10.1016/j.kisu.2021.11.003.
- O’Brien TD, Roberts J, Brackenridge GR, et al. Some aspects of community care of the frail and elderly: the need for assessment. Gerontol Clin. 1968;10(4):215–227. doi: 10.1159/000245187.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58.
- Donatelli NS, Somes J. What is frailty? J Emerg Nurs. 2017;43(3):272–274. doi: 10.1016/j.jen.2017.03.003.
- Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005(31):pe24. doi: 10.1126/sageke.2005.31.pe24.
- Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x.
- Ozturk S, Cetin DG, Cetin M, et al. Prevalence and associates of frailty status in different stages of chronic kidney disease: a cross-sectional study. J Nutr Health Aging. 2022;26(9):889–895. doi: 10.1007/s12603-022-1839-z.
- Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221.
- Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022.
- Lorenz EC, Kennedy CC, Rule AD, et al. Frailty in CKD and transplantation. Kidney Int Rep. 2021;6(9):2270–2280. doi: 10.1016/j.ekir.2021.05.025.
- Chowdhury R, Peel NM, Krosch M, et al. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135–142. doi: 10.1016/j.archger.2016.10.007.
- Yoo J, Ruppar T, Wilbur J, et al. Effects of home-based exercise on frailty in patients with end-stage renal disease: systematic review. Biol Res Nurs. 2022;24(1):48–63. doi: 10.1177/10998004211033031.
- Pritchard A. Statistical bibliography or bibliometrics? J Doc. 1969;(25):348–349.
- Van Raan AF. Sleeping beauties in science. Scientometrics. 2004;59(3):467–472. doi: 10.1023/B:SCIE.0000018543.82441.f1
- Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. 2006;57(3):359–377. doi: 10.1002/asi.20317.
- Chen S, Zhang Y, Dai W, et al. Publication trends and hot spots in postoperative cognitive dysfunction research: a 20-year bibliometric analysis. J Clin Anesth. 2020;67:110012. doi: 10.1016/j.jclinane.2020.110012.
- Ying J, Tan GMY, Zhang MW. Intellectual disability and COVID-19: a bibliometric review. Front Psychiatry. 2022;13:1052929. doi: 10.3389/fpsyt.2022.1052929.
- Bao X, Chung LYF, Wen Y, et al. A visualization analysis of hotspots and frontiers of cardiovascular diseases with frailty. Front Public Health. 2022;10:915037. doi: 10.3389/fpubh.2022.915037.
- Andrassy KM. Comments on “KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease”. Kidney Int. 2013;84(3):622–623. doi: 10.1038/ki.2013.243.
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5.
- Li LL, Ding G, Feng N, et al. Global stem cell research trend: bibliometric analysis as a tool for mapping of trends from 1991 to 2006. Scientometrics. 2009;80(1):39–58. doi: 10.1007/s11192-008-1939-5.
- Kelly JC, Glynn RW, O’Briain DE, et al. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338–1343. doi: 10.1302/0301-620X.92B10.24867.
- van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3.
- Chen K, Zhao J, Yang Y, et al. Global research trends of adult degenerative scoliosis in this decade (2010–2019): a bibliometric study. Eur Spine J. 2020;29(12):2970–2979. doi: 10.1007/s00586-020-06574-6.
- Aria M, Cuccurullo C. Bibliometrix : an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–975. doi: 10.1016/j.joi.2017.08.007.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569–16572. doi: 10.1073/pnas.0507655102.
- Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001.
- Foley RN, Parfrey PS, Morgan J, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58(3):1325–1335. doi: 10.1046/j.1523-1755.2000.00289.x.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European Consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034.
- Cruz-Jentoft AJ, Kiesswetter E, Drey M, et al. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43–48. doi: 10.1007/s40520-016-0709-0.
- Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007.
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9.
- Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003.
- Akner G. Critical appraisal of the concept frailty: rating of frailty in elderly people has weak scientific basis and should not be used for managing individual patients. Aging Dis. 2023;14(1):21–24. doi: 10.14336/AD.2022.0506.
- Anderson BM, Qasim M, Correa G, et al. Correlations, agreement and utility of frailty instruments in prevalent haemodialysis patients: baseline cohort data from the FITNESS study. Clin Kidney J. 2022;15(1):145–152. doi: 10.1093/ckj/sfab137.
- Cesari M, Gambassi G, van Kan GA, et al. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. doi: 10.1093/ageing/aft160.
- Wilhelm-Leen ER, Hall YN, K Tamura M, et al. Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. Am J Med. 2009;122(7):664–671.e2. doi: 10.1016/j.amjmed.2009.01.026.
- Lee S, Lee S, Harada K, et al. Relationship between chronic kidney disease with diabetes or hypertension and frailty in community-dwelling Japanese older adults. Geriatr Gerontol Int. 2017;17(10):1527–1533. doi: 10.1111/ggi.12910.
- Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017.
- Chen JCY, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172–1179. doi: 10.2215/CJN.00590118.
- Segall L, Nistor I, Pascual J, et al. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: a literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation. 2016;100(10):e55–e65. doi: 10.1097/TP.0000000000001367.
- Haugen CE, Thomas AG, Chu NM, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20(4):1170–1180. doi: 10.1111/ajt.15709.
- McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266(6):1084–1090. doi: 10.1097/SLA.0000000000002025.
- Ye L, Elstgeest LEM, Zhang X, et al. Factors associated with physical, psychological and social frailty among community-dwelling older persons in Europe: a cross-sectional study of Urban Health Centres Europe (UHCE). BMC Geriatr. 2021;21(1):422. doi: 10.1186/s12877-021-02364-x.
