Abstract
Background
Inflammation plays a crucial role in occurrence of kidney injury, and specific dietary patterns can influence systemic inflammation levels. However, the relationship between dietary inflammatory potential and early-stage kidney damage remains unclear.
Method
2,108 participants was recruited from 2001–2002 National Health and Nutrition Examination Survey (NHANES). Dietary Inflammatory Index (DII) is utilized to assess dietary inflammatory potential, calculated through a 24-h dietary recall questionnaire. Early renal injury was evaluated using urinary albumin to creatinine (UACR), cystatin C (CysC), β-2 microglobulin (β2M), and estimated glomerular filtration rate (eGFR) based on serum creatinine (eGFRs), cystatin C (eGFRc), and both Scr and CysC (eGFRs&c). Participant characteristics were analyzed, and association between DII, hypertension, and early renal injury markers was explored using multiple linear and logistic regression models.
Results
The average age of participants was 53.9 years. DII exhibited a positive correlation with UACR (β = −0.048[0.017,0.078]), β2M (β = 0.019[0.010,0.027]), CysC (β = 0.012 [0.004,0.021]). Conversely, a negative correlation was observed between DII and eGFRc (β = −1.126[−1.554, −0.699]), eGFRs&c (β=-1.101[−1.653, −0.549]). A significant association was observed between hypertension and abnormality of early kidney damage markers. Subgroup analysis reveals that the positive correlation between DII and the occurrence of abnormal markers of early kidney damage is only observed in individuals with hypertension. Furthermore, an interaction between DII and hypertension was detected in eGFRs&c (OR:1.250[1.042, 1.499], p for interaction = 0.03).
Conclusion
Higher levels of DII may be associated with occurrence of early kidney damage. For individuals with hypertension, avoiding excessive consumption of pro-inflammatory foods may reduce the risk of renal injury.
1 Introduction
Epidemiological studies have provided valuable insights into the disease burden associated with kidney diseases. Analysis of data from the NHANES in the United States (US) from 1999 to 2004 revealed a prevalence rate of 8.1% for kidney dysfunction, 9.5% for albuminuria, and 13.1% for chronic kidney disease (CKD) [Citation1]. In contrast, more recent data from China (2018–2019) reported lower prevalence rates of 2.2%, 6.7%, and 8.2%, respectively [Citation2]. Prolonged exposure to these risk factors increases the likelihood of developing CKD [Citation3]. CKD is recognized as a significant risk factor for various adverse outcomes, including acute kidney injury, cardiovascular disease, cancer, and premature mortality [Citation4–6]. Early renal injury often goes unnoticed because the decline in renal function is not obvious and there are no clear clinical manifestations. Therefore, studying the risk factors of early renal injury is very important, as it can provide new evidence for the primary prevention of kidney diseases.
In the pathogenesis of the majority of renal diseases, immune-inflammatory responses are implicated, with inflammation playing a substantial role in both the initiation and resolution of kidney injury [Citation7–9]. Previous cross-sectional studies have demonstrated associations between systemic inflammation markers, such as interleukin, tumor necrosis factor, highly sensitive C-reactive protein, and fibrinogen, with decreased glomerular filtration rates and increased urinary protein excretion, indicating a connection between systemic inflammation and renal health [Citation10]. Among the factors influencing systemic inflammation, diet has been extensively investigated. Research has revealed that specific dietary components including coffee, tea, certain peptides, nuts, and dietary fiber, as well as dietary patterns such as the Mediterranean and Western diets, exert diverse impacts on systemic inflammation levels [Citation11–18]. However, due to the extremely rich variety of foods that people consume in modern society, studying the relationship between a single food or nutrient and the level of inflammation in the human body is not convincing enough. Therefore, the research on pro-inflammatory dietary patterns has gradually become a trend. The dietary inflammatory index (DII) is a scoring tool that can quantify the overall inflammatory potential of an individual’s diet [Citation19]. Recently, the DII has been widely utilized in clinical studies to reveal the relationship between inflammatory potential of diet and multiple disease outcomes. Previous studies have revealed associations between DII and cardiovascular diseases, metabolic disorders, oral diseases and other conditions [Citation20–22].
Previous studies have revealed the association of the dietary inflammatory index (DII) with the incidence of chronic kidney disease (CKD), renal cancer, and mortality in CKD populations. However, no one has studied the relationship between DII and early kidney damage so far [Citation23–25]. CysC and β2M are considered more specific and sensitive markers for evaluating early renal injury due to their stable production rates within the human body, as opposed to the traditional marker of serum creatinine (Scr), which primarily assesses renal filtration function [Citation26,Citation27]. Moreover, damage to the glomerular filtration barrier caused by primary or secondary factors can result in protein leakage without significantly impacting glomerular filtration function. The UACR is frequently employed to assess the extent of proteinuria during renal injury [Citation28]. Therefore, these indicators would be used to evaluate early renal injury in the study.
