Abstract
This study aimed to investigate the correlation between ultrafiltration rate (UFR) and hemoglobin levels and erythropoietin (EPO) response in patients receiving maintenance hemodialysis (MHD). 225 MHD patients were divided into three groups according to the UFR: < 10 ml/h/kg, 10–13 ml/h/kg, and >13 ml/h/kg. Clinical parameters and prognosis were compared among the groups. Multiple linear correlation and regression analyses were conducted. SPSS 26.0 (IBM, Chicago, IL, USA) was used to analyze all statistics. The UFR < 10 ml/h/kg group was older than the other groups (p < 0.05). The UFR > 13 ml/h/kg group had the highest SpKt/V (p < 0.05), monthly EPO dose/weight (p < 0.001), and EPO resistance index (p < 0.001), as well as the lowest dry weight (p < 0.001), BMI (p < 0.001), hemoglobin (p < 0.001), hematocrit (p < 0.05), and red blood cell count (p < 0.05). Multiple linear regression analysis showed that sex, dry weight, UFR, calcium, phosphorus, albumin, and C-reactive protein levels were associated with hemoglobin levels. Multivariate logistic regression analysis revealed that a higher UFR was associated with lower hemoglobin levels, while male sex and higher levels of calcium and albumin were associated with higher hemoglobin levels. High UFR is associated with more severe anemia and EPO resistance in MHD. This study provides new insights into anemia management in patients undergoing hemodialysis.
Introduction
Hemodialysis (HD) is the most common kidney replacement therapy for end-stage kidney disease (ESKD) patients worldwide [Citation1]. In HD, fluid balance is achieved via ultrafiltration (UF), which can lead to hemodynamic instability and unphysiological fluid transfer. Despite continuous improvements in HD treatment, the mortality rate of HD patients remains high [Citation2,Citation3]. One potential risk factor for mortality is the ultrafiltration rate (UFR), which is the rate at which fluid is removed during HD. Studies have shown that UFRs greater than 10 mL/h/kg are associated with a higher risk of mortality and intradialytic hypotension [Citation4]. Additionally, UFRs greater than 10 mL/h/kg, particularly > 13 mL/kg/h have been linked to all-cause and cardiovascular death [Citation5–7]. Despite these findings, there are currently no established guidelines for optimal UFR.
Anemia is a common complication of hemodialysis that directly affects patients’ medical costs and quality of life and increases mortality in dialysis patients [Citation8–11]. Decreased production of erythropoietin (EPO) is the primary cause of renal anemia. EPO therapy is the primary treatment for renal anemia, but some patients cannot achieve or maintain their target goals even after active treatment, presenting with low EPO reactivity [Citation8]. Based on our clinical experience, we have observed that anemia in patients with high UFRs is usually more severe and difficult to correct. We believe that this is due to the lower responsiveness of patients with higher UFR to EPO treatment. Currently, the effect of UFR on hemoglobin levels and erythropoietin response has not yet been reported. We speculate that UFR levels may be related to hemoglobin levels and EPO treatment responsiveness in dialysis patients. Therefore, we designed this study to analyze clinical data of HD patients and examine the correlation between UFR and Hb levels, as well as the response to EPO, to better understand the impact of UFR on anemia management.
Materials and methods
Study population
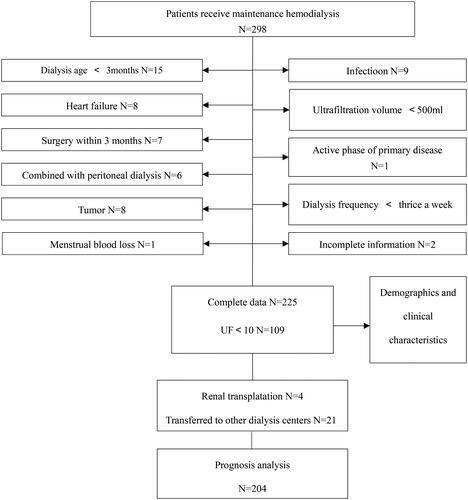
This was a single-center cross-sectional longitudinal study that included patients who met the following inclusion criteria: (1) receiving regular hemodialysis three times per week for four hours each time in our hemodialysis center, (2) having a hemodialysis treatment duration of more than three months with a stable dialysis process and no related complications, (3) having an ultrafiltration volume of more than 500 mL during each session, (4) no surgical history within three months, and (5) in the inactive phase of their primary disease. Patients were excluded if they had symptomatic infections, tumors, symptomatic heart failure, or were undergoing peritoneal dialysis. All patients have not received blood transfusions in the last three months. Additionally, they undergo monthly blood routine monitoring to make necessary adjustments to their EPO dosage. None of the patients have modified their EPO dosage in the past month. A total of 225 patients were included in the study as shown in . The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, and all patients provided informed consent.
