 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to investigate ultrasound features of arteriovenous fistula stenosis and their relationship with primary patency after percutaneous transluminal angioplasty (post-intervention primary patency) and compare this classification with that using lesion location. Hemodialysis patients who underwent ultrasound-guided percutaneous transluminal angioplasty for arteriovenous fistula stenosis from July 2020 to December 2021 were retrospectively evaluated. Lesions (excluding inflow arteries) were categorized into five groups based on ultrasound features, and the clinical characteristics and risk factors affecting the post-intervention primary patency of the arteriovenous fistula were analyzed. Among 185 patients, 100 (54.05%), 36 (19.46%), 22 (11.89%), 11 (5.95%), and 16 (8.65%) were classified into the intima-dominant, non-intima-dominant, valve obstruction, vascular calcification, and mixed groups, respectively. The dialysis duration and arteriovenous fistula use time were the highest in the vascular calcification group at 86 (interquartile range: 49–140) and 77 (interquartile range: 49–110) months, respectively. Diabetes mellitus was most common in the intima-dominant group (42.0%). In Kaplan–Meier and univariate Cox analysis, type III lesion location (stenosis in the venous confluence site) was associated with the lower post-intervention primary patency. In the multivariate Cox analysis, percutaneous transluminal angioplasty times (the number of times patients were treated with percutaneous transluminal angioplasty for arteriovenous fistula stenosis dysfunction), vascular calcification, calcification at the lesion site requiring percutaneous transluminal angioplasty, and serum parathyroid hormone levels were independent risk factors for post-intervention primary patency. Ultrasound features showed that calcification of the arteriovenous fistula was detrimental to the post-intervention primary patency of arteriovenous fistula.
Introduction
Arteriovenous fistula (AVF) is the preferred vascular access in hemodialysis (HD) patients [Citation1]. However, after AVF creation, stenosis often occurs, leading to AVF dysfunction or occlusion. Endovascular techniques, such as percutaneous transluminal angioplasty (PTA), are preferred modalities for the treatment of AVF stenosis [Citation1,Citation2]. However, restenosis requires PTA to be repeatedly performed to maintain AVF patency.
AVF stenosis was previously classified into four types according to lesion location: type I (stenosis in the juxta-anastomotic area), type II (stenosis in the region of the draining vein, especially in the region of the puncture), type III (stenosis in the venous confluence site [cephalo-axillary vein and the cephalic arch] or the central vein), and type IV (stenosis in the inflow artery) [Citation3,Citation4]. Recently, vascular ultrasound has become increasingly common for PTA application, with the advantages of being highly sensitive, reproducible, and noninvasive for the evaluation of vascular lesions. In recent years, new AVF stenosis classifications based on ultrasound features have been explored [Citation5,Citation6]. These classifications are focussed on vascular neointimal hyperplasia (NIH) and correlate NIH with the histological features and pathophysiological mechanisms of AVF stenosis, suggesting that AVF stenosis is not homogeneous and that different stenosis features may influence treatment strategies [Citation5–8].
Nevertheless, no unified criteria are available for classifying AVF stenosis based on ultrasound features. The relationship between NIH or intima-media thickness and the clinical outcomes of AVF is controversial [Citation9,Citation10]. Along with NIH and vascular adventitial fibrosis, valve sclerosis and vascular calcification are also common in AVF stenosis. In this study, the ultrasound features of AVF stenosis were classified into five groups, and the clinical characteristics of the five sonographic groups and their impact on post-intervention primary patency of AVF were analyzed.
