Abstract
Aim
Tripterygium wilfordii Hook F (TwHF) has been shown to substantially reduce proteinuria in patients with diabetic kidney disease (DKD); however, the effect of TwHF on renal outcomes in DKD remains unknown. Accordingly, we aimed to establish the effects of TwHF on renal outcomes in patients with DKD.
Methods
Overall, 124 patients with DKD, induced by type 2 diabetes mellitus, with 24-h proteinuria > 2 g, and an estimated glomerular filtration rate > 30 mL/min/1.73 m2 were retrospectively investigated. The renal outcomes were defined as doubling serum creatinine levels or end-stage kidney disease. Kaplan-Meier curves and Cox regression analyses were performed to analyze prognostic factors for renal outcomes.
Results
By the end of the follow-up, renal outcomes were observed in 23 and 11 patients in the non-TwHF and TwHF groups, respectively (p = 0.006). TwHF significantly reduced the risk of renal outcomes (adjusted hazard ratio [HR] 0.271, 95% confidence interval [CI] 0.111–0.660, p = 0.004) in patients with chronic kidney disease (CKD) G3 (adjusted HR 0.274, 95%CI 0.081–0.932, p = 0.039). Based on the Kaplan-Meier analysis, 1- and 3-year proportions of patients without renal outcomes were significantly lower in the non-TwHF group than those in the TwHF group (92.8% vs. 95.5% and 47.2% vs. 76.8%, respectively; p = 0.0018).
Conclusion
In DKD patients with severe proteinuria, TwHF could prevent DKD progression, especially in patients with CKD G3. A randomized clinical trial is needed to elucidate the benefits of TwHF on renal outcomes in patients with DKD.
Introduction
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease (ESKD) worldwide [Citation1]. Patients with DKD and severe proteinuria have a substantially increased risk of progression to ESKD, and proteinuria is regarded as both a prognostic predictor and a therapeutic target in DKD [Citation2,Citation3]. In addition to renin-angiotensin system (RAS) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1RA), and non-steroidal mineralocorticoid receptor antagonists (MRAs) have been shown to exert renal and cardiovascular (CV) benefits and are recommended by current treatment guidelines [Citation4–7]. Although the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) have lowered the threshold of estimated glomerular filtration rate (eGFR) for initiating SGLT2 inhibitors to ≥20 mL/min/1.73 m2, the effect of these inhibitors in DKD patients with severe renal impairment remains unclear [Citation8]. Furthermore, the effectiveness of SGLT-2 inhibitors in DKD patients with heavy proteinuria has not been comprehensively clarified. Current evidence is limited to case reports [Citation9] and secondary analyses of randomized controlled trials (RCTs) [Citation10,Citation11]. GLP-1RA can reduce proteinuria and retard CKD progression in patients with type 2 diabetes; however, its direct renal protective efficacy remains poorly elicidated [Citation8,Citation12]. In addition to SGLT2 inhibitors and GLP-1RA, finerenone, a selective MRA, elicited renal benefits in patients with CKD and type 2 diabetes [Citation6]. However, MRA-induced hyperkalaemia remains a major concern in patients with DKD presenting an eGFR <60 mL/min/1.73 m2 [Citation8,Citation12]. Likewise, the effectiveness of GLP-1RA and MRA in treating DKD with severe proteinuria remains inconclusive. Therefore, effective treatment for DKD patients with severe proteinuria remains uncertain and such research is urgently needed.
Tripterygium wilfordii Hook F (TwHF), as a herbal medicinal extract, has been extensively used by Chinese nephrologists to treat glomerulonephritis for nearly 40 years owing to its anti-inflammatory, anti-proliferative, and podocyte protective effects attributed to its monomeric component triptolide [Citation13]. Moreover, TwHF has been increasingly explored in treating DKD as an adjunct medicinal agent [Citation14]. TwHF has been shown to considerably reduce proteinuria in patients with DKD. However, the effects of TwHF on renal outcomes in patients with DKD are yet to be established [Citation15,Citation16]. In this retrospective cohort study, we examined the effects of TwHF on renal outcomes in DKD patients with severe proteinuria.
