Abstract
Background
Idiopathic membranous nephropathy (IMN) with deposits of phospholipase A2 receptor (PLA2R) antigen in glomerular tissue (GAg+) but no circulating serum PLA2R antibody (SAb−) has been reported. However, little is known about the clinicopathological characteristics and prognosis of this subtype.
Methods
A total of 74 IMN patients with GAg + identified by kidney biopsy were enrolled in this study. We categorized patients into two groups based on the presence or absence of serum PLA2R antibody. Data on clinical features, pathological features, and outcomes were collected. Kaplan–Meier analysis of complete remission (CR) and partial remission (PR) comparing SAb−/GAg + and SAb+/GAg + patients. Cox proportional hazards models was used to examine factors associated with CR and PR.
Results
Among 74 IMN patients, 14 were SAb−/GAg+. Compared with SAb+/GAg + patients, SAb−/GAg + patients presented with higher levels of albumin, lower levels of cholesterol and low density lipoprotein cholesterol (all p < .01), but similar pathological manifestations of kidney biopsy. Multivariate logistic analyses indicated that low albumin (0.79 [95%CI: 0.66–0.95], p = .01) and high cholesterol (1.81 [95%CI: 1.02–3.19], p = .04) were correlated with seropositivity of PLA2R antibody. SAb−/GAg + patients exhibited a significantly higher probability of CR (p = .03) than patients who were SAb+/GAg+. However, no difference was found in the PR rate. Cox regression analyses showed that compared to SAb+/GAg + patients, SAb−/GAg + was more predictive of complete remission (4.28 [95%CI: 1.01–18.17], p = .04).
Conclusion
IMN with PLA2R staining on kidney biopsy but without serum PLA2R antibody has milder clinical manifestations and a better prognosis.
Background
Idiopathic membranous nephropathy (IMN) is a common glomerulonephritis disorder pathologically characterized by an apparent thickening of the capillary walls due to immune complex deposits [Citation1]. The presence of serum phospholipase A2 receptor (PLA2R) antibody (SAb+) and glomerular phospholipase A2 receptor antigen (GAg+) deposits have been widely observed in IMN. Serum PLA2R antibody level is a good marker for IMN diagnosis, disease activity monitoring, prognosis, and treatment decision making [Citation2,Citation3]. Sustained glomerular PLA2R antigen deposits also closely related to disease relapse [Citation4].
SAb + usually coexists with the GAg+. However, recent studies have found that some patients can detected GAg deposits without SAb (SAb−/GAg+) [Citation4–7]. The underlying mechanism is far from clear, and may be related to the speculation that circulating anti-PLA2R antibodies are prevalent only when they are saturated in kidney tissues [Citation7]. The understanding of the clinicopathological characteristics and prognosis of this subtype of PLA2R-associated IMN is worth exploring, but is limited currently.
Therefore, we divided patients with PLA2R related IMN into different subgroups based on the circulating PLA2R antibody status to explore the clinical and pathological characteristics and prognosis of SAb–/GAg + patients.
Methods
Study design and population
A total of 203 patients with biopsy-proven IMN were diagnosed in the Nephrology Department of Beijing Chaoyang Hospital, Capital Medical University, between 2019 and 2022. Among all patients with IMN, 129 patients were excluded due to lack of renal PLA2R detection, or absence of PLA2R deposition in the glomeruli, or lack of serological PLA2R testing. Finally, 74 patients with both PLA2R glomerular deposition and PLA2R serological test results were included in the study. 42 patients who were followed up for more than 6 months or achieved complete or partial remission were included in the cohort study. The therapeutic regimen was determined by the nephrologist and the patient according to the disease severity. Supportive therapy included angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, and immunosuppressive therapy included corticosteroids, cyclophosphamide, calcineurin inhibitors, and rituximab.
This study was in accordance with the Helsinki declaration. The Ethics Review Board of Beijing Chaoyang Hospital, Capital Medical University, waived the requirement of informed consent due to the observational and retrospective design of the study.