We used data from the NHANES 2001–2002, as serum concentrations of CysC and β2M were only measured in that cycle. The main purpose of this study was to explore the relationship between DII and early renal injury. Since hypertension is an important risk factor for renal injury and affects a large population, this study also aimed to explore the joint effect of DII and hypertension on early renal injury, providing new evidence for the primary prevention of kidney diseases among population with hypertension.
2 methods
2.1. Study population and data source
This cross-sectional study enrolled a total of 2108 adults from NHANES 2001–2002. The NHANES is a nationally conducted project in the US that aims to investigate the health and nutrition status of Community residents. It gathers comprehensive health information through questionnaire surveys, physical examinations, and laboratory tests from community residents across different regions of the country. In this study, individuals who were below 20 years of age and those with missing data on laboratory tests (such as β2M, CysC, UACR, and Scr), date of 24-h dietary recall, and other covariates were uncovered in analysis. The research protocol received approval from the Ethics Committee of the National Center for Health Statistics, and written informed consents were obtained from all participants. The study’s flowchart is presented in .
2.2. Dietary inflammatory index
The DII serves as a scoring index that quantifies the overall inflammatory capacity of individual’s diet. Shivappa et al. conducted a comprehensive review of published literature to ascertain the impact of whole foods and dietary constituents on six inflammatory markers, namely interleukin −1β, interleukin −4, interleukin −6, interleukin −10, tumor necrosis factor -α, and C-reactive protein. To calculate the overall inflammatory potential of an individual’s diet, Shivappa et al. assigned pro-inflammatory and anti-inflammatory scores to each food parameter based on the characteristics of the studies reviewed. These scores were then weighted to obtain an overall inflammatory effect score for each specific food parameter. A lower DII score indicates a diet with a stronger capacity to reduce inflammation, while a higher DII score indicates a diet with a stronger capacity to promote inflammation [Citation19]. DII can evaluate the inflammatory effects of 45 food components, but previous studies have shown that using less than 30 food components does not affect the evaluation ability of DII [Citation20]. The DII was computed in this investigation utilizing data from 24-h dietary recall, and a total of 27 nutrients were employed in the computation of the DII score containing alcohol, vitamins B12/B6, β-carotene, caffeine, carbohydrates, cholesterol, total fat, fiber, folate, iron, magnesium, zinc, selenium, mono unsaturated fat acid (MUFA), niacin, n-3 fatty acids, n-6 fatty acids, protein, polyunsaturated fatty acid (PUFA), riboflavin, saturated fat, thiamin, and vitamins A/C/D/E. The food parameters used to calculate DII are shown in Supplementary Table 1.
2.3. Early renal injury indicators
All laboratory indicators were tested by NHANES professionals. Serum β2M and CysC were measured using an immunoassay (Siemens Healthcare Diagnostics) on an automated multi-channel analyzer, Siemens Dimension Vista 1500 (Siemens Healthcare Diagnostics). The details of detection of serum β2M and CysC are shown on the website of https://www.cdc.gov/nchs/nhanes/index.htm. Urine creatinine was analyzed by a Jaffé rate reaction [Citation29] and the urinary albumin value was detected by a solid-phase fluorescent immunoassay described by Chavers et al. [Citation30]. The calculation of UACR was based on the following formula: UACR (mg/g) = urinary albumin (mg/dL)/urine creatinine (g/dL) [Citation31]. In this study, a natural logarithm transformation was applied to the three indicators to ensure their adherence to a normal distribution. The eGFR-EPI equation based on Scr has been extensively utilized in clinical practice for more than a decade. Its application has contributed significantly to enhancing knowledge and comprehension of CKD. However, it is important to note that the inclusion of race as a variable in the eGFR-EPI equation (as indicated by reference [Citation32]) has raised concerns about the potential for overlooking the diversity within and between racial groups, which could inadvertently contribute to systemic racism in medicine [Citation33]. Therefore, an alternative eGFR-EPI formula based on CysC was developed [Citation34], which has been shown to have higher specificity and sensitivity for detecting early CKD in previous studies [Citation35]. In this study, three eGFR equations based on Scr or/and CysC were used to estimate the GFR of the participants.