Methods
Study participants and design
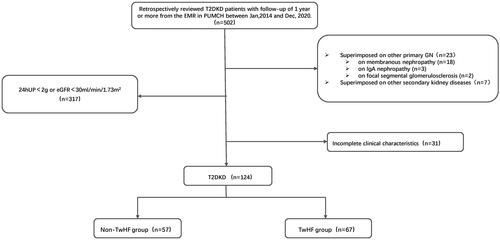
We retrospectively reviewed electronic medical records from the Department of Nephrology, Peking Union Medical College Hospital, from 1 January 2014, to 31 December 2020. Type 2 DKD was diagnosed based on a history of type 2 diabetes with persistent urinary albumin-to-creatinine ratio ≥30 mg/g or decreased eGFR, with or without diabetic retinopathy, and exclusion of other causes of chronic kidney disease [Citation17]. Other inclusion criteria were as follows: (1) receiving standard treatments for diabetes with CKD in accordance with the current guidelines [Citation18]; (2) 24-h proteinuria (24hUP) > 2 g and eGFR > 30 mL/min/1.73 m2 (CKD-EPI-Asia) [Citation19]; (3) and at least a one-year follow-up, including three outpatient visits. Patients with type 1 diabetes, other glomerular diseases, severe liver disease, and systemic diseases, such as systemic lupus erythematosus and cancers, were excluded. Based on renal pathology, 48 patients in this cohort were diagnosed with diabetic nephropathy. Two renal pathologists examined the renal specimens independently. All patients received the comprehensive standard treatments for DKD, including nutritional management, glycemic control, blood pressure control, lipid management, and proteinuria management, such as RAS inhibitors. TwHF therapy was administered to patients who did not experience a reduction in proteinuria after two months of the maximum tolerable dose of RAS inhibitors. The usage of TwHF among patients in the cohort strictly adhered to our department’s standard treatment protocol. TwHF was initiated at a dose of 1 mg/kg/day and tapered after two months. The maintenance dose was 10 mg/day. The cumulative dose of TwHF was 24.83 ± 5.99g. TwHF (10 mg/tablet) was manufactured by Zhejiang DND Pharmaceutical Co., Ltd. (Zhejiang, China). A flowchart of the patient screening process is illustrated in .
Figure 1. The flowchart of patient screening.
T2DKD: type 2 diabetic kidney disease; EMR: electronic medical records; GN: glomerulonephritis; 24hUP: 24-h proteinuria; eGFR: estimated glomerular filtration rate; TwHF: Tripterygium wilfordii Hook F.
This study was approved by the Ethics Committee of the Peking Union Medical College Hospital (I-22PJ483). The requirement for informed consent was waived, given that this study was a retrospective cohort study and all clinical information was de-identified. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data collection
The following baseline clinical characteristics were collected: age; sex; body mass index (BMI); diabetes duration; history of smoking; use of oral glycemic drugs, GLP-1RA, and insulin; use of RAS inhibitors; complications of diabetic peripheral neuropathy or diabetic retinopathy; and other comorbidities such as hypertension, cardiovascular diseases, and peripheral artery disease. Cardiovascular disease history included coronary artery disease, congestive heart failure, and stroke. Laboratory data included hemoglobin level, serum creatinine (Scr), eGFR, serum albumin, fasting blood glucose (FBG), glycated hemoglobin (HbA1c), serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), uric acid (UA), and 24hUP. The reduction in 24hUP was calculated by subtracting the follow-up 24hUP value from the baseline 24hUP value. The decline in eGFR was calculated by subtracting the follow-up eGFR value from the baseline eGFR value.
Follow-up and endpoint
The deadline for follow-up was 31 December 2021. Patients who did not reach the endpoint during the follow-up were censored. The renal outcomes were defined as doubling Scr or ESKD (dialysis or renal transplant), whichever occurred first [Citation20].
Sample size
As shown in Supplementary Table 1 based on previous reports [Citation15,Citation20], the estimated sample size was 53 patients per group based on α = 0.05 and study power at 0.80, assuming that 43.5% of patients in the non-TwHF group and 21% in the TwHF group would reach the endpoint [Citation21].