Serology
Patient baseline data including sex, age, albumin, serum creatinine, 24-h urine protein, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglycerides were measured during the hospitalization for renal biopsy. Serum samples were collected before treatment at the time of biopsy. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines. Serum anti-PLA2R antibody was measured by enzyme-linked immunosorbent assay or indirect immunofluorescence assay. The results were considered negative when the concentration was <20 relative units/mL or the determination was negative [Citation8].
Kidney histopathology
The immunofluorescent assay was utilized to detect the deposition of IgG, IgG subclasses, IgA, IgM, C3, C1q, and the PLA2R antigen in kidney tissue. And staining intensity was evaluated as negative and positive. We divided IMN into 4 stages. If two stages were present at the same time, we defined the highest stage as the final stage. Meanwhile, we also recorded renal pathological features including glomerulosclerosis, mesangial proliferation, tubular atrophy, interstitial fibrosis, and arterial lesions.
Outcome definitions
Based on KDIGO guidelines, complete remission (CR) was defined as proteinuria <0.3 g/d, and partial remission (PR) was defined as proteinuria <3.5 g/d, which concurrently achieved a ≥ 50% reduction compared to the baseline; the kidney function was stable in both conditions [Citation9].
Statistical analyses
Continuous variables were summarized using mean ± standard deviation for normally distributed variables and median (interquartile range) for non-normally distributed variables. Frequency (percentages) were used for categorical variables. Comparison among the two subgroups was performed using 2-sample t tests or Mann–Whitney U-test for continuous variables and chi-square test for categorical variables respectively. Logistic regression analyses were used to explore factors associated with seropositive PLA2R antibody. Covariates with p < .05 in univariate analyses were included in multivariate analyses. Kaplan–Meier analysis and the log-rank method were used to compare the probability of CR and PR between the two groups. Cox proportional hazards models were used to examine the association between potential risk factors and outcomes. Covariates with p < .05 in univariate analyses were included in multivariate analyses. Statistical analyses were performed using SPSS version 25 (IBM Corp.) and R Version 3.6.2. p-value <.05 were considered statistically significant.
Results
Baseline characteristics
A total of 74 participants were included in this study, including 14 (18.9%) patients in the SAb−/GAg + group and 60 (81.1%) patients in the SAb+/GAg + group. The mean age was 46.9 ± 15.5 years, and 55.4% were male. The baseline characteristics are shown in . The patients in the SAb−/GAg + group had higher levels of albumin, lower levels of cholesterol and LDL-C, compared with patients in the SAb+/GAg + group (p < .01). The proportion of immunosuppressive therapy in SAb+/GAg + group was higher than that in SAb−/GAg + group. The proportions of renal pathological stages, glomerulosclerosis, mesangial proliferation, tubular atrophy, interstitial fibrosis, and arterial lesions were similar between the two groups. In addition, among the 69 patients tested for IgG subclasses, there was no significant difference in the deposition of IgG, IgG subclasses, IgM, IgA, C3, or C1q between SAb−/GAg + group and SAb+/GAg + group (Supplementary Table 1).
Table 1. Comparison Of baseline characteristics between the two groups.
Factors associated with anti-PLA2R antibody
In univariate logistic analyses, decreased albumin, increased cholesterol and LDL-C were risk factors for seropositivity of anti-PLA2R antibody (p < .01). And the use of immunosuppressive therapy was positively associated with serum anti-PLA2R antibody positivity (p = .03). Multivariate analyses still indicated that SAb+/GAg + was associated with higher risk of low albumin (0.79 [95%CI: 0.66–0.95], p = .01) and high cholesterol (1.81 [95%CI: 1.02–3.19], p = .04) compared to the patients in SAb−/GAg + group ().
Table 2. Logistic regression analysis evaluating the risk factors on seropositive PLA2R antibody.