The CKD-EPI equation based solely on Scr can be represented as follows: 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] _ 1.159 [if black], where Scr represents the serum creatinine level, κ is a constant value of 0.7 for females and 0.9 for males, α is a constant value of −0.329 for females and −0.411 for males, min denotes the minimum of Scr/κ or 1, and max denotes the maximum of Scr/κ or 1(32). The CKD-EPI equation based on CysC can be expressed as: 133 × min(Scys/0.8, 1)−0.499 × max (Scys/0.8, 1)−1.328 × 0.996Age [× 0.932 if female], where Scys represents serum cystatin C level, min denotes the minimum of Scr/κ or 1, and max denotes the maximum of Scys/κ or 1 [Citation36]. The CKD-EPI equation based on Scr and CysC can be expressed as: 135 × min(Scr/κ, 1)α × max(Scr/κ, 1)−0.601 × min(Scys/0.8, 1)−0.375 × max(Scys/0.8, 1)−0.711 × 0.995Age [× 0.969 if female] [× 1.08 if black], where Scr denotes the concentration of serum creatinine, while Scys represents the concentration of serum cystatin C. The value of κ is set at 0.7 for females and 0.9 for males, indicating the gender-specific adjustment factor. Similarly, the value of α is −0.248 for females and −0.207 for males, representing the gender-specific coefficient, min denotes the minimum of Scr/κ or 1, and max denotes the maximum of Scr/κ or 1 [Citation36]. As there is currently no universally recognized threshold for CysC and β2M to define early renal injury, values exceeding the highest quartile were regarded as indicative of early renal damage in this research. In addition, values of UACR >30 mg/g and eGFR < 60 mL/min/1.73 m2 were also considered indicative of early renal injury [Citation31].
2.4. Hypertension
The measurement method for blood pressure in this context followed the recommendations from the American Heart Association for measuring human blood pressure used a sphygmomanometer [Citation37]. Participants received three blood pressure measurements including systolic and diastolic blood pressure and using a mercury sphygmomanometer at the Mobile Examination Center (MEC) or at home. The measurements were performed by NHANES examiners. We took the average of the three blood pressure measurement values as the participant’s blood pressure number. According to latest American College of Cardiology/American Heart Association Hypertension Guideline [Citation38], high blood pressure was characterized by a systolic blood pressure exceeding 130 mmHg or a diastolic blood pressure surpassing 80 mmHg.
2.5. Covariates
Information on demographic characteristics, lifestyle, and health status was collected. Sociological characteristics included age, gender, race (non-Hispanic white, Mexican American, non-Hispanic black, other Hispanics, other races), education status (less than 9th grade, 9-11th grade, high school graduate or equivalent, some college or AA degree, college graduate or above), and the poverty impact ratio (PIR). Lifestyle information included smoking status (never smoker, former smoker, current smoker), and drinking status (non-drinker, drinker). Health information included body mass index (BMI), history of diabetes (hemoglobin A1C concentration ≥6.5%, fasting plasma glucose ≥126 mg/dL, or self-reported diabetes).
2.6. Statistical analysis
To demonstrate the characteristics of participants with different dietary patterns, we divided all participants into pro-inflammatory and anti-inflammatory dietary groups (DII value greater than zero was considered a pro-inflammatory diet) [Citation19]. Continuous variables that followed a normal distribution were presented in terms of their mean and standard deviation, whereas categorical variables were displayed using the sample size and percentage. The significance of differences between the means of continuous variables and categorical variables was determined by analysis of variance (ANOVA) and
Pearson’s χ2 test, respectively. The analysis used sample weights to make the results representative of the US community population. Unweighted characteristics are shown in Supplementary Table 2. To assess the non-linear relationship between continuous values of DII and early renal injury indicators, restricted cubic splines (RCS) were employed. Four knots were strategically placed at the 5th, 35th, 65th, and 95th percentiles to identify and exclude any non-linear terms. The association between DII and early renal injury indicators was examined using linear regression and logistic regression models.
Logistic regression analysis was employed to investigate the potential relationship between hypertension and the risk of early renal injury. Finally, the interaction between DII and hypertension on the risk of early renal injury was analyzed. To verify the robustness of the results, we conducted an analysis to test the linear relationship between quartile values of DII and early renal injury indicators, as presented in Supplementary Table 3. Furthermore, we investigated the interaction between DII and hypertension on early renal damage indicators, which is summarized in Supplementary Table 4. It is important to note that the associations between DII, early renal damage indicators, and hypertension remained statistically significant in the sensitivity analysis. The statistical analyses in this study were conducted using R version 4.2.2, and all statistical tests were two-sided. A significance level of 0.05 was used to determine statistical significance.