Patients and methods
Study population
This study retrospectively analyzed the data of consecutive HD patients who underwent PTA for AVF stenosis from July 2020 to December 2021. A total of 289 patients were screened at the Department of Nephrology, Wuhan Central Hospital, and evaluated using physical and vascular ultrasound examinations before PTA and every 3–6 months after PTA. All PTA procedures were performed by the same two physicians. Patients’ demographic and pre-and postoperative data were obtained from the hospital medical record system, and all information was reviewed by another physician. The inclusion criteria were: (1) patients requiring HD due to chronic kidney disease; (2) age >18 years; (3) AVF dysfunction due to stenosis; and (4) patients who cooperated with the investigation and long-term follow-up. The exclusion criteria were: (1) AVF occlusion (n = 41); (2) lesions located in central veins or other sites that cannot be sonographically explored (n = 24); (3) life expectancy <6 months (n = 3); (4) AVF infection (n = 1); (5) patients with left ventricular ejection fraction (LVEF) <30% (n = 2); (6) AVF that did not reach the clinical maturation criteria [Citation1] (n = 17); and (7) arterial and juxta-arterial inflow lesions (n = 16). Echocardiography was mandatorily in patients with NYHA class III-IV heart failure or a documented LVEF <40% and had to be performed within the previous 6 months. Echocardiographic data within 6 months before or after enrollment were collected from 110 patients and used in this study.
All patients provided written informed consent. The Ethics Committee of the Wuhan Central Hospital approved this study (Approval document: 2016 Medical Research No. 03 and Hospital-Heng-Lun letter-2021 [Citation9]). All the study procedures were performed in accordance with the Declaration of Helsinki.
Vascular ultrasound examination
AVF was assessed using a GE LOGIQTM E11 and L2-9-D probe (2–10 mHz frequency, GE Healthcare, USA) by two experienced vascular ultrasound physicians. Inflow arteries, anastomotic sites, and outflow veins were scanned, and vascular access blood flow (Qa) was measured at a relatively straight section of the brachial artery 3–5 cm above the elbow. The Doppler angle was <60°. The average blood flow of the brachial artery was automatically calculated using the system flow calculation software according to the following formula:
When measuring the residual lumen diameter, in conjunction with the physical examination, an experienced sonographer repeated the scanning process in the transverse and longitudinal planes and selected the narrowest residual lumen for measurement. The percentage of stenosis was calculated based on a reduction in the linear diameter. All Doppler ultrasound parameters were measured three times, and the average value was calculated.
Stenosis classification based on lesion location [Citation3,Citation4]
Type I: stenosis in the juxta-anastomotic area, within 2 cm in inflow artery and 5 cm in outflow vein; type II: stenosis in the region of the draining vein, especially in the region of the puncture; type III: stenosis in the venous confluence site (cephalo-axillary vein and the cephalic arch) or the central vein; and mixed type, AVF with two or more types of stenosis.
Ultrasound features and AVF stenosis classification
Ultrasound features, including NIH thickness, valve morphology and function, inner and outer diameters at stenosis sites, and adjacent normal vasculature, were recorded. The typical ultrasound features are shown in . All lesions were categorized into five groups, and all stenoses were >50%. The intima thickness was measured at the thickest part of the stenotic region of a single vein. Based on a previous study, the intimal thickness was ≤0.6 mm at the stenosis site in the non-intima-dominant group and >0.6 mm in intima-dominant group [Citation5]. The venous valve obstruction group included venous valve dysfunction-induced stenosis. Lesions in the vascular calcification group had vascular calcification at the lesion site in the venous segment, appearing as a hyperechoic ‘white’ spot of the intimal and/or medial layer with a posterior acoustic shadow [Citation10,Citation11]. At the discretion of the two physicians, calcified lesions without circumferential extension of 360° and length of <2 cm were considered suitable for PTA and included in this study. The key lesions causing AVF dysfunction were used to classify the stenosis group. The mixed group was defined as having more than two patterns of stenosis on ultrasound examination, believed to be the key lesions. To further assess the impact of vascular calcification on the post-intervention primary patency, the vascular calcification of non-key lesions, including calcification in the feeding artery, was used as an influential factor in the Cox analysis.
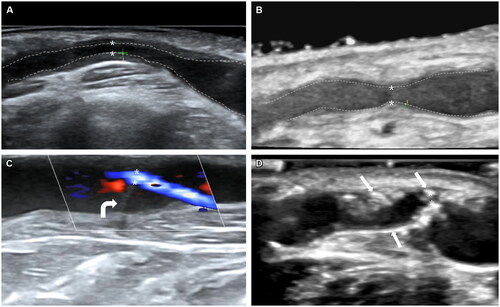
Figure 1. Ultrasound features of typical AVF lesions.