Treatment and outcome
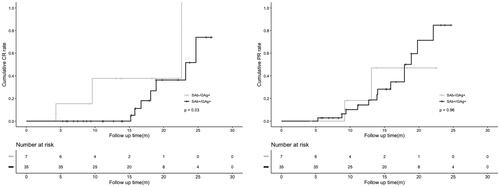
Of the 74 patients, 42 patients were followed up for more than 6 months or achieved complete or partial remission, including 7 patients in the SAb−/GAg + group and 35 patients in the SAb+/GAg + group, with a median follow-up time of 15.2 (9.3-19.1) months. Among the 42 patients who were followed, immunosuppressive regimens included cyclosporin A (2.4%), glucocorticoids and cyclosporin A (40.5%), glucocorticoids and cyclophosphamide (7.1%), rituximab (23.8%), rituximab and cyclosporin A (4.8%) (Supplementary Table 2). Kaplan–Meier analysis indicated that SAb−/GAg + patients had a higher complete remission rate (p = .03) than SAb+/GAg + patients. However, there was no difference in the partial remission rate (p = .96) ().
Relationship between PLA2R and CR rate
Univariate Cox regression analyses showed that only seropositivity of anti-PLA2R antibody was a risk factor for lack of complete remission (4.28 [95%CI: 1.01–18.17], p = .04), and SAb−/GAg + was a better predictor for complete remission. There was no relationship between serum PLA2R antibody and partial remission in Cox regression analyses (1.03 [95%CI: 0.23–4.63], p = .96).
Discussion
M-type phospholipase A2 receptor was identified as a major target antigen involved in IMN in adults [Citation10]. The absence of circulating PLA2R antibody at the time of kidney biopsy should not rule out a diagnosis of PLA2R related IMN5. Moreover, different state of SAb/GAg may represent different clinical characteristics and prognosis of IMN. In the present study, we found that SAb−/GAg + patients had higher level of albumin, and lower levels of total cholesterol and LDL-C compared to SAb+/GAg + patients. Hypoalbuminemia and hypercholesterolemia are independently associated with seropositive PLA2R antibody in PLA2R related IMN. In addition, SAb−/GAg + patients seem to be more likely to achieve clinical complete remission. Seropositive PLA2R antibody is an important predictor of lack of remission. It can be seen that SAb−/GAg + patients tend to have milder clinical manifestations and better prognosis compared with SAb+/GAg + patients.
In our study, the SAb−/GAg + rate of 74 patients with IMN was 18.9%, which was similar to that reported in previous studies [Citation4,Citation5,Citation11]. The possible mechanism has not been explained. One explanation is the “kidney as a sink” hypothesis: antibodies become detectable only after the buffer capacity of the kidney is exceeded [Citation12]. While others speculate that the lack of circulating antibody detection may also be due to the high avidity of anti-PLA2R antibodies to the podocytes [Citation7], or antibodies are quickly cleared from the blood and deposited in the glomeruli [Citation5]. The above studies all remind us that GAg deposition combined with SAb may be more sensitive to the diagnosis of IMN than SAb alone, so as to avoid missing the IMN manifested as SAb−/GAg+.
Current data also indicated that decreased albumin and increased TC at baseline were independent risk factors for positive serum PLA2R antibody in IMN with glomerular PLA2R deposit. Similarly, a meta-analysis, including 20 studies involving 2,224 IMN patients, also reached the conclusion that TC was correlated with serum anti-PLA2R and glomerular PLA2R deposit [Citation13]. In addition, a study including 495 IMN patients, not only showed that high TC, non-HDL-C, and LDL-C were independent risk factors for seropositivity of anti-PLA2R antibody, but also suggested that increased TC was an independent risk factor for the IgG4 deposit [Citation14]. Given that IgG4 has been speculated to activate the lectin pathway and induce podocyte injury in anti-PLA2R-associated MN [Citation15,Citation16], which may mediate hypercholesterolemia to induce a stronger immune responses in the kidney, leading to more severe clinical symptoms. Therefore, increased TC is an important indicator that cannot be ignored when evaluating the PLA2R antibody status and disease severity.