Clinical and biochemical data collection
Data on sex, age, dialysis age, primary etiology, height, and dry body weight were collected, and body mass index (BMI, weight [kg]/height2 [m2]) was calculated. In this study, laboratory measurements were obtained during routine clinical examination. In our dialysis center, the hemodialysis schedule was defined as Monday-Wednesday-Friday or Tuesday-Thursday-Saturday. According to the standard operating procedures for blood purification in China, we conduct monthly and quarterly biochemical tests on patients. Blood samples were obtained from patients after long interdialytic intervals (3-day interval between sessions), and before starting the procedure. We collected patient laboratory results of whole blood red blood cell (RBC), hemoglobin (Hb), hematocrit (HCT), and serum potassium (K), calcium (Ca), phosphorus (P), parathyroid hormone (PTH), C-reactive protein (CRP), albumin, and ferritin levels in December 2020, and calculated the average UFR for that month. The UFR was calculated as follows: [pre-dialysis weight (kg) – post-dialysis weight (kg)]/dialysis session length (h)/post-dialysis body weight (kg). The urea reduction ratio (URR) was calculated as follows: [pre-dialysis blood urea nitrogen BUN (mmol/L) – post-dialysis BUN (mmol/L)]/pre-dialysis BUN (mmol/L) × 100%. SpKt/V (Single pool Kt/V, ‘K’ is dialyzer urea clearance, ‘t’ is total dialysis session time, and ‘V’ is the volume of distribution of urea which is approximately equal to total body water) was calculated as follows: In [post-dialysis BUN (mmol/L)/pre-dialysis BUN (mmol/L)-0.008 × therapy time(h)] + [4-3.5 × post-dialysis BUN (mmol/L)/pre-dialysis BUN (mmol/L)] × [pre-dialysis weight (kg) − post-dialysis weight (kg)]/post-dialysis weight (kg), and the result was automatically calculated by the hemodialysis electronic system. We calculated the total recombinant human erythropoietin (rHuEPO) dose and average rHuEPO dose per week in December 2020. Erythropoietin (EPO) resistance index (ERI) was calculated as the total rHuEPO dose per week (IU)/body weight (kg) /hemoglobin (g/L).
The patient’s laboratory test results were obtained in December 2020. We conducted a two-year follow-up to obtain patient prognosis data. During the two-year follow-up period from December 31, 2020, to December 31, 2022, 21 patients were transferred to other dialysis centers, and 4 patients underwent kidney transplantation and withdrew from dialysis. The final number of patients with prognostic data (the number of emergency department visits, hospitalizations, and deaths) was 204.
Statistical analysis
A non–parametric test (Kruskal–Wallis with multiple comparisons) was used to identify significant differences among the three groups. Continuous variables are presented as medians and quartiles, whereas categorical variables are expressed as numbers with percentages. Multiple linear correlation and regression analyses were conducted. Correlation analysis was performed using Spearman correlation analysis. Statistical significance was set at p < 0.05. SPSS 26.0 (IBM, Chicago, IL, USA) was used to analyze all statistical data.
Results
A total of 225 patients were divided into three groups based on their UFR: the low UFR group (UFR < 10 mL/h/kg), the middle UFR group (UFR 10–13 mL/h/kg), and the high UFR group (UFR > 13 mL/h/kg). The demographic and clinical characteristics of patients are presented in . The primary etiology and dialysis age were not significantly different between the groups. There were significant differences in BMI among the three groups. The higher the UFR, the lower the BMI (high vs. middle vs. low UFR group: 20.28 vs. 22.91 vs. 24.71 kg/m2, p < 0.001). The low UFR group (68.5 vs. 54 kg, p < 0.001) and middle UFR group (65.1 vs. 54 kg, p < 0.001) had higher dry weights than the high UFR group. The middle UFR group (49 vs. 60 years, p < 0.001) and the high UFR group (51 vs. 60 years, p = 0.007) were younger than the low UFR group. The proportion of males was lower in the high UFR group than in the middle UFR group (44.4% vs. 70.3%, p = 0.043). High UFR group had the highest monthly EPO dose/weight (high vs. middle group:940 vs. 678 IU/kg, p = 0.001; high vs. low group:940 vs. 629 IU/kg, p < 0.001) and highest EPO resistance index (high vs. middle group:2.29 vs. 1.5 IU/kg/w/g/L, p = 0.001; high vs. low group:2.29 vs. 1.5 IU/kg/w/g/L, p < 0.001). When re-grouping the patients based on gender, we obtained similar results in age, dry weight, BMI, monthly EPO dose/weight, and EPO resistance index (Supplementary Tables 1 and 2).