(A) Type intima-dominant. Intimal hyperplasia at the proximal vein: distance shows the thickness of the intima (1.5 mm), and the minimum luminal diameter is 1.3 mm. The PSV at the stenosis site is 567 cm/s. (B) Type Non-intima-dominant: distance shows the thickness of intima (0.3 mm), and minimum luminal diameter is 1.7 mm. The PSV at the stenosis site is 592 cm/s. (C) Type Valve obstruction (In color mode). The valve is stiff, and the lead vascular lumen shows severe stenosis. The minimum luminal diameter is 1.0 mm. The white curved arrow points to the stiff valve. The PSV at the stenosis site is 534 cm/s. (D) Type vascular calcification. Vein calcification at venous outflow near the anastomosis, and the minimum luminal diameter is 0.8 mm. The PSV at the stenosis site is 511 cm/s. Sheet-like calcified plaques break into the lumen of the vessel and are hyperechoic. The white straight arrows point to calcified plaques. The border between intima and lumen is marked on the figures as dotted lines, * residual lumen, + intima. AVF: arterial-venous fistula; PSV: peak systolic velocity.
Laboratory examinations
Laboratory data, including serum concentrations of albumin, calcium, phosphorus, intact parathyroid hormone (PTH), and high-sensitivity C-reactive protein (hs-CRP), were collected. All patients were examined at baseline and at least every 3–6 months (as required by the local HD Quality Control Committee). The serum calcium level was adjusted for the serum albumin concentration using the formula:
A time-averaged value was obtained as values averaged over the follow-up period and was used in the Cox analysis.
PTA indications
PTA was indicated based on clinical symptoms of AVF dysfunction (difficulty in puncturing, blood flow <250 mL/min during hemodialysis, or difficulty in stopping bleeding) detected by experienced physicians, combined with vascular ultrasound evaluation. More stringent intervention indications [Citation12] were implemented based on the criteria for significant stenosis [Citation13,Citation14], i.e., if at least two of the following criteria were met: (1) stenosis ratio ≥70%; (2) peak systolic velocity >500 cm/s or peak systolic velocity ratio >2-fold at the stenosis; (3) Qa <500 mL/min and (4) minimum luminal diameter <1.7 mm.
PTA procedure
All PTA procedures were performed under ultrasound guidance. Briefly, the patient was administered local anesthesia; a 6-F vascular sheath (Terumo Inc., Japan) was inserted into the vessel, and 20 mg of heparin was injected. A 0.035-inch guidewire (Terumo Inc., Japan) was introduced, and the lesion was traversed under ultrasound guidance. In most cases, the tip of the guidewire was delivered at the level of the proximal arteries (e.g., brachial artery) to ensure stability of the working guidewire. A high-pressure balloon (Mustang, Boston Scientific Inc., USA) of appropriate size (usually 5–7 mm for veins) was placed along the guidewire to dilate the lesion, and the balloon was brought to the working pressure (usually 10 atm × 60 s). If the ‘girdle sign’ or residual stenosis >30% was still visible, the balloon pressure was gradually increased until it reached a rated pressure of 24 atm × 60 s × 2-times. If the high-pressure balloon still did not open completely, re-dilatation, such as with the parallel wire technique, could be chosen, or surgical revision was considered. Cutting balloons, drug-coated balloons, and stents were not used.
Endpoints and related definitions
Primary endpoints:
Post-intervention primary patency, defined as the interval after PTA wherein functional AVF continued successfully, and dialysis sessions were effective without the need for repeat endovascular therapy or surgical revision. The follow-up period ended on 31 October 2022.
Secondary endpoints:
Anatomic success [Citation15] was defined as residual lumen stenosis of <30% measured within 15 min after PTA and AVF blood flow >500 mL/min. Clinical success [Citation15] was defined as the ability to complete more than one HD session with a pump-controlled blood flow ≥250 mL/min after PTA.