Statistical analysis
Continuous data were expressed as mean ± standard deviation if normally distributed, with an independent sample t-test used to compare the means between the two groups. Continuous data were expressed as medians and interquartile quartile ranges (IQR) if non-normally distributed, with nonparametric analysis used to compare the means between two groups. Categorical variables were expressed as proportions, with the chi-square test used to compare the two groups. Kaplan-Meier curve analysis and Cox proportional hazards regression with a stepwise selection procedure were performed to analyze risk factors for renal outcomes. Multivariate Cox regression analysis calculated the hazard ratio (HR) stratified by eGFR. To account for the difference in follow-up times between the two groups, we calculated the restricted mean survival time (RMST) and restricted mean time lost (RMTL) at τ = 36 months using the area under the Kaplan-Meier curves [Citation22]. Statistical significance was set at p < 0.05. All data analyses were performed using R, version 4.0.2.
Results
Baseline characteristics
Overall, 124 patients were included in the current study, with 67 and 57 in the TwHF and non-TwHF groups, respectively. For the overall cohort, the median follow-up time was 2.01 (Q25–Q75 1.23–2.85) years. summarized the baseline characteristics of the two groups. Patients in the TwHF group had a higher 24hUP level (p = 0.008) and longer follow-up duration (p = 0.017) than those in the non-TwHF group. The two groups did not demonstrate differences in the remaining characteristics, including age, sex, BMI, smoking, the proportion of diabetic complications and other comorbidities, the use of RAS inhibitors and anti-glycemic drugs, hemoglobin, serum albumin, FBG, HbA1c, Scr, eGFR, and other laboratory tests.
Table 1. The baseline characteristics of the TwHF and non-TwHF groups.
Follow-up and renal outcomes
As shown in , 34 patients reached the defined renal outcomes, including 23 in the non-TwHF group and 11 in the TwHF group (p = 0.006). At the end of the follow-up period, the eGFR decline in the TwHF group was significantly lower than that in the non-TwHF group, and the 24hUP reduction in the TwHF group was significantly higher than that in the non-TwHF group. The two groups had no differences in other clinical characteristics, including Scr, eGFR, serum albumin, FBG, UA, TC, TG, and LDL-c levels.
Table 2. The characteristics of the TwHF and non-TwHF groups at the end of the follow-up period.
Univariate and multivariate Cox regression analyses were performed to explore prognostic factors for renal outcomes (). Variables with a p-value <0.10 in univariate analysis, including BMI, age, application of TwHF, SGLT2 inhibitors, eGFR, serum albumin, hemoglobin, and 24-hUP, were included in the multivariate Cox regression. Scr was not included in the multivariate regression analysis owing to its collinearity with eGFR. In the multivariate regression analysis, age, TwHF use, SGLT2 inhibitor use, serum albumin level, 24-hUP, and eGFR were independent prognostic factors for renal outcomes. The use of TwHF was significantly associated with a lower risk of renal outcomes after adjusting for confounding factors (age, BMI, hemoglobin, serum albumin, eGFR, 24hUP, and the use of SGLT2i) in multivariate regression analysis (HR 0.271, 95% confidence interval [CI] 0.120–0.613).
Table 3. The univariate and multivariate Cox regression analysis for renal outcomes.
