Abstract
Background
To evaluate the efficacy, effectiveness and safety of fermented Ophiocordyceps sinensis mycelium (FOSM) products for preventing contrast-associated acute kidney injury (CA-AKI).
Methods
Randomized controlled trials were searched from four Chinese and four English electronic databases and three clinical trial registries up to July 2023. Methodological quality was assessed by using the Cochrane risk-of-bias tool 2.0. Risk difference (RD) or risk ratio (RR) and mean difference (MD) were calculated along with the 95% confidence intervals (CIs).
Results
Fourteen trials testing three types of FOSM products (Bailing, Zhiling, and Jinshuibao capsules) involving 1271 participants injected contrast agents were included. For the risk of bias, all trials were rated as some concerns. Compared with routine preventive procedure (RPP) (saline hydration and alprostadil), FOSM products plus RPP showed beneficial effects in reducing the incidence of CA-AKI (14.62% and 5.35%, respectively; RD −0.06, 95% CI −0.09 to −0.03). Subgroup analysis showed that Bailing/Jinshuibao plus RPP demonstrated lower incidence of CA-AKI compared to RPP. However, there was no statistically significant difference between Zhiling with RPP and RPP in the incidence of CA-AKI. Additionally, only when FOSM products were taken before injection of the contrast, it was superior to RPP in reducing the incidence of CA-AKI. There was no statistical difference in adverse events between these two groups.
Conclusions
Low certainty evidence suggests that preventive oral use of FOSM products as an adjuvant agent was safe and might decrease the incidence of CA-AKI. However, high-quality placebo-controlled trials are needed to confirm its benefit.
1. Introduction
Contrast agents are widely used in radiology, but are associated with increased risk of renal impairment [Citation1,Citation2]. Contrast-associated acute kidney injury (CA-AKI) is defined as an increase in serum creatinine (Scr) levels by at least 25% above baseline or 44 µmol/L from the pre-contrast value within 48–72 h of administering a contrast agent [Citation3,Citation4]. The incidence of CA-AKI depends on the population and risk factors, with rates as high as 30–50% in high-risk groups [Citation5–7]. CA-AKI was once regarded a leading cause of hospital-acquired renal failure, resulting in longer hospital stays, higher costs, and increased mortality [Citation8]. With better design of contrast agents, improved recognition of risk factors, and preventive care, the prevalence of CA-AKI has reduced; however, it remains an important clinical concern, for which new prophylactic and therapeutic strategies are needed [Citation1,Citation2].
Prevention remains the most important strategy against CA-AKI. Current preventive strategies include risk assessment, selection of low osmolality and iso-osmolality, instead of high osmolality, contrast materials and discontinuation of nephrotoxic drugs, as well as using the lowest necessary doses of contrast agents and administering intravenous isotonic sodium chloride in high-risk populations, such as those with estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 [Citation3]. Drugs like antioxidants (N-acetylcysteine, ascorbic acid) and statins have been reported to be effective in preventing CA-AKI in small clinical trials [Citation9,Citation10], but none of them have shown benefits in properly designed large randomized controlled trials (RCTs) and current evidence supports hydration as the standard care for preventing CA-AKI in high-risk populations [Citation1,Citation11–14], although a RCT published in The Lancet in 2017 did not find any difference in the incidence of CA-AKI between the intravenous hydration group (2.7%) and the non-hydrated control group (2.6%) [Citation15]. Besides, whether bicarbonate-based hydration is superior to normal saline for preventing CA-AKI is still controversial [Citation16–18] and no conventional drugs can be not recommended for preventing CA-AKI [Citation19,Citation20].
Emerging preclinical and clinical evidence suggests that herbal and fungal drugs could be an important resource for the prevention and treatment of AKI, including CA-AKI [Citation21]. For example, Cordyceps is a genus of fungi that include Ophiocordyceps sinensis, also widely known by its synonym Cordyceps sinensis, Cordyceps militaris, and Cordyceps cicadae, etc. [Citation22,Citation23]. The most famous and historically influential species of Cordyceps is O. sinensis, a precious tonic Chinese medicine, which was first recorded in the eighth century AD in Somaratsa [Citation24]. O. sinensis has various physiological properties, such as anti-inflammatory, anti-oxidative, anti-fibrogenic, and renoprotective effects [Citation21,Citation25,Citation26]. Due to the scarcity of natural resources and technical challenges in cultivating O. sinensis, there has been growing interests in exploring possible substitutes for natural O. sinensis, for example, using fermented O. sinensis mycelia (FOSM) or C. militaris, cultivation of which have been successfully, as discussed elsewhere [Citation22,Citation25,Citation27]. At the moment, O. sinensis-based Chinese patent medicines sold in China all contain FOSM rather than natural O. sinensis, and these FOSM products include Jinshuibao, Bailing, Zhiling, Xinganbao, and Ningxinbao, none of which contains any ingredients other than FOSM. Additionally, North America was the largest region in the Cordyceps sinensis market in 2022. The regions covered in the Cordyceps sinensis market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa, said the business research company [Citation28].