3 results
3.1. Baseline characteristics of the participants
Among the initial 11,039 participants, a total of 2108 individuals met the inclusion criteria after applying the exclusion criteria outlined in . presents a summary of the participant characteristics. The mean age of the participants was 53.9 years, with females comprising 50.47% of the sample. The mean BMI was 28.2, and non-Hispanic white individuals accounted for 58.25% of the sample. Additionally, participants with a pro-inflammatory diet exhibited lower levels of education and PIR, higher rates of smoking and alcohol consumption, as well as higher prevalence of diabetes and hypertension.
Table 1. Characteristics of participants aged 20 years and older from the NHANES (2001-2002).
3.2. DII And early renal injury
The RCS results (Supplementary Figure 1) revealed a linear relationship between DII and various indicators, except for eGFR based on Scr. Consequently, eGFR based on Scr was excluded from our model. We developed three multiple linear regression models and three multiple logistic regression models, all accounting for weights. Model 1 was unadjusted for covariates, model 2 was adjusted for covariates including gender, age, race, education level, and poverty index, and model 3 added hypertension, diabetes, smoking, and drinking as additional covariates based on model 2. In linear regression Model 3 (), a positive correlation was observed between DII and UACR (β = 0.048, 95% CI: 0.017, 0.078, p-value = 0.004), β2M (β = 0.019, 95% CI: 0.010, 0.027, p-value < 0.001), and CysC (β = 0.012, 95% CI: 0.004, 0.021, p-value = 0.006). A negative correlation was observed between DII and eG*FR based on CysC (β= −1.126, % CI: −1.554, −0.699, p-value < 0.0001) and eGFR based on Scr and CysC (β= −1.101, 95% CI: −1.653, −0.549, p-value < 0.001). In logistic regression model 3 (), elevated DII values were correlated with a heightened susceptibility to early renal injury, with the following risk estimates for abnormality of indicators: β2M (OR = 1.206, 95% CI: 1.114, 1.307, p-value < 0.001), CysC (OR = 1.203, 95% CI:1.100, 1.316, p-value < 0.001), eGFR based on CysC (OR = 1.186,% CI: 1.063, 1.323, p-value = 0.005), and eGFR based on Scr and CysC (OR = 1.204, 95% CI: 1.082, 1.339, p-value = 0.002). However, no similar results were found for UACR.
Table 2. The linear relationship between DII and early renal injury indicators among participants aged 20 years and older from the NHANES (2001–2002).
Table 3. The logistic regression model between DII and the abnormality of early renal injury indicators among participants aged 20 years and older from the NHANES (2001–2002).
3.3. Hypertension and early renal injury
We constructed a multiple logistic regression model to examine the correlation between hypertension and the risk of early renal injury (). Our analysis revealed that individuals with hypertension had an elevated risk of early kidney damage, with the following risk estimates for abnormality of indicators: β2M (OR = 1.560, 95% CI: 1.182, 2.061, p-value = 0.004), CysC (OR = 1.408, 95% CI: 1.061, 1.868, p-value = 0.021), eGFR based on CysC (OR = 1.875, 95% CI: 1.339, 2.626, p-value = 0.001), and eGFR based on Scr and CysC (OR = 1.691, 95% CI: 1.106, 2.586, p-value = 0.019). However, no similar results were found for UACR and eGFR based on Scr.
Table 4. The logistic regression model between hypertension status and the abnormality of early renal injury indicators among participants aged 20 years and older from the NHANES (2001–2002).
3.4. The correlation between DII and early renal damage showed variations based on the presence of hypertension
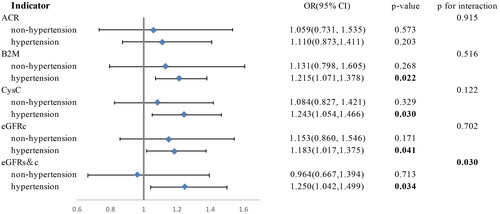
The correlation between DII and early renal impairment showed variations based on the presence of hypertension in individuals following either an anti-inflammatory or pro-inflammatory diet (). Among hypertensive individuals, higher DII scores were correlated with an elevated risk of abnormality of β2M (OR = 1.215, 95% CI: 1.071, 1.378, p-value = 0.022), CysC (OR = 1.243, 95% CI: 1.054, 1.466, p-value = 0.030), eGFR based on CysC (OR = 1.183, 95% CI: 1.071, 1.375, p-value = 0.041), and eGFR based on Scr and CysC (OR = 1.250, 95% CI: 1.042, 1.499, p-value = 0.034). However, in non-hypertensive individuals, there were no notable correlations found between DII and the abnormality of these indicators. Additionally, we found an interaction between higher DII scores and hypertensive status, which significantly increased the risk of abnormal eGFR based on Scr and CysC (p for interaction = 0.03). Notably, no similar associations were observed between DII and the abnormality of UACR.