Table 1. Baseline demographics and clinical characteristics among groups according to ultrafiltration rate.
Table 2. Biochemical characteristics among groups according to ultrafiltration rate.
shows the biochemical characteristics of the three groups. The high UFR group had the lowest hemoglobin level (high vs. middle group: 99 vs. 111 g/L, p = 0.001; high vs. low group:99 vs. 108 g/L, p = 0.005). The hematocrit and red blood cell count levels were consistent with the hemoglobin results. SpKt/V in the high UFR group was higher than in the middle UFR group (1.49 vs. 1.29, p = 0.004) and low UFR group (1.49 vs. 1.23, p < 0.001). Ca, P, PTH, albumin, ferritin, CRP, and URR were not statistically significant among the three groups. We also re-grouped the patients based on gender and compared these biochemical indicators, however, most indicators did not show statistical differences among the three groups. Hemoglobin level was lower in the high UFR group than in the low UFR group in females, but not statistically significant in males. (Supplementary Tables 3 and 4).
Table 3. Multiple linear correlations between hemoglobin and other clinical parameters.
Table 4. Multivariate logistic regression analyses of related factors for hemoglobin.
shows the correlation analysis between hemoglobin levels and related parameters. The results indicated that UFR and CRP levels were negatively correlated with hemoglobin levels. Male sex, dry weight, Ca, P, and albumin levels were positively correlated with hemoglobin. For females, UFR was negatively correlated with hemoglobin levels. Age and Ca were positively correlated with hemoglobin. For males, Ca, P, and albumin were positively correlated with hemoglobin (Supplementary Tables 5 and 6). In multivariate logistic regression analyses, lower hemoglobin levels were associated with higher UFR, while male sex and high levels of Ca and albumin were associated with high hemoglobin levels, as shown in . In both male and female groups, a high level of Ca was associated with high hemoglobin levels (Supplementary Tables 7 and 8).
Table 5. Prognostic value of UFR.
shows the prognostic value of the UFR. We analyzed the relationship between UFR and the average number of hospitalizations and emergency department visits, as well as the number of deaths. The high UFR group had the highest number of hospitalizations, emergency department visits, and the highest rate of death, whereas the middle UFR group had the opposite result. The statistical significance of prognosis only appeared between the average number of hospitalizations in the middle and low UFR groups (0.47 vs. 1.1, p = 0.010).
Discussion
The number of patients starting HD and relying on it is increasing annually. While HD can help patients survive the otherwise fatal complications of ESKD, it is also associated with cardiovascular events and mortality [Citation12,Citation13]. Rapid fluid removal during hemodialysis likely contributes to poor outcomes [Citation14]. It is crucial to explore and understand dialysis practices to improve the prognosis of patients undergoing hemodialysis.
UFR is a modifiable parameter in dialysis prescriptions, determined by pre- and post-dialysis weight and treatment time. Short hemodialysis sessions and high weight gain often result in a high UFR. Previous studies have investigated the relationship between UFR, cardiovascular events, and mortality associated with dialysis. Higher ultrafiltration volumes have been linked to hemodialysis-induced cardiac injury presence and severity, resulting in higher risks of death from cardiovascular disease and all-cause mortality [Citation4–7,Citation15]. Previously published literature indicates that a high UFR rate typically refers to a rate greater than 10 ml/kg/h, and the maximum safe UFR is 13 mL/kg/h [Citation6,Citation16,Citation17]. However, the implementation of a UFR threshold remains controversial [Citation18]. Based on these studies, we compared the clinical characteristics of patients with UFR < 10 mL/kg/h, 10-13 mL/kg/h, and > 13 mL/kg/h in our dialysis center. As expected, a significant association was found between hemoglobin levels and UFR, with a higher UFR being linked to more severe anemia. Interestingly, it was observed that patients with the highest UFR had the lowest hemoglobin levels and required the highest monthly EPO dose per weight. These results indicate that patients with higher UFR require more EPO treatment and still cannot achieve the desired hemoglobin levels, suggesting that patients with higher UFR have a poorer response to EPO. After re-grouping the patients based on gender, we also found that the high UFR group required more EPO treatment, while hemoglobin level still showed a decreasing trend.