Stratification analysis according to eGFR revealed that 10 of 61 patients with CKD G1-2 and 24 of 63 patients with CKD G3 reached renal outcomes. In the adjusted multivariate Cox proportional hazards regression model, TwHF use was significantly associated with a lower risk of renal outcomes in patients with CKD G3 (adjusted HR 0.274, 95%CI 0.081–0.932, p = 0.039) but not in those with CKD G1-2 (adjusted HR 0.166, 95%CI 0.015–1.859, p = 0.077) (). Based on the Kaplan-Meier analysis, 1- and 3-year proportions of patients without renal outcomes were significantly lower in the non-TwHF group than those in the TwHF group (92.8% vs. 95.5% and 47.2% vs. 76.8%, respectively; p = 0.0018) (). The TwHF group had a lower incidence of renal outcomes, whether doubling Scr or ESKD, than the non-TwHF group (Supplementary Figure 1). The RMST with τ = 36 months in the TwHF and non-TwHF groups was 35.32 and 28.26, respectively; the RMTL was 0.68 and 7.74, respectively. Results showed that RMST (TwHF) – (non-TwHF)= 7.06 (95% CI 4.32–9.80; p < 0.005), RMST (TwHF)/(non-TwHF)= 1.25 (95% CI 1.14- 1.38; p < 0.005), and RMTL (TwHF)/(non-TwHF)= 0.088 (95% CI 0.027- 0.291; p < 0.005). After a three-year follow-up, the incidence of renal outcomes in the TwHF group was lower than that in the non-TwHF group ().
Figure 2. Kaplan-Meier curves for the renal outcomes in the overall cohort.
The renal outcomes were defined as doubling serum creatinine level or ESKD (dialysis or renal transplant), whichever occurred first. The non-TwHF group has a significantly lower proportion of patients without renal outcomes than the TwHF group (p = 0.0018). TwHF, Tripterygium wilfordii Hook F.
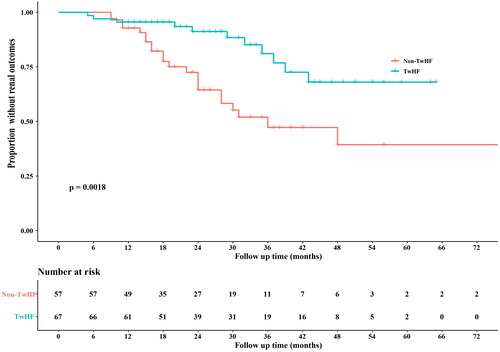
Figure 3. Comparison of the proportion of patients without renal outcomes between the two groups at three years using the restricted mean survival time method.
The pink part indicates RMST, and the orange part indicates RMTL. The y-axis denotes the proportion of patients without an outcome, and the x-axis shows the number of months. Abbreviations: RMST, restricted mean survival time; RMTL, restricted mean time lost.
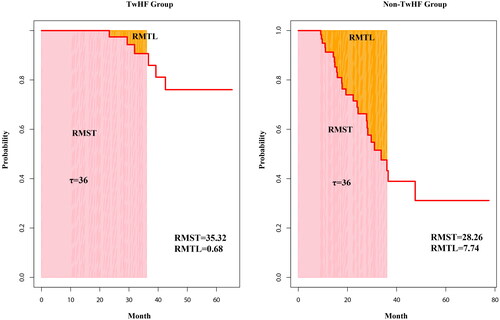
Table 4. Multivariate Cox regression analysis of renal outcomes in two subgroups according to TwHF use.
The overall incidence of adverse events in the TwHF group was 17.91%. Two cases of liver dysfunction were observed, with one necessitating discontinuation of TwHF; another case warranted TwHF discontinuation owing to the development of a rash. Furthermore, three cases of irregular menstruation, five gastrointestinal reactions, and one leukopenia (<3.5 × 109/L) were recorded (). These adverse reactions gradually subsided upon TwHF dose reduction.
Table 5. Adverse events of TwHF during the follow-up period.
Discussion
To the best of our knowledge, this is the first study to utilize renal outcomes of ESKD and doubling Scr level as endpoints to explore the effects of TwHF in patients with type 2 DKD. In this cohort, 44% of patients exhibited proteinuria >5 g/day, 33% had progressed to CKD G3b (eGFR 30–45 mL/min/1.73 m2), and 50% were followed for over 2 years (). Multivariate Cox regression analysis revealed that the use of TwHF was associated with a reduced risk of renal outcomes. Kaplan-Meier analysis demonstrated that treatment with TwHF could reduce the 3-year risk of renal outcomes by nearly 30%. Furthermore, the stratified analysis showed that treatment with TwHF could delay the progression of DKD in patients with CKD G3.