Figure 2. The relationship between DII and abnormality of early renal injury indicators differed by hypertension status among participants aged 20 years and older from the NHANES (2001–2002). Model was adjusted for covariates including gender, age, race, education level, poverty index, hypertension, diabetes, smoking, and drinking. Sampling weights were considered in logistic regression analyses to obtain nationally representative estimates.
4 Discussion
The present study employed data from NHANES conducted in 2001–2002 to investigate the correlation between DII and early renal injury indicators, specifically UACR, β2M, CysC, and eGFR based on Scr, eGFR based on CysC, eGFR based on Scr and CysC. Additionally, the study examined the potential interaction between DII and hypertension in the context of the risk of early renal injury. The findings revealed significant associations between DII and the various early renal injury indicators. Specifically, DII demonstrated positive correlations with UACR, β2M, and CysC, and negative correlations with eGFR based on CysC and eGFR based on Scr and CysC. Even after controlling for potential confounders, the statistical significance of these associations persisted. Notably, no linear relationship was observed between DII and eGFR based on Scr. Furthermore, the logistic regression analysis revealed a positive correlation between elevated DII levels and an augmented likelihood of early renal impairment. Additionally, the study identified hypertension as a risk factor for early renal injury, as individuals with hypertension had a higher likelihood of experiencing such injury. However, further analysis stratified by hypertension status revealed a distinct pattern in the relationship between DII and the risk of early renal damage. Specifically, among participants with hypertension, individuals with higher DII scores exhibited a greater susceptibility to early renal injury. This observation suggests an interaction effect between higher DII scores and hypertension, indicating that the coexistence of a pro-inflammatory diet and hypertension may synergistically increase the risk of early renal injury. In summary, the findings of this investigation offer substantiation that a diet rich in pro-inflammatory components may pose a potential hazard for the development of early renal impairment in the adult population, particularly in the presence of hypertension. This study reveals the relationship between pro-inflammatory diet and early kidney injury from the perspective of dietary patterns, providing new evidence for primary prevention of kidney disease in hypertensive populations. According to our literature review, this study is the first to reveal this relationship.
The correlation between levels of inflammatory in the body and renal health has been extensively investigated. Numerous studies have demonstrated that elevated levels of systemic inflammatory biomarkers such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), pentraxin-3 (PTX3), interleukin-6 (IL), interleukin-10 (IL-10), and fibrinogen are inversely correlated with renal function [Citation10,Citation39,Citation40]. Inflammation plays a critical role in the pathogenesis and progression of renal disease, as the kidneys are highly metabolically active organs that are particularly vulnerable to inflammation-induced oxidative damage and hypoxia [Citation40]. Additionally, the excessive release of pro-inflammatory cytokines can lead to pathological changes in the kidney [Citation40]. The promotion of inflammatory cytokines leads to renal injury by inducing mesangial cell activation and proliferation, renal tubular epithelial cell damage and basement membrane destruction, dysfunction of glomerular endothelial cells, and disappearance of podocytes [Citation41]. In addition, studies have indicated that the elevated levels of inflammatory mediators are closely associated with poor prognosis and increased complications in renal disease. Conversely, renal damage itself can trigger an inflammatory response, leading to a self-perpetuating cycle that further exacerbates disease progression [Citation39,Citation40]. Previous studies have found that renal inflammation may serve as an important initial response to injury, and in individuals with higher eGFR levels, the association between elevated inflammatory biomarkers and adverse renal outcomes is particularly pronounced [Citation42]. These findings suggest that factors influencing systemic inflammation levels may be correlated with the risk of early renal impairment.
The impact of dietary habits on renal health has been a subject of extensive investigation. There is substantial evidence on dietary management for CKD and end-stage renal disease (ESRD), but there is still a lack of clear understanding regarding which specific foods increase the risk of renal damage. The findings of a cross-sectional investigation conducted among the elderly population residing in a Swedish community unveiled a significant correlation between an increased consumption of dietary fiber and improved renal function, as well as reduced levels of inflammation [Citation43]. An analysis of 18 prospective cohort studies encompassing 630,108 adults conducted a meta-analysis, which revealed that augmenting the intake of vegetables, fruits, legumes, nuts, whole grains, fish, and low-fat dairy products, while reducing the consumption of red and processed meats, sodium, and sugary beverages, exhibited a significant correlation with a decreased occurrence of CKD and proteinuria [Citation44]. Additionally, a Mendelian randomization study indicated that a relatively high protein intake and relatively lower fat intake may reduce the risk of CKD in the general population [Citation45]. Previous studies have revealed the relationship between DII and the risk of chronic kidney disease in adults and population with diabetes, the risk of diabetes nephropathy, and the all-cause mortality of CKD population [Citation23,Citation46–48]. However, no study has yet revealed the relationship between DII and early renal injury in hypertensive/non hypertensive populations. In our study, we employed DII as a measure of overall dietary inflammatory potential and observed a positive correlation between DII and indicators of renal impairment in the adult population. Higher DII scores were found to be significantly correlated with a heightened risk of renal injury, suggesting that the role of dietary inflammatory potential should be taken into consideration in the renal disease prevention strategies. Interestingly, we found no linear relationship between DII and eGFR based on Scr. This may be attributed to the fact that Scr levels can be influenced by various factors such as diet, metabolic status, and muscle condition of people that are not accurate enough to identify early renal injury [Citation49].