Anemia is a complication that affects most individuals with advanced chronic kidney disease (CKD), and its presence predicts morbidity, mortality, and hospitalization in patients undergoing hemodialysis [Citation9,Citation19,Citation20]. It has been proven to be a good predictor of adverse effects, including cardiovascular complications and death [Citation21]. According to DOPPS data, higher hemoglobin concentrations are associated with improved health outcomes [Citation9]. In maintenance hemodialysis patients, anemia is generally caused by iron deficiency and relative or absolute erythropoietin deficiency [Citation22]. EPO is the primary treatment for renal anemia. However, some patients cannot achieve or maintain their target goals even after active treatment, presenting with low EPO reactivity [Citation8]. Only 43.1% of patients in the China Dialysis Outcomes and Practice Patterns Study (DOPPS) 5 had Hb> 110 g/l, whereas 18.8% of patients had Hb < 90 g/L [Citation23]. A high EPO resistance index is an important marker of cardiovascular and all-cause mortality [Citation24]. Our study revealed that the >13 mL/h/kg group had the highest EPO resistance index. In view of the significantly increased female composition of the high UFR subgroup, we re-grouped the patients based on gender and obtained the same result in both the male and female groups.
Iron deficiency is recognized as the most common cause of ineffective EPO treatment, and maintaining sufficient iron stores is considered the most important strategy for reducing the need for EPO [Citation25]. In our study, we did not find any significant differences in ferritin levels, indicating that there were no significant differences in iron reserves among the groups. It is worth noting that chronic inflammation is one of the most important causes of iron utilization disorders [Citation26], and CRP levels have been associated with a decreased response to EPO [Citation27]. Additionally, hyperparathyroidism has been identified as a predictive factor for reduced EPO responsiveness [Citation28]. In our study, we did not observe any signs of inflammation in the patients (CRP < 3mg/L), and there were no statistically significant differences in CRP and PTH levels among the groups. Malnutrition may also contribute to EPO resistance, as the malnutrition-inflammation complex can lead to a decreased response to EPO [Citation29]. In our study, although we did not evaluate the nutritional intake of the patients, all groups maintained adequate nutrition status (albumin > 35g/L), and there were no statistical differences in calcium, phosphorus, and albumin levels among the three groups. Furthermore, our correlation analysis indicated that hemoglobin levels were not affected by dialysis adequacy in this study. These findings suggest that the ultrafiltration rate may be an independent factor related to the responsiveness of HD patients to EPO treatment.
Previous studies have shown that high ultrafiltration rates are associated with ischemia in the heart, brain, and intestines [Citation30]. We have reason to believe that patients with high ultrafiltration rates may also experience ischemia in hematopoietic organs such as bone marrow, liver, and spleen during dialysis. In addition, UF-induced intestinal ischemia may lead to the transfer of intestinal bacteria into the bloodstream, causing an increase in blood endotoxin [Citation31–33], which may lead to the destruction and dissolution of red blood cells, exacerbating the severity of anemia. Moreover, patients with high ultrafiltration rates may also experience greater transmembrane pressure during hemodialysis, resulting in more damage to red blood cells, microthrombosis, and more drastic changes in blood composition. These factors may all affect the degree of anemia and responsiveness of dialysis patients to EPO treatment. Further research is needed to confirm the correlation and possible mechanisms between UFR and hemoglobin levels and EPO response-ability.
To further evaluate the influencing factors of hemoglobin levels, we conducted correlation and regression analysis. The results showed a positive correlation between Hb and Ca, Alb, and male, while a negative correlation with UFR. Regression analysis further confirms a negative correlation between UFR and hemoglobin levels. These results confirm that nutritional status and UFR level are related to anemia in patients undergoing dialysis. Compared with men, menstrual bleeding in women can affect the number of red blood cells, and renal anemia is increased by this factor. Interestingly, in the female group, we found a positive correlation between age and hemoglobin, which we believe may be related to the significantly higher age in the low UFR group compared to the other two groups. Additionally, it is plausible that the absence of menstruation in older women could also contribute to this correlation.