Several systematic reviews of clinical trials have documented the effects of FOSM products in treating patients with chronic kidney disease, including diabetic nephropathy, and patients with end-stage kidney disease on hemodialysis and after renal transplantation [Citation29–32]. An earlier scoping review has found FOSM products among those herbal medicines that could be of value in AKI patients [Citation21], but to date, there is no systematic review evaluating the clinical effects of FOSM products on CA-AKI. Hence, we conducted a synthesis of relevant RCTs to examine the efficacy, effectiveness, and safety of FOSM products in the prevention and treatment of CA-AKI.
2. Methods
This systematic review was reported following the PRISMA 2020 statement [Citation33]. The protocol was registered with INPLASY (INPLASY202260098; https://inplasy.com/inplasy-2022-6-0098/).
2.1. Eligibility criteria
Original studies were included when they meet the following inclusion criteria: (1) participants: all participants who received intravascular injection of contrast agent, irrespective of age or presence of kidney disease; (2) interventions: FOSM products alone or in combination with other preventive procedure. Other Cordyceps species, such as C. militaris, were also included; (3) comparators: no intervention or any intervention that aimed to reduce the incidence of CA-AKI; (4) outcomes: primary: the incidence of CA-AKI; secondary: Scr level, eGFR, the need for dialysis, blood urea nitrogen (BUN) level, interleukin 18 (IL-18) level, kidney injury molecule-1 (KIM-1) level, superoxide dismutase (SOD) level, neutrophil gelatinase-associated lipocalin (NGAL) level, cystatin C (Cys C) level, malondialdehyde (MDA) level; safety outcomes: adverse events. (5) Studies: parallel-group RCTs.
2.2. Search strategies
PubMed, the Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), SinoMed, and WanFang Data were searched from inception to June 2022. We also explored the World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, and the Chinese Clinical Trial Registry for ongoing or unpublished trials to July 2023. Search terms include ‘Cordyceps’ AND ‘contrast agent’ AND ‘acute kidney injury’. The additional search terms and strategies in different databases are displayed in Supplementary Appendix A1.
2.3. Study selection and data extraction
NoteExpress (Beijing Aegean Software company, Rev 3.4.0.8878, Beijing, China) was used to manage and check the literature. Two authors independently screened titles and abstracts for potential eligible studies. These selected studies were then assessed again independently in full text by the same authors to eliminate the duplicated studies and identify eligible studies. Any disagreement was resolved by discussion with third author.
Data were extracted independently by two authors using standard data extraction forms. Where specific data were not available from the included studies, more detailed information was sought by contacting the corresponding author of the trial.
2.4. Quality assessments and certainty of evidence
The version 2 of the Cochrane risk-of-bias tool (RoB 2) [Citation34] was used to assess the risk of bias for included studies by two authors independently. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) evidence grading system was used to evaluate the evidence quality of the main outcome indicators [Citation35].
2.5. Data synthesis
This study used STATA (version 15.0, Stata SE, College Station, TX) for statistical analysis. Dichotomous data were expressed by risk ratio (RR) with 95% confidence interval (CI), and continuous data were expressed by mean difference (MD) with 95% CI. The standard mean difference (SMD) was used for results reported by various measurement methods or different measurement scales.
For multi-arm trials that met the inclusion criteria, we divided the shared control groups equally or combined all relevant experimental interventions from the study into one group. In addition, we used the risk difference (RD) to analyze studies with no events in either arm [Citation36].
2.6. Assessment of heterogeneity and publication bias
Heterogeneity was determined by the Q-statistical test and I2-statistical test. According to the Cochrane Handbook of Heterogeneity Analysis, I2 values between 50% and 90% may represent substantial heterogeneity; 75–100% means considerable heterogeneity [Citation37]. A fixed-effects model was adopted if I2 <50%; otherwise, a random-effects model would be applied. If 10 or more studies were included in the meta-analysis, the publication bias in these studies would be assessed using Egger’s test and funnel plots.
2.7. Subgroup analysis and sensitivity analysis
If sufficient study data were available, subgroup analyses of the primary outcomes were performed in the following groups: type and the starting time of application of FOSM products. p Value <.05 is considered statistically significant. We performed a sensitivity analysis for indicators with significant heterogeneity (I2 >50%) to test the robustness of the results.
3. Results
3.1. Eligible studies