As observed in this study, SAb−/GAg + patients tend to have higher levels of albumin and lower levels of TC than SAb+/GAg + patients. Therefore, it can be seen that SAb−/GAg + patients tend to have milder clinical manifestations than SAb+/GAg + patients. However, as previous studies have shown, patients with negative or low serum PLA2R antibody titers at diagnosis are generally considered to have a high probability of spontaneous remission [Citation17,Citation18]. Therefore, it is uncertain whether SAb−/GAg + is an early stage of the disease or has achieved spontaneous remission, and these hypotheses must be tested in future studies.
Regarding the therapeutic response and prognosis of the two MN subtypes, our results showed that SAb−/GAg + patients had a higher probability of remission than SAb+/GAg + patients. The probability of CR after treatment was 42.9% in SAb−/GAg + patients, compared with 25.7% in SAb+/GAg + patients. In cox regression analyses, patients who were SAb−/GAg + had a higher chance of reaching complete remission than patients who were SAb+/GAg+ (hazard ratio, 4.28; 95% CI, 1.01–18.17; p = .04). Seropositive PLA2R antibody was an independent predictor of lack of remission. This is consistent with the poor prognosis of IMN patients with positive anti-PLA2R antibody mentioned in other studies [Citation11,Citation19–21]. As mentioned earlier, at baseline, SAb+/GAg + patients had lower serum albumin level and higher TC and LDL level, all of which indicate a poor prognosis [Citation14,Citation22]. Therefore, different PLA2R related IMN subtypes may have different clinical behaviors, and we strongly recommend the combined detection of PLA2R-Ab serology and PLA2R antigen staining of the kidney biopsy to accurately diagnose and evaluate the disease activity, treatment response, and prognosis of IMN.
Certain limitations exist to this study. First, this is a retrospective single center study with a small sample size and a lack of uniform treatment. Second, most patients were treated with immunosuppressive agents, which limited our observation of spontaneous remission. Third, the short follow-up time is relatively short, and the lack of repeated monitoring of PLA2R antibodies during follow-up also makes it difficult for us to systematically understand the dynamic relationship between PLA2R antibodies and clinical outcomes. Additional studies with multi-center and large sample data focusing on the relationship between consecutive changes in serum PLA2R antibody and prognosis of IMN are needed.
Conclusion
In conclusion, IMN with glomerular PLA2R antigen deposition but without serum PLA2R antibody is not rare, which presenting with milder clinical features and a better prognosis. Renal tissue PLA2R antigen testing should be considered for patients with seronegative PLA2R antibody to improve diagnostic efficiency. Seropositive PLA2R antibody is an independent predictor of lack of remission in PLA2R related IMN. More attention should be paid to the serum PLA2R antibody of PLA2R related IMN.
Authors contributions
XL conceived and designed the study, performed the data collection, data analysis and manuscript writing. QMS were involved in the design of the study and led the study. YS, YCL and LJM performed the recruitment and data collection. All authors contributed to this article, and all have approved the final manuscript.
Ethics approval
This study was approved by the ethics committees of Beijing Chaoyang Hospital, Capital Medical University, Beijing China (approval notice number 2023-KE-419). The Institutional Review Board waived the requirement of informed consent due to the retrospective design of the study.
Supplemental Material
Download PDF (95.3 KB)Supplemental Material
Download PDF (85.6 KB)Acknowledgements
We would like to thank all patients and their families for participating in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets of the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6):1–6. doi: 10.2215/CJN.11761116.
- Tesar V, Hruskova Z. Autoantibodies in the diagnosis, monitoring, and treatment of membranous nephropathy. Front Immunol. 2021;12:593288. doi: 10.3389/fimmu.2021.593288.
- Bobart SA, De Vriese AS, Pawar AS, et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019;95(2):429–438. doi: 10.1016/j.kint.2018.10.021.