In our study, a significant interaction between DII and hypertension was observed in relation to the likelihood of early renal injury. Specifically, among individuals with hypertension, an elevated DII score was found to be significantly associated with an augmented risk of early renal injury. Conversely, no such association was detected among those without hypertension. Hypertension is widely acknowledged as a pivotal determinant for the advancement of CKD and the emergence of ESRD [Citation50]. Numerous cross-sectional studies have demonstrated that hypertensive patients exhibit elevated levels of CysC and UACR, as well as decreased eGFR based on CysC, indicating a correlation between hypertension and early renal function decline [Citation51,Citation52]. The mechanisms underlying the renal injury caused by hypertension involve multiple factors, including endothelial dysfunction, increased renal sympathetic nerve activity (RSNA), activation of the renin-angiotensin-aldosterone system (RAAS), oxidative stress, and inflammatory response [Citation53,Citation54]. Inflammation is believed to play a pivotal role in the intricate relationship between hypertension, diet, and renal health. Animal studies have demonstrated that hypertension can induce glomerular injury by compromising the integrity of the glomerular capillaries and exacerbating local renal inflammation [Citation55]. The tissue damage triggered by hypertension can stimulate the generation or presentation of antigens, leading to an immune response and stimulates the release of inflammatory mediators [Citation53]. Furthermore, a cross-sectional study involving individuals with primary hypertension revealed a positive correlation between CysC levels and serum levels of IL-6 and TNF-α [Citation56]. These factors collectively contribute to the observed interaction between hypertension and a pro-inflammatory diet in the development of renal injury.
This study possesses several strengths. Firstly, the utilization of weighted data enhances the representativeness of the findings, allowing for generalizability to the broader population. Secondly, the inclusion of multiple indicators to define early renal injury enhances the reliability of the results, capturing a comprehensive assessment of renal health. Thirdly, the calculation of DII based on numerous dietary components provides a more accurate evaluation of the actual inflammatory potential of the diet compared to examining individual nutrients.
Nevertheless, it is imperative to acknowledge the various constraints that should be taken into account when interpreting the findings of this study. First, this study cannot establish a causal relationship among DII, hypertension, and early renal injury and future studies can use methods such as Mendelian randomization and cohort studies to explore the causality. Second, potential confounders may affect the observed association and future studies can validate the results by using data from different databases and including more comprehensive covariates. Third, due to recall bias, it is difficult to accurately quantify the dietary intake of the population and future studies need to optimize the strategies for collecting dietary information from the population.
5 Conclusion
DII exhibited a positive correlation with early renal injury indicators, wherein higher DII scores were linked to an elevated risk of such injury. Notably, individuals with hypertension experienced an intensified association between DII and renal injury. Consequently, modifying dietary patterns could potentially mitigate the inflammatory impact of the diet on renal health. Specifically, for individuals with hypertension, minimizing the consumption of pro-inflammatory foods may serve to lower the risk of renal injury.
Ethics approval and consent to participate
The CDC/NCHS Research Ethics Review Board granted approval for the detailed methods and protocols employed in the NHANES study. These procedures, including informed consent protocols for participants, can be accessed on the CDC.gov website. All methods adhered to the applicable guidelines and regulations. Since the data used in this study are publicly available, it was exempt from ethical review involving human subjects.
Consent for publication
Not applicable.
Authors’ contributions
JD.H and MD.S conceptualized the research topic and framework; JD.H and YH.W identified the methodological details of the research; JD.H, HM.L and X.Y used statistical software for data analysis; JD.H, HM.L and CY.Q wrote the main manuscript text; JD.H and MD.S conducted Writing-review and editing. All authors reviewed the manuscript.
Supplemental Material
Download PDF (223.9 KB)Acknowledgments
We thank to the National Center for Health Statistics at the CDC for their excellent work in collecting the NHANES data and for making it accessible to the public.
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
All data can be downloaded from the NHANES website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).