In an RCT that included 65 DKD patients with proteinuria ≥2.5 g/day and Scr < 3 mg/dl, TwHF combined with valsartan was found to reduce proteinuria substantially. The level of proteinuria reduction in the TwHF group was 1.7 g/day, although the authors did not examine the progression to renal failure [Citation15]. Another study used proteinuria reduction as a surrogate endpoint to evaluate the efficacy and safety of TwHF at a 24-week follow-up in patients with DKD exhibiting overt proteinuria and normal eGFR; however, the authors did not assess the progression of renal function [Citation16].
Although RAS inhibitors, SGLT2 inhibitors, and GLP1-RAs have been shown to exert renoprotective effects in patients with DKD, the proportions of use of these three drugs were similar between the two groups in our cohort. It is well-known that persistent severe proteinuria is an important risk factor for renal progression in DKD [Citation2,Citation3]. The CANVAS-R trial has demonstrated the renal benefit of canagliflozin [Citation23]; the urinary albumin/creatinine ratio (UACR) of 763.2 (451.5–1394.1) mg/g in the severally elevated albuminuria group was lower than the proteinuria levels of our cohort. Likewise, in the FIDELIO-DKD trial, which illustrated the renoprotective effects of finerenone, the enrolled patients had UACR levels of 852 (446–1634) mg/g, with a number exhibiting proteinuria levels > 5 g/day [Citation6]. The objective of the FLOW trial was to clarify the renal effects of semaglutide in patients with DKD (NCT03819153). Therefore, the renal outcomes in DKD patients with severe proteinuria (>2g/day) have not improved substantially. It is promising that TwHF can be used as an adjunctive therapy for patients with DKD and severe proteinuria who have poorly responded to the above treatment.
The RMST represents the area under the Kaplan-Meier curve up to a predetermined time point (τ) and considers all information within that period. The RMST is effective for comparing the impacts of interventions with inconsistent follow-up periods, as τ can be determined arbitrarily [Citation22,Citation24]. In our study, over 36 months of follow-up, the TwHF exhibited a significantly higher RMST than the non-TwHF group, further demonstrating the renoprotective effect of TwHF.
Root extracts of TwHF have been used to treat rheumatoid arthritis [Citation25,Citation26], Crohn’s disease [Citation27], IgA nephropathy [Citation28], and other diseases [Citation29]. Triptolide is one of the active components of TwHF, and its protective mechanisms against DKD progression remain elusive. Previous studies have suggested that the beneficial effects of TwHF might be partially mediated via its protective effect on podocytes. In vitro studies have confirmed that extracts of TwHF could protect podocytes through the deactivation of NADPH oxidase, inhibition of ROS generation, p38 mitogen-activated protein kinase, and restoration of RhoA signaling activity [Citation30,Citation31]. Moreover, an in vivo study has reported improvements in podocyte density, foot process width, and nephrin expression in db/db diabetic mice treated with triptolide [Citation32]. Other in vitro studies and animal experiments have shown that the active components of TwHF, such as triptolide and Wolforlide A, can suppress inflammation [Citation33–35] and exert anti-fibrotic effects [Citation36–38].
The major TwHF-induced side effects include skin itching, rash, mild gastrointestinal symptoms, decreased white blood cell count, liver dysfunction, and menstrual disorders in female patients [Citation15,Citation26,Citation39]. In an RCT assessing the efficacy of TwHF on rheumatoid arthritis, patients received 60 mg of TwHF daily for 6 months. The authors found that 23.2% of patients developed gastrointestinal discomfort, 10.1% developed skin and mucous membrane abnormalities, 12.7% experienced irregular menstruation, and 5.8% developed liver dysfunction [Citation26]. Although most patients who developed adverse events completed treatment, a few discontinued the study owing to liver injury accompanied by elevated levels of alanine aminotransferase. In routine practice at our department, we followed up with patients regularly and tapered the dose to maintenance therapy after two months of treatment. TwHF was generally well tolerated in this cohort; however, one patient developed liver dysfunction, and one developed a rash that warranted discontinuation of TwHF.