Although patients with UFR > 13 mL/h/kg had the highest number of hospitalizations, emergency department visits, and deaths, our study did not reveal statistically significant differences, which may be due to the limited sample size. It is worth noting that the UFR 10–13 mL/kg/h group had lower hospitalizations than the UFR <10 mL/h/kg group, which indicates that a low ultrafiltration rate may also adversely affect dialysis patients. One possible reason is that UFR reflects not only volume flux but also interdialytic weight gain; the low ultrafiltration rate may represent insufficient intake during dialysis, leading to malnutrition. Previous studies have also shown that better nutritional status indicates higher food and fluid intake, which may be associated with higher UFR [Citation16,Citation34]. Therefore, we believe that maintaining a UFR of 10-13 mL/h/kg may be beneficial for improving prognosis. UFR over 13 mL/h/kg should be avoided when correcting for anemia in hemodialysis patients. Prospective research or larger clinical trials are needed in the later stages to validate this viewpoint.
In summary, our study provides valuable data on the relationship between UFR and anemia and EPO response in patients undergoing maintenance hemodialysis for the first time. UFR > 13 mL/h/kg is associated with more severe anemia and EPO resistance in maintenance hemodialysis patients. Patients with a higher UFR should undergo more frequent or prolonged dialysis sessions to prevent the deleterious effects of excessive UF. Future trials are needed to clarify the mechanism of UFR in anemia and EPO resistance in patients undergoing hemodialysis.
This study has some limitations. First, this was a single-center observational retrospective study. The analysis may have contained uncontrolled confounding factors. A short observation time and a limited number of researchers have led to insufficient research and interpretation of prognosis. As an observational study, although we have determined the association and correlation between UFR and hemoglobin levels and EPO reactivity, we cannot confirm its causal relationship or explain the pathological and physiological mechanisms behind this phenomenon. Second, without information on serum transferrin saturation rate, functional iron deficiency, and vitamin B12 levels, we cannot evaluate the iron utilization status of patients, nor can we diagnose whether they have EPO resistance. Finally, we did not have records of urine volume and could not assess the patient’s residual kidney function based on hemoglobin levels.
Conclusions
UFR over 13 mL/h/kg might be associated with severe anemia and low EPO reactivity in maintenance hemodialysis patients. This may provide new insights into anemia management in patients undergoing hemodialysis.
Author contributions
CL and LZ designed the study. All data were collected and monitored using LC and YL. The manuscript was drafted by JL and LZ, proofread, and reviewed by YX and CL
Supplemental Material
Download PDF (87.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
This published article and its Online Resources include all the data generated or analyzed during this study.
Additional information
Funding
References
- Parekh VB, Niyyar VD, Vachharajani TJ. Lower extremity permanent dialysis vascular access. Clin J Am Soc Nephrol. 2016;11(9):1–7. doi: 10.2215/CJN.01780216.
- Sumida K, Yamagata K, Iseki K, et al. Different impact of hemodialysis vintage on cause-specific mortality in long-term hemodialysis patients. Nephrol Dial Transplant. 2016;31(2):298–305. doi: 10.1093/ndt/gfv402.
- Saran R, Robinson B, Abbott KC, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3 Suppl 1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001.
- Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–1228. doi: 10.1038/sj.ki.5000186.
- Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–257. doi: 10.1038/ki.2010.383.
- Assimon MM, Wenger JB, Wang L, et al. Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2016;68(6):911–922. doi: 10.1053/j.ajkd.2016.06.020.
- Lee YJ, Okuda Y, Sy J, et al. Ultrafiltration rate, residual kidney function, and survival among patients treated with Reduced-Frequency hemodialysis. Am J Kidney Dis. 2020;75(3):342–350. doi: 10.1053/j.ajkd.2019.08.019.
- KDOQI. KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50(3):471–530. doi: 10.1053/j.ajkd.2007.06.008.
- Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five european countries: association with morbidity and mortality in the dialysis outcomes and practice patterns study (DOPPS) [published correction appears in nephrol dial transplant. 2004 jun;19(6):1666]. Nephrol Dial Transplant. 2004;19(1):121–132. doi: 10.1093/ndt/gfg458.
- Buttarello M, Pajola R, Novello E, et al. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol. 2010;133(6):949–954. doi: 10.1309/AJCPQAX0JFHFS0OA.
- Eschbach JW. The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney Int. 1989;35(1):134–148. doi: 10.1038/ki.1989.18.
- Chan KE, Maddux FW, Tolkoff-Rubin N, et al. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642–2649. doi: 10.2215/CJN.03680411.
- Hu PJ, Chen YW, Chen TT, et al. Impact of dialysis modality on major adverse cardiovascular events and all-cause mortality: a national population-based study. Nephrol Dial Transplant. 2021;36(5):901–908. doi: 10.1093/ndt/gfaa282.