Additional information
Funding
References
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298(17):1–10. doi: 10.1001/jama.298.17.2038.
- Wang L, Xu X, Zhang M, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–310. doi: 10.1001/jamainternmed.2022.6817.
- Siddiqui K, George TP, Joy SS, et al. Risk factors of chronic kidney disease among type 2 diabetic patients with longer duration of diabetes. Front Endocrinol. 2022;13:1079725. doi: 10.3389/fendo.2022.1079725.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031.
- Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20(6):1341–1350. doi: 10.1681/ASN.2008090998.
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X.
- Fu Y, Xiang Y, Li H, et al. Inflammation in kidney repair: mechanism and therapeutic potential. Pharmacol Ther. 2022;237:108240. doi: 10.1016/j.pharmthera.2022.108240.
- Feng YL, Yang Y, Chen H. Small molecules as a source for acute kidney injury therapy. Pharmacol Ther. 2022;237:108169. doi: 10.1016/j.pharmthera.2022.108169.
- Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, et al. Inflammation in renal diseases: new and old players. Front Pharmacol. 2019;10:1192. doi: 10.3389/fphar.2019.01192.
- Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7(12):1938–1946. doi: 10.2215/CJN.03500412.
- Thomas MS, Calle M, Fernandez ML. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv Nutr. 2023;14(1):44–54. doi: 10.1016/j.advnut.2022.10.002.
- Hall RL, George ES, Tierney AC, et al. Effect of dietary intervention, with or without cointerventions, on inflammatory markers in patients with nonalcoholic fatty liver disease: a systematic review and Meta-Analysis. Adv Nutr. 2023;14(3):475–499. doi: 10.1016/j.advnut.2023.01.001.
- Lv R, Sun N, Mao C, et al. Prevention and potential repair of colitis: beneficial effects and regulatory mechanisms of food-derived anti-inflammatory peptides. Crit Rev Food Sci Nutr. 2023;63:1–19. doi: 10.1080/10408398.2023.2197068.
- Surma S, Sahebkar A, Banach M. Coffee or tea: anti-inflammatory properties in the context of atherosclerotic cardiovascular disease prevention. Pharmacol Res. 2023;187:106596. doi: 10.1016/j.phrs.2022.106596.
- Ramos-Lopez O, Martinez-Urbistondo D, Vargas-Nuñez JA, et al. The role of nutrition on meta-inflammation: insights and potential targets in communicable and chronic disease management. Curr Obes Rep. 2022;11(4):305–335. doi: 10.1007/s13679-022-00490-0.
- Mateș L, Popa DS, Rusu ME, et al. Walnut intake interventions targeting biomarkers of metabolic syndrome and inflammation in middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Antioxidants. 2022;11(7):1412. doi: 10.3390/antiox11071412.
- Arouca AB, Meirhaeghe A, Dallongeville J, et al. Interplay between the mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin Nutr. 2020;39(6):1919–1926. doi: 10.1016/j.clnu.2019.08.016.
- Malesza IJ, Malesza M, Walkowiak J, et al. High-Fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10(11):3164. doi: 10.3390/cells10113164.
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115.
- Zhang J, Jia J, Lai R, et al. Association between dietary inflammatory index and atherosclerosis cardiovascular disease in U.S. adults. Front Nutr. 2022;9:1044329. doi: 10.3389/fnut.2022.1044329.
- Zhang X, Guo Y, Yao N, et al. Association between dietary inflammatory index and metabolic syndrome: analysis of the NHANES 2005-2016. Front Nutr. 2022;9:991907. doi: 10.3389/fnut.2022.991907.
- Botelho J, Leira Y, Viana J, et al. The role of inflammatory diet and vitamin D on the link between periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. 2021;13(3):924. doi: 10.3390/nu13030924.
- Yan LJ, Zhang FR, Ma CS, et al. Higher dietary inflammatory index is associated with increased all-cause mortality in adults with chronic kidney disease. Front Nutr. 2022;9:883838. doi: 10.3389/fnut.2022.883838.
- Mazidi M, Shivappa N, Wirth MD, et al. Greater dietary inflammatory index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. 2018;120(2):204–209. doi: 10.1017/S0007114518001071.
- Lu DL, Ren ZJ, Zhang Q, et al. Meta-analysis of the association between the inflammatory potential of diet and urologic cancer risk. PLOS One. 2018;13(10):e0204845. doi: 10.1371/journal.pone.0204845.
- Zhang L, Sun J, Zhang M, et al. The significance of combined detection of CysC, urinary mAlb and β(2)-MG in diagnosis of the early renal injury in pregnancy-induced hypertension syndrome. Saudi J Biol Sci. 2019;26(8):1982–1985. doi: 10.1016/j.sjbs.2019.07.013.