Clinical maturation [Citation1]:
Puncture of AVF is easy during HD. It can provide sufficient blood flow throughout the HD process and can meet the needs of three times or more HD treatments weekly. Inadequate blood flow was defined as pump-controlled blood flow ≤200 mL/min during HD.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). Quantitative variables are expressed as means ± standard deviations or medians (interquartile ranges [IQR]), whereas categorical variables as percentages or ratios. Clinical and demographic parameters were compared among the groups using the χ2 and Fisher’s exact tests or Student’s t- and Mann–Whitney U tests for categorical or continuous variables, respectively. Post-intervention primary patency was estimated using the Kaplan–Meier method, whereas intergroup comparisons were assessed using the log-rank test. A Cox proportional hazards model was used to identify independent factors associated with post-intervention primary patency after adjusting for confounders. p < 0.05 was considered statistically significant.
Results
Patients
A total of 185 patients (mean age: 58.51 ± 11.73 years, men: 96, women: 89) were included. The duration of dialysis was 34 (IQR: 13–67) months, and the AVF use time was 24 (IQR: 11–49) months. There were 161 (87.03%) cases of forearm AVF, among which 127 were radial-cephalic AVF, 29 ulnar-basilic AVF, 5 basilic transposition AVF, and the other 24 (12.97%) of upper arm AVF (brachial-cephalic AVF). A total of 153 patients (82.70%) were treated with PTA for the first time, and PTA was required in 24 (12.97%) cases with two or more lesions. Regarding comorbidities, 60 (32.43%) patients had diabetes mellitus (DM) and 1 (0.54%) had peripheral arterial disease, as reported in the hospital medical record system. Of the 185 patients, 100 (54.05%) were intima-dominant, 36 (19.46%) were non-intima-dominant, 22 (11.89%) had valve obstruction, 11 (5.95%) had vascular calcification, and 16 (8.65%) had mixed type. The duration of dialysis and AVF use time were the highest in the vascular calcification group, reaching 86 (IQR: 49–140) and 77 (IQR: 49–110) months, respectively (p = 0.000). More patients in the intima and non-intima groups underwent PTA for the first time (p = 0.034). DM was the most common baseline disease in the intima-dominant group (42.00%) and the least common in the mixed group (12%). PTH levels were higher (p = 0.048), and upper arm AVFs were lower (p = 0.035) in the calcification group than in the other groups. The remaining indicators, such as age, sex, hypertension, cerebrovascular disease, coronary artery disease, systolic blood pressure (SBP), diastolic blood pressure (DBP), left ventricular ejection fraction (LVEF), and other laboratory examinations at enrollment were not significantly different among the five ultrasound morphological groups (p > 0.05). The baseline demographic and clinical characteristics of the patients are presented in .
Table 1. Baseline demographics, clinical, and laboratory examination characteristics of all 185 patients in the cohort.
Ultrasound features of AVF stenosis
Of the 185 patients, 122 (65.95%) had type I stenosis. There were no significant differences in the distribution of stenosis types according to location among the ultrasound morphological groups (p = 0.146). The minimum luminal diameters in the non-intima-dominant and intima-dominant groups were significantly lower than those in the other groups (p = 0.013). Calcification in AVF or at the PTA site (100%) were the highest in vascular calcification group (p = 0.000). Other indications, including the diameter of the brachial artery, diameter of the inflow artery near the anastomosis, pressure required for PTA to open the stenosis, and blood flow in the brachial artery after PTA were not significantly different among the groups (p > 0.05). These details are provided in .
Table 2. Ultrasound characteristics of different AVF lesion types (n = 185).
Anatomical and clinical success
The anatomical and clinical success rates were 98.37% and 100%, respectively. Residual stenosis >30% was still visible in three patients, including two with cephalic arch stenosis (type III when classified using stenosis location and mixed group when classified using stenosis ultrasound appearance) and one other patient (type II when classified using stenosis location and valve obstruction when classified using stenosis ultrasound appearance). All patients completed at least one successful and efficient dialysis session after PTA.