- Brown SA, Tyrer FC, Clarke AL, et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10(6):788–796. doi: 10.1093/ckj/sfx057.
- Wilkinson TJ, Clarke AL, Nixon DGD, et al. Prevalence and correlates of physical activity across kidney disease stages: an observational multicentre study. Nephrol Dial Transplant. 2021;36(4):641–649. doi: 10.1093/ndt/gfz235.
- Clyne N, Jogestrand T, Lins LE, et al. Factors limiting physical working capacity in predialytic uraemic patients. Acta Med Scand. 1987;222(2):183–190. doi: 10.1111/j.0954-6820.1987.tb10657.x.
- Roshanravan B, Patel KV, Robinson-Cohen C, et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis. 2015;65(5):737–747. doi: 10.1053/j.ajkd.2014.10.016.
- Hellberg M, Höglund P, Svensson P, et al. Decline in measured glomerular filtration rate is associated with a decrease in endurance, strength, balance and fine motor skills. Nephrology. 2017;22(7):513–519. doi: 10.1111/nep.12810.
- Tentori F, Elder SJ, Thumma J, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25(9):3050–3062. doi: 10.1093/ndt/gfq138.
- Sheng K, Zhang P, Chen L, et al. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40(5):478–490. doi: 10.1159/000368722.
- Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24(3):337–351. doi: 10.1681/ASN.2012010047.
- Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585.
- Han SH, Han DS. Nutrition in patients on peritoneal dialysis. Nat Rev Nephrol. 2012;8(3):163–175. doi: 10.1038/nrneph.2012.12.
- Nixon AC, Bampouras TM, Pendleton N, et al. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11(2):236–245. doi: 10.1093/ckj/sfx134.
- Farrington K, Covic A, Nistor I, et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR < 45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant. 2017;32(1):9–16. doi: 10.1093/ndt/gfw411.
- McDonnell T, Wu HHL, Kalra PA, et al. COVID-19 in elderly patients receiving haemodialysis: a current review. Biomedicines. 2023;11(3):926. doi: 10.3390/biomedicines11030926.
- Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368:l6964. doi: 10.1136/bmj.l6964.
- MacRae C, Mercer SW, Guthrie B, et al. Comorbidity in chronic kidney disease: a large cross-sectional study of prevalence in Scottish Primary Care. Br J Gen Pract. 2021;71(704):e243–e249. doi: 10.3399/bjgp20X714125.
- Noels H, Jankowski J. Increased risk of cardiovascular complications in chronic kidney disease: introduction to a compendium. Circ Res. 2023;132(8):899–901. doi: 10.1161/CIRCRESAHA.123.322806.
- Carracedo J, Alique M, Vida C, et al. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: a process related to accelerated senescence. Front Cell Dev Biol. 2020;8:185. doi: 10.3389/fcell.2020.00185.
- Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3.
- Chapter 8: cardiovascular disease in patients with ESRD. Am J Kidney Dis. 2018;71(3):S417–S432. doi: 10.1053/j.ajkd.2018.01.021.
- Johns TS, Yee J, Smith-Jules T, et al. Interdisciplinary care clinics in chronic kidney disease. BMC Nephrol. 2015;16(1):161. doi: 10.1186/s12882-015-0158-6.
- Wilkinson TJ, Watson EL, Gould DW, et al. Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: a secondary analysis of the ‘ExTra CKD’ trial. Clin Kidney J. 2019;12(1):113–121. doi: 10.1093/ckj/sfy071.
- Nixon AC, Bampouras TM, Pendleton N, et al. Frailty is independently associated with worse health-related quality of life in chronic kidney disease: a secondary analysis of the frailty assessment in chronic kidney disease study. Clin Kidney J. 2020;13(1):85–94. doi: 10.1093/ckj/sfz038.
- Stenholm S, Ferrucci L, Vahtera J, et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies. J Gerontol A Biol Sci Med Sci. 2019;74(5):667–674. doi: 10.1093/gerona/gly132.
- Clarke AL, Young HML, Hull KL, et al. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30(11):1885–1892. doi: 10.1093/ndt/gfv208.
- Walston J, Buta B, Xue QL. Frailty screening and interventions. Clin Geriatr Med. 2018;34(1):25–38. doi: 10.1016/j.cger.2017.09.004.
- Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4.
- Li Y, Xue QL, Odden MC, et al. Linking early life risk factors to frailty in old age: evidence from the China Health and Retirement Longitudinal Study. Age Ageing. 2020;49(2):208–217. doi: 10.1093/ageing/afz160.
- McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266.
- Seeley A, Glogowska M, Hayward G. ‘Frailty as an adjective rather than a diagnosis’—identification of frailty in primary care: a qualitative interview study. Age Ageing. 2023;52(6):afad095. doi: 10.1093/ageing/afad095.
- Turner G, Clegg A, British Geriatrics Society, et al. Best practice guidelines for the management of frailty: a British Geriatrics Society, age UK and Royal College of General Practitioners Report. Age Ageing. 2014;43(6):744–747. doi: 10.1093/ageing/afu138.
- Noor H, Reid J, Slee A. Resistance exercise and nutritional interventions for augmenting sarcopenia outcomes in chronic kidney disease: a narrative review. J Cachexia Sarcopenia Muscle. 2021;12(6):1621–1640. doi: 10.1002/jcsm.12791.
- Hoshino J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients. Nutrients. 2021;13(5):1444. doi: 10.3390/nu13051444.
- Moorman D, Suri R, Hiremath S, et al. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol. 2019;14(2):268–276. doi: 10.2215/CJN.09700818.