- Dieterle F, Perentes E, Cordier A, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28(5):463–469. doi: 10.1038/nbt.1622.
- Ruilope LM, Ortiz A, Lucia A, et al. Prevention of cardiorenal damage: importance of albuminuria. Eur Heart J. 2023;44(13):1112–1123. doi: 10.1093/eurheartj/ehac683.
- Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020.
- Chavers BM, Simonson J, Michael AF. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984;25(3):576–578. doi: 10.1038/ki.1984.57.
- Stevens PE, Levin A, Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006.
- Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–882. doi: 10.1056/NEJMms2004740.
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953.
- Lees JS, Rutherford E, Stevens KI, et al. Assessment of cystatin C level for risk stratification in adults with chronic kidney disease. JAMA Netw Open. 2022;5(10):e2238300. doi: 10.1001/jamanetworkopen.2022.38300.
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248.
- Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460–2470. doi: 10.1161/01.cir.88.5.2460.
- Carey RM, Whelton PK,. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American college of cardiology/American heart association hypertension guideline. Ann Intern Med. 2018;168(5):351–358. doi: 10.7326/M17-3203.
- Machowska A, Carrero JJ, Lindholm B, et al. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res. 2016;167(1):204–213. doi: 10.1016/j.trsl.2015.06.012.
- Tinti F, Lai S, Noce A, et al. Chronic kidney disease as a systemic inflammatory syndrome: update on mechanisms involved and potential treatment. Life. 2021;11(5):11. doi: 10.3390/life11050419.
- Brennan E, Kantharidis P, Cooper ME, et al. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol. 2021;17(11):725–739. doi: 10.1038/s41581-021-00454-y.
- Amdur RL, Feldman HI, Gupta J, et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11(9):1546–1556. doi: 10.2215/CJN.13121215.
- Xu H, Huang X, Risérus U, et al. Dietary fiber, kidney function, inflammation, and mortality risk. Clin J Am Soc Nephrol. 2014;9(12):2104–2110. doi: 10.2215/CJN.02260314.
- Bach KE, Kelly JT, Palmer SC, et al. Healthy dietary patterns and incidence of CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2019;14(10):1441–1449. doi: 10.2215/CJN.00530119.
- Park S, Lee S, Kim Y, et al. Causal effects of relative fat, protein, and carbohydrate intake on chronic kidney disease: a mendelian randomization study. Am J Clin Nutr. 2021;113(4):1023–1031. doi: 10.1093/ajcn/nqaa379.
- Zeng S, Qi L, Sun Y, et al. Association of chronic kidney disease with dietary inflammatory index in adults aged 50 years and older: dose-response analysis of a nationally representative population-based study. J Ren Nutr. 2023:S1051-2276(23)00157-7. doi: 10.1053/j.jrn.2023.09.007.
- Wang YJ, Du Y, Chen GQ, et al. Dose-response relationship between dietary inflammatory index and diabetic kidney disease in US adults. Public Health Nutr. 2022;26(3):1–9. doi: 10.1017/S1368980022001653.
- Guo C, Lin Y, Wu S, et al. Association of the dietary inflammation index (DII) with the prevalence of chronic kidney disease in patients with type-2 diabetes mellitus. Ren Fail. 2023;45(2):2277828.
- Zheng H, Liu H, Hao A, et al. Association between serum cystatin C and renal injury in patients with chronic hepatitis B. Medicine . 2020;99(32):e21551. doi: 10.1097/MD.0000000000021551.
- Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension. 2017;70(4):687–694. doi: 10.1161/HYPERTENSIONAHA.117.08314.
- Yin LH, Yan WJ, Guo ZX, et al. Relation between blood pressure variability and early renal damage in hypertensive patients. Eur Rev Med Pharmacol Sci. 2017;21(9):2226–2231.
- Wali U, Hussain MM, Wali N, et al. Comparison of serum levels of cystatin-C and traditional renal biomarkers for the early detection of pre-hypertensive nephropathy. JPMA. 2019;69(3):313–319.
- Hall JE, Mouton AJ, da Silva AA, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi: 10.1093/cvr/cvaa336.
- Mennuni S, Rubattu S, Pierelli G, et al. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28(2):74–79. doi: 10.1038/jhh.2013.55.
- Huyan Y, Wang C, Kang H, et al. Single-cell transcriptome sequencing reveals molecular mechanisms of renal injury in essential hypertension. Kidney Blood Press Res. 2023;48(1):297–313. doi: 10.1159/000530624.
- Okura T, Jotoku M, Irita J, et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol. 2010;14(6):584–588. doi: 10.1007/s10157-010-0334-8.