Post-intervention primary patency of AVF according to stenosis classification methods
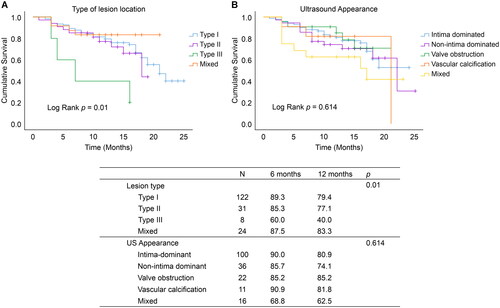
When stenosis was classified according to lesion location, post-intervention primary patency at 6 and 12 months was lower in type III than in types I, II, and mixed (log-rank p = 0.01, ). Meanwhile, no significant difference was observed in the post-intervention primary patency at 6 and 12 months among the five ultrasound morphological groups (log-rank p = 0.614, ). The details are presented in .
Figure 2. Kaplan–Meier survival analysis of AVF primary patency after PTA.
(A) Kaplan–Meier survival analysis of the type of AVF stenosis location on AVF primary patency after PTA; (B) Kaplan–Meier survival analysis of the type of ultrasound appearance on AVF primary patency after PTA; type I: stenosis near the anastomosis; type II: stenosis in the region of the draining vein, particularly in the area of the puncture; type III: stenosis in the venous confluence site or the central vein, particularly in the region of the cephalo-axillary vein or the cephalic arch); mixed type: AVF with two or more types of stenosis. AVF: Arterial-venous fistula; PTA: percutaneous transluminal angioplasty.
Multivariate Cox regression of risk factors predicting post-intervention primary patency of AVF
In the univariate analysis, PTA times (the number of times patients were treated with PTA for AVF dysfunction within the life of the AVF) (hazard ratio [HR] = 0.196, 95% confidence interval [CI]: 0.108–0.358, p = 0.000), PTH level (HR = 1.001, 95%CI 1.000–1.001, p = 0.023), and type of lesion locations (p = 0.023) were independent risk factors for post-intervention primary patency. In the multivariate analysis, after adjusting for sex, C-reactive protein, calcium, albumin, SBP, DBP, and LVEF, PTH level (HR = 1.001, 95%CI: 1.000–1.002; p = 0.024), PTA times (HR = 0.101, 95%CI: 0.046–0.222; p = 0.000), vascular calcification (HR = 2.014, 95%CI: 1.104–3.676, p = 0.023), and calcification at the lesion site that required PTA (HR = 3.306, 95%CI: 1.129–9.854; p = 0.033) were independent risk factors for post-intervention primary patency. Type of lesion location was not an independent risk factor for post-intervention primary patency (p = 0.612). Details are provided in .
Table 3. Multivariate Cox regression model of factors predicting post-intervention primary patency of AVF in patients receiving HD (n = 185).
Discussion
Adventitial fibrosis and NIH are currently considered the main reasons for AVF dysfunction [Citation16]. A few studies have discussed valve-induced stenosis; however, AVF calcification or other patterns were not addressed. Clinically, AVF calcification is common, including preoperative inflow artery calcification and postoperative calcification of the anastomosis, venous outflow, or other AVF areas. However, studies on the grading of AVF calcification are lacking, and there is no consensus on the indications for PTA or revision surgery for calcified lesions. This study did not include calcified lesions that were not suitable for PTA treatment based on the operators’ judgment, such as large calcifications of >2 cm or circumferential extension of 360°. In these cases, surgical excision of calcified lesions or other surgical revision is preferred. Additionally, cases of arterial lesions were excluded from this study due to the significant differences between arteries and veins. Arterialized veins differ from arteries even with AVF establishment and usage. The clinical characteristics of the five ultrasound morphological groups in this study appeared to reflect the different pathophysiological mechanisms of AVF stenosis. AVF stenosis results from endothelial injury and failure of outwards remodeling in response to multiple factors, such as abnormal haemodynamics, repeated cannulation, uremic toxins, inflammation, and surgical injury [Citation9,Citation16,Citation17].