- Qin HZ, Zhang MC, Le WB, et al. Combined assessment of phospholipase A2 receptor autoantibodies and glomerular deposits in membranous nephropathy. J Am Soc Nephrol. 2016;27(10):3195–3203. doi: 10.1681/ASN.2015080953.
- Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364(7):689–690. doi: 10.1056/NEJMc1011678.
- Hoxha E, Kneißler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82(7):797–804. doi: 10.1038/ki.2012.209.
- Svobodova B, Honsova E, Ronco P, et al. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28(7):1839–1844. doi: 10.1093/ndt/gfs439.
- Bobart SA, Han H, Tehranian S, et al. Noninvasive diagnosis of PLA2R-associated membranous nephropathy: a validation study. Clin J Am Soc Nephrol. 2021;16(12):1833–1839. doi: 10.2215/CJN.05480421.
- KDIGO. Chapter 7: idiopathic membranous nephropathy. Kidney Int Suppl. 2011;2(2):186–197.
- Beck LH, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457.
- Yin P, Wang J, Liang W, et al. Outcomes of primary membranous nephropathy based on serum anti-phospholipase A2 receptor antibodies and glomerular phospholipase A2 receptor antigen status: a retrospective cohort study. Ren Fail. 2020;42(1):675–683. doi: 10.1080/0886022X.2020.1792315.
- Fresquet M, Jowitt TA, Gummadova J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2015;26(2):302–313. doi: 10.1681/ASN.2014050502.
- Rao SJ, Shen Q, Wang HM, et al. The association of anti-PLA2R with clinical manifestations and outcomes in idiopathic membranous nephropathy: a meta-analysis. Int Urol Nephrol. 2020;52(11):2123–2133. doi: 10.1007/s11255-020-02588-7.
- Dong L, Li YQ, Guo SM, et al. Hypercholesterolemia correlates with glomerular phospholipase A2 receptor deposit and serum anti-phospholipase A2 receptor antibody and predicts proteinuria outcome in idiopathic membranous nephropathy. Front Immunol. 2022;13:905930. doi: 10.3389/fimmu.2022.905930.
- Borza DB. Alternative pathway dysregulation and the conundrum of complement activation by IgG4 immune complexes in membranous nephropathy. Front Immunol. 2016;7:157. doi: 10.3389/fimmu.2016.00157.
- Haddad G, Lorenzen JM, Ma H, et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest. 2021;131(5):e140453.
- Jullien P, Seitz Polski B, Maillard N, et al. Anti-phospholipase A2 receptor antibody levels at diagnosis predicts spontaneous remission of idiopathic membranous nephropathy. Clin Kidney J. 2017;10(2):209–214. doi: 10.1093/ckj/sfw121.
- Rodas LM, Matas-Garcia A, Barros X, et al. Antiphospholipase 2 receptor antibody levels to predict complete spontaneous remission in primary membranous nephropathy. Clin Kidney J. 2019;12(1):36–41. doi: 10.1093/ckj/sfy005.
- Jurubita R, Obrisca B, Sorohan B, et al. Clinical phenotypes and predictors of remission in primary membranous nephropathy. J Clin Med. 2021;10(12):2624.
- Guo W, Zhang Y, Gao C, et al. Retrospective study: clinicopathological features and prognosis of idiopathic membranous nephropathy with seronegative anti-phospholipase A2 receptor antibody. PeerJ. 2020;8:e8650. doi: 10.7717/peerj.8650.
- Sun Y, Lan P, Feng J, et al. Analysis of glomerular PLA2R efficacy in evaluating the prognosis of idiopathic membranous nephropathy in the background of different serum anti-PLA2R levels. Ren Fail. 2022;44(1):731–740. doi: 10.1080/0886022X.2022.2068442.
- Huh H, Lee H, Lee JP, et al. Factors affecting the long-term outcomes of idiopathic membranous nephropathy. BMC Nephrol. 2017;18(1):104. doi: 10.1186/s12882-017-0525-6.