The current study had some limitations. First, this was a single-center observational retrospective study; hence, the presence of selection bias cannot be ignored. Confounding variables may arise owing to variations in follow-up time. Secondly, there were no MRA data in the cohort study, as most patients were enrolled three years ago, which may confound the finding as the interaction between MRA and TwHF cannot be tested. Third, the diagnosis of DKD in the current study was based on clinical presentation, potentially resulting in misclassification of exposure. However, all patients in our cohort were followed up and treated by experienced nephrologists and were comprehensively evaluated to exclude other kidney diseases. In addition, 90% of patients in our cohort had diabetic retinopathy, consistently indicating that the majority of patients had microvascular complications of diabetes. Lastly, our study did not discuss the potential for increased side effects with prolonged TwHF administration. It is imperative to prospectively explore the benefits of TwHF combined with SGLT2 inhibitors, GLP1 RA, or MRAs in patients with advanced DKD and severe proteinuria to achieve the maximal renoprotective effect.
Conclusion
This retrospective cohort study demonstrated that TwHF could reduce proteinuria in patients with DKD and severe proteinuria. Our findings revealed that TwHF could improve renal outcomes in CKD G3 patients. A multicenter RCT is needed to generate robust evidence on the renal benefits of TwHF in patients with advanced DKD.
Author contributions
Yaqi Cheng designed the study, collected the data, performed the statistical analysis, and wrote the manuscript. Danni Li, Liling Lin, and Liying Peng collected the data. Yuhao Liu, Ke Zheng, and Jianling Tao revised the manuscript accordingly. Mingxi Li conceived the study, supervised the study design, and drafted the manuscript. All authors have read and approved the final manuscript.
Supplemental Material
Download MS Word (205.4 KB)Supplemental Material
Download JPEG Image (2.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data are not shared due to privacy restrictions.
Additional information
Funding
References
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):1–8. doi: 10.1053/j.ajkd.2019.09.003.
- Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270.
- Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. doi: 10.1016/S2213-8587(18)30313-9.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827.
- Bakris GL, Agarwal R, Anker SD, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816.
- de Boer IH, Khunti K, Sadusky T, et al. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090. doi: 10.2337/dci22-0027.
- Tanaka A, Nakamura T, Sato E, et al. Therapeutic potential of tofogliflozin on nephrotic syndrome secondary to diabetic nephropathy. J Cardiol Cases. 2017;16(1):30–33. doi: 10.1016/j.jccase.2017.04.003.
- Kalay Z, Sahin OE, Copur S, et al. SGLT-2 inhibitors in nephrotic-range proteinuria: emerging clinical evidence. Clin Kidney J. 2023;16(1):52–60. doi: 10.1093/ckj/sfac189.
- Ruggenenti P, Kraus BJ, Inzucchi SE, et al. Nephrotic-range proteinuria in type 2 diabetes: effects of empagliflozin on kidney disease progression and clinical outcomes. EClinicalMedicine. 2021;43:101240. doi: 10.1016/j.eclinm.2021.101240.
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–S184. doi: 10.2337/dc22-S011.
- Zhong Y, Deng Y, Chen Y, et al. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013;84(6):1108–1118. doi: 10.1038/ki.2013.276.
- Liu P, Zhang J, Wang Y, et al. The Active Compounds and Therapeutic Target of Tripterygium wilfordii Hook. f. in Attenuating Proteinuria in Diabetic Nephropathy: A Review. Front Med. 2021;8:747922. doi: 10.3389/fmed.2021.747922.
- Ge Y, Xie H, Li S, et al. Treatment of diabetic nephropathy with Tripterygium wilfordii Hook F extract: a prospective, randomized, controlled clinical trial. J Transl Med. 2013;11(1):134. doi: 10.1186/1479-5876-11-134.
- Xiong C, Li L, Bo W, et al. Evaluation of the efficacy and safety of TWHF in diabetic nephropathy patients with overt proteinuria and normal eGFR. J Formos Med Assoc. 2020;119(3):685–692. doi: 10.1016/j.jfma.2019.11.001.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116.
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005.
- Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462.
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161.