In this study, the proportion of DM was the highest and AVF use time was the shortest in the intima-dominant group. Previous studies also suggested that DM was disadvantageous for AVF maturation and primary patency [Citation15,Citation16,Citation18,Citation19]. PTH level, vascular calcification, and calcification at the PTA sites were independent risk factors in the multivariate Cox analysis, which suggests disturbed uremic metabolism of glucose, calcium, phosphorus, or other uremic toxins in the pathogenesis of AVF stenosis in HD patients.
No significant differences were noted among the five sonographic groups in terms of the post-intervention primary patency of AVF (p = 0.614) and the multivariate Cox analysis results (p = 0.532). In recent years, different studies have derived different conclusions regarding the relationship between different sonographic classifications and AVF function [Citation7,Citation20]. Chen et al. [Citation5] claimed that the primary patency and secondary patency of AVF after PTA in the NIH group were significantly lower than those in the non-NIH group; however, they did not report the PTA times before the study. Suemitsu et al. [Citation21] suggested that valvular stenosis may be a favorable factor for AVF patency. Meanwhile, all studies claimed that different ultrasound features of AVF stenosis were valuable for choosing treatment strategies. For example, drug-coated balloons may be beneficial in the NIH-dominant group owing to their antiproliferative actions, and stents may be chosen for constriction restenosis [Citation5,Citation6]. Sonographic classification studies attempted to refine the morphological features of AVF lesions and link them to the pathophysiological mechanisms of AVF stenosis. However, clinical studies correlating ultrasound morphological features with different treatment strategies are lacking, and present study did not demonstrate a relationship between different ultrasound morphologic features of AVF stenosis and primary patency after PTA.
The effect of vascular calcification on post-intervention primary patency was further independently analyzed using Cox analysis. Both calcification through the AVF (HR = 2.014, 95%CI: 1.104–3.676, p = 0.023) and calcification at the PTA sites (HR = 3.306, 95%CI: 1.129–9.854, p = 0.033) were independent risk factors in the multivariate Cox analysis. AVF vascular calcification is the part of systemic vascular calcification, which predicts higher cardiovascular mortality and all-cause mortality [Citation22,Citation23]. Previous studies suggested that preoperative arterial microcalcifications affected AVF maturation and unassisted patency [Citation24,Citation25]. The present study suggests that postoperative AVF calcification, including calcification at the PTA site, was unfavorable for post-intervention primary patency. The minimum luminal diameter of the vascular calcification group was significantly larger than that of other groups; however, calcified plaques can interfere with ultrasound measurement of the minimum luminal diameter, and accurate measurement often requires digital subtraction angiography.
Regarding the type of lesion location, type III was associated with lower post-intervention primary patency of AVF, as confirmed in the Kaplan–Meier analysis (log-rank p = 0.01) and univariate Cox analysis (HR = 7.657, 95%CI: 1.898–30.883, p = 0.004), but not in the multivariate Cox analysis (HR = 2.354, 95%CI: 0.447–11.605, p = 0.293). Lesions located in central veins that could not be detected by ultrasound were excluded from this study, and only eight patients with type III were included. This may have influenced the results of the Cox analysis. The post-intervention primary patency of AVF was higher than that previously reported [Citation26,Citation27]. Several reasons were considered for this difference. First, 153 patients (82.70%) in this study were treated with PTA for the first time. PTA for the first time (PTA ≥1 as a reference, HR = 0.101, 95%CI: 0.046–0.222, p = 0.000) had a lower risk for post-intervention primary patency. Second, indications for PTA in this study were more strictly defined [Citation12] compared to the previously reported criteria [Citation13,Citation14], which may prolong the post-intervention primary patency of AVF.
Ideally, the best time to intervene in vascular access with PTA is when the lesions that may lead to AVF dysfunction are detected early to prolong the patency of the AVF while avoiding unnecessary intervention. Blood flow of AVF (Qa) is one of the important indications for PTA; if Qa is >500 mL/min pre-PTA, but a stenosis is present, clinical decisions will be made based on the indication, in conjunction with the individual situation, such as: (1) the Qa of the brachial artery measured by ultrasound is >500 mL/min, but there are branches in the patient’s AVF vein, and the actual flow in the main trunk of the AVF draining vein cannot meet the requirements for dialysis. If stringent intervention indications and criteria for significant stenosis are met, PTA should be performed; (2) if this is not the case, clinical and ultrasound evaluations will be followed while observing the vascular ultrasound parameters, and PTA will be performed if there is a decrease in blood flow of >25% from the last vascular ultrasound, which is one of the criteria for significant stenosis [Citation13,Citation14]; and (3) clinical decisions for other situations are based on the experienced physician’s discretion.