- Navarrete JE, Rajabalan A, Cobb J, et al. Proportion of hemodialysis treatments with high ultrafiltration rate and the association with mortality. Kidney360. 2022;3(8):1359–1366. Published 2022 May 5. doi: 10.34067/KID.0001322022.
- Lertdumrongluk P, Streja E, Rhee CM, et al. Changes in pulse pressure during hemodialysis treatment and survival in maintenance dialysis patients. Clin J Am Soc Nephrol. 2015;10(7):1179–1191. doi: 10.2215/CJN.09000914.
- Kuipers J, Verboom LM, Ipema KJR, et al. The prevalence of intradialytic hypotension in patients on conventional hemodialysis: a systematic review with Meta-Analysis. Am J Nephrol. 2019;49(6):497–506. doi: 10.1159/000500877.
- European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association. Section II. Haemodialysis adequacy. Nephrol Dial Transplant. 2002;17Suppl(7):16–31.
- Kramer H, Yee J, Weiner DE, et al. Ultrafiltration rate thresholds in maintenance hemodialysis: an NKF-KDOQI controversies report. Am J Kidney Dis. 2016;68(4):522–532. doi: 10.1053/j.ajkd.2016.06.010.
- Lankhorst CE, Wish JB. Anemia in renal disease: diagnosis and management. Blood Rev. 2010;24(1):39–47. doi: 10.1016/j.blre.2009.09.001.
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116(2):288–296. doi: 10.1172/JCI27699.
- Weiner DE, Tighiouart H, Vlagopoulos PT, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16(6):1803–1810. doi: 10.1681/ASN.2004070597.
- Mikhail A, Brown C, Williams JA, et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18(1):345. Published 2017 Nov 30. doi: 10.1186/s12882-017-0688-1.
- Zhao X, Niu Q, Gan L, et al. Baseline data report of the China dialysis outcomes and practice patterns study (DOPPS). Sci Rep. 2021;11(1):873. Published 2021 Jan 13. doi: 10.1038/s41598-020-79531-4.
- Lu X, Zhang J, Wang S, et al. High erythropoiesis resistance index is a significant predictor of cardiovascular and all-cause mortality in chinese maintenance hemodialysis patients. Mediators Inflamm. 2020;2020:1027230–1027237. Published 2020 Nov 26. doi: 10.1155/2020/1027230.
- Ogawa T, Nitta K. Erythropoiesis-stimulating agent hyporesponsiveness in end-stage renal disease patients. Contrib Nephrol. 2015;185:76–86. doi: 10.1159/000380972.
- Batchelor EK, Kapitsinou P, Pergola PE, et al. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31(3):456–468. doi: 10.1681/ASN.2019020213.
- Kimachi M, Fukuma S, Yamazaki S, et al. Minor elevation in C-Reactive protein levels predicts incidence of Erythropoiesis-Stimulating agent hyporesponsiveness among hemodialysis patients [published correction appears in nephron. 2016;132(4):342]. Nephron. 2015;131(2):123–130. doi: 10.1159/000438870.
- Al-Hilali N, Al-Humoud H, Ninan VT, et al. Does parathyroid hormone affect erythropoietin therapy in dialysis patients? Med Princ Pract. 2007;16(1):63–67. doi: 10.1159/000096143.
- Rattanasompattikul M, Molnar MZ, Zaritsky JJ, et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013;28(7):1936–1945. doi: 10.1093/ndt/gfs368.
- Assimon MM, Flythe JE. Rapid ultrafiltration rates and outcomes among hemodialysis patients: re-examining the evidence base. Curr Opin Nephrol Hypertens. 2015;24(6):525–530. doi: 10.1097/MNH.0000000000000174.
- McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–141. doi: 10.2215/CJN.04610510.
- Jefferies HJ, Crowley LE, Harrison LE, et al. Circulating endotoxaemia and frequent haemodialysis schedules. Nephron Clin Pract. 2014;128(1-2):141–146. doi: 10.1159/000366519.
- McIntyre CW, Odudu A. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial. 2014;27(2):87–97. doi: 10.1111/sdi.12197.
- Hussein WF, Arramreddy R, Sun SJ, et al. Higher ultrafiltration rate is associated with longer dialysis recovery time in patients undergoing conventional hemodialysis. Am J Nephrol. 2017;46(1):3–10. doi: 10.1159/000476076.