- Little RJA. Testing the equality of two independent binomial proportions. The American Statistician. 1989;43(4):283–288. doi: 10.2307/2685390.
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13(1):152. doi: 10.1186/1471-2288-13-152.
- Neuen BL, Ohkuma T, Neal B, et al. Effect of Canagliflozin on Renal and Cardiovascular Outcomes across Different Levels of Albuminuria: Data from the CANVAS Program. J Am Soc Nephrol. 2019;30(11):2229–2242. doi: 10.1681/ASN.2019010064.
- Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385. doi: 10.1200/JCO.2014.55.2208.
- Zhou Y-Z, Zhao L-D, Chen H, et al. Comparison of the impact of Tripterygium wilfordii Hook F and Methotrexate treatment on radiological progression in active rheumatoid arthritis: 2-year follow up of a randomized, non-blinded, controlled study. Arthritis Res Ther. 2018;20(1):70. doi: 10.1186/s13075-018-1563-6.
- Lv Q-W, Zhang W, Shi Q, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74(6):1078–1086. doi: 10.1136/annrheumdis-2013-204807.
- Sun J, Shen X, Dong J, et al. Tripterygium wilfordii Hook F as Maintenance Treatment for Crohn’s Disease. Am J Med Sci. 2015;350(5):345–351. doi: 10.1097/MAJ.0000000000000591.
- Wang Z, Yu C, Zhou L-N, et al. Effects of Tripterygium wilfordii Induction Therapy to IgA Nephropathy Patients with Heavy Proteinuria. Biol Pharm Bull. 2017;40(11):1833–1838. doi: 10.1248/bpb.b17-00134.
- Liu X, Lin L, Lv T, et al. Combined multi-omics and network pharmacology approach reveals the role of Tripterygium Wilfordii Hook F in treating HIV immunological non-responders. Phytomedicine. 2022;101:154103. doi: 10.1016/j.phymed.2022.154103.
- Zheng C-X, Chen Z-H, Zeng C-H, et al. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 2008;74(5):596–612. doi: 10.1038/ki.2008.203.
- Chen Z-H, Qin W-S, Zeng C-H, et al. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 2010;77(11):974–988. doi: 10.1038/ki.2010.41.
- Gao Q, Shen W, Qin W, et al. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25(11):3539–3547. doi: 10.1093/ndt/gfq245.
- Guo H, Pan C, Chang B, et al. Triptolide Improves Diabetic Nephropathy by Regulating Th Cell Balance and Macrophage Infiltration in Rat Models of Diabetic Nephropathy. Exp Clin Endocrinol Diabetes. 2016;124(6):389–398. doi: 10.1055/s-0042-106083.
- Ma R, Liu L, Liu X, et al. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med. 2013;6(3):649–656. doi: 10.3892/etm.2013.1226.
- Zhang M, Chen Y, Yang M-J, et al. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-κB pathway. Phytother Res. 2019;33(4):1191–1198. doi: 10.1002/ptr.6314.
- Han F, Wang S, Chang Y, et al. Triptolide prevents extracellular matrix accumulation in experimental diabetic kidney disease by targeting microRNA-137/Notch1 pathway. J Cell Physiol. 2018;233(3):2225–2237. doi: 10.1002/jcp.26092.
- Li X-Y, Wang S-S, Han Z, et al. Triptolide Restores Autophagy to Alleviate Diabetic Renal Fibrosis through the miR-141-3p/PTEN/akt/mTOR pathway. Mol Ther Nucleic Acids. 2017;9:48–56. doi: 10.1016/j.omtn.2017.08.011.
- Chang B, Chen W, Zhang Y, et al. Tripterygium wilfordii mitigates hyperglycemia-induced upregulated Wnt/β-catenin expression and kidney injury in diabetic rats. Exp Ther Med. 2018;15(4):3874–3882. doi: 10.3892/etm.2018.5901.
- Ru Y, Luo Y, Zhou Y, et al. Adverse Events Associated With Treatment of Tripterygium wilfordii Hook F: A Quantitative Evidence Synthesis. Front Pharmacol. 2019;10:1250. doi: 10.3389/fphar.2019.01250.