This study has some limitations. It was a retrospective study with no pathological examination, compared to other published studies [Citation6,Citation8,Citation25]. In the present study, lesions at inflow arteries and those located in central veins or other sites that could not be detected by ultrasound were excluded, indicating that only arterialized venous segments of the AVF and veins that could be detected using ultrasound were included in this study. However, the arterialized venous segments of the AVF were quite different from simple veins and arteries.
This study described the clinical characteristics of five sonographic features of AVF stenosis and compared the relationship between two different AVF stenosis classifications and post-intervention primary patency. The findings indicate that ultrasound features indicate different clinical characteristics. Furthermore, the sonographic classification of calcification and a type III lesion location were detrimental to post-intervention primary AVF patency.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
PTA times indicates the number of times patients are treated with PTA for AVF dysfunction within the life of the AVF.
Data are expressed as mean ± standard deviation for normally distributed measures and as median (interquartile range) for non-normally distributed measures, except where noted. a: Mann–Whitney U non-parameter test; b: Fisher exact probability method is used for comparison.
Data forms are expressed as mean ± standard deviation for normally distributed measures and median for non-normally distributed measures, except where noted; a: Fisher exact probability method is used for comparison.
Additional information
Funding
References
- Branch of Vascular Access. Expert consensus on vascular access for hemodialysis in China (2nd edition). Chin J Blood Purif. 2019;18(6):1–9. doi: 10.3969/j.issn.1671-4091.2019.06.001.
- Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4 Suppl 2):S1–S164. doi: 10.1053/j.ajkd.2019.12.001.
- Sun X, He Y, Ma Y, et al. A Single-Center retrospective analysis of the efficacy of a new balloon catheter in autogenous arteriovenous fistula dysfunction resistant to conventional balloon angioplasty. Ann Vasc Surg. 2018;48:79–88. doi: 10.1016/j.avsg.2017.09.025.
- Beathard GA, Arnold P, Jackson J, et al. Aggressive treatment of early fistula failure. Kidney Int. 2003;64(4):1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x.
- Chen L, Zhang W, Tan J, et al. Morphological lesion types are associated with primary and secondary patency rates after high-pressure balloon angioplasty for dysfunctional arteriovenous fistulas. Blood Purif. 2022;51(5):425–434. doi: 10.1159/00051688366.
- Krishnan P, Purushothaman KR, Purushothaman M, et al. Histological features of restenosis associated with paclitaxel drug-coated balloon: implications for therapy. Cardiovasc Pathol. 2019;43:107139. doi: 10.1016/j.carpath.2019.06.003.
- Allon M, Robbin ML, Young CJ, et al. Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol. 2013;8(10):1750–1755. doi: 10.2215/CJN.02740313.
- Steiner K, Ramanarayanan S, Metcalfe M, et al. Ultrasound appearances and histological correlation of native arteriovenous fistula stenoses - A retrospective case series. Semin Dial. 2021;34(3):224–228. doi: 10.1111/sdi.12947.
- Vazquez-Padron RI, Duque JC, Tabbara M, et al. Intimal hyperplasia and arteriovenous fistula failure: looking beyond size differences. Kidney360. 2021;2(8):1360–1372. doi: 10.34067/KID.0002022021.
- Flore R, Ponziani FR, Tinelli G, et al. New modalities of ultrasound-based intima-media thickness, arterial stiffness and non-coronary vascular calcifications detection to assess cardiovascular risk. Eur Rev Med Pharmacol Sci. 2015;19(8):1430–1441.
- Flore R, Zocco MA, Ainora ME, et al. A novel ultrasound-based vascular calcification score (CALCS) to detect subclinical atherosclerosis. Eur Rev Med Pharmacol Sci. 2018;22(3):736–742. doi: 10.26355/eurrev_201802_14304.
- Xiaomei H, Yin W, Yi ZH, et al. The effects of stricter stenosis intervention threshold and surveillance by doppler ultrasound on artenovenous graft(AVG)patency rate. Chin J Blood Purif. 2018;17(6):397–400. doi: 10.3969/j.issn.1671-4091.2018.06.009.
- Kudlicka J, Kavan J, Tuka V, et al. More precise diagnosis of access stenosis: ultrasonography versus angiography. J Vasc Access. 2012;13(3):310–314. doi: 10.5301/jva.5000047.
- Lomonte C, Meola M, Petrucci I, et al. The key role of color doppler ultrasound in the work-up of hemodialysis vascular access. Semin Dial. 2015;28(2):211–215. doi: 10.1111/sdi.12312.
- Mingxi L, Jian F, Xinxin J, et al. Percutaneous transluminal angioplasty treatment under ltrasound-guiding. Chin J Nephrol. 2012;28(1):63–64. doi: 10.3760/cma.j.issn.1001-7097.2012.01.015.
- Martinez L, Duque JC, Tabbara M, et al. Fibrotic venous remodeling and nonmaturation of arteriovenous fistulas. J Am Soc Nephrol. 2018;29(3):1030–1040. doi: 10.1681/ASN.2017050559.
- Tonia C, Rothuizen CW, Paul HA, et al. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013;28(5):1085–1092. doi: 10.1093/ndt/gft068.
- Yan Y, Ye D, Yang L, et al. A meta-analysis of the association between diabetic patients and AVF failure in dialysis. Ren Fail. 2018;40(1):379–383. doi: 10.1080/0886022X.2018.1456464.
- Gan W, Shao D, Xu L, et al. Maturation and survival of arteriovenous fistula: the challenge starts from the preoperative assessment stage. Semin Dial. 2022;35(3):228–235. doi: 10.1111/sdi.13036.
- Tabbara M, Duque JC, Martinez L, et al. Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis. 2016;68(3):455–464. doi: 10.1053/j.ajkd.2016.02.044.
- Suemitsu K, Shiraki T, Iida O, et al. Impact of lesion morphology on durability after angioplasty of failed arteriovenous fistulas in hemodialysis patients. J Endovasc Ther. 2018;25(5):649–654. doi: 10.1177/1526602817748316.
- Bountouris I, Kristmundsson T, Dias N, et al. Is repeat PTA of a failing hemodialysis fistula durable? Int J Vasc Med. 2014;2014:369687. doi: 10.1155/2014/369687.
- Brunet MC, Chen SH, Sur S, et al. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J Neurointerv Surg. 2019;11(7):710–713. doi: 10.1136/neurintsurg-2019-014718.
- Georgiadis GS, Georgakarakos EI, Antoniou GA, et al. Correlation of pre-existing radial artery macrocalcifications with late patency of primary radiocephalic fistulas in diabetic hemodialysis patients. J Vasc Surg. 2014;60(2):462–470. doi: 10.1016/j.jvs.2014.02.042.
- Choi SJ, Yoon HE, Kim YS, et al. Pre-existing arterial micro-calcification predicts primary unassisted arteriovenous fistula failure in incident hemodialysis patients. Semin Dial. 2015;28(6):665–669. doi: 10.1111/sdi.12365.
- Bountouris I, Kritikou G, Degermetzoglou N, et al. A review of percutaneous transluminal angioplasty in hemodialysis fistula. Int J Vasc Med. 2018;2018:1420136. doi: 10.1155/2018/1420136.
- Agarwal SK, Nadkarni GN, Yacoub R, et al. Comparison of cutting balloon angioplasty and percutaneous balloon angioplasty of arteriovenous fistula stenosis: a Meta-Analysis and systematic review of randomized clinical trials. J Interv Cardiol. 2015;28(3):288–295. doi: 10.1111/joic.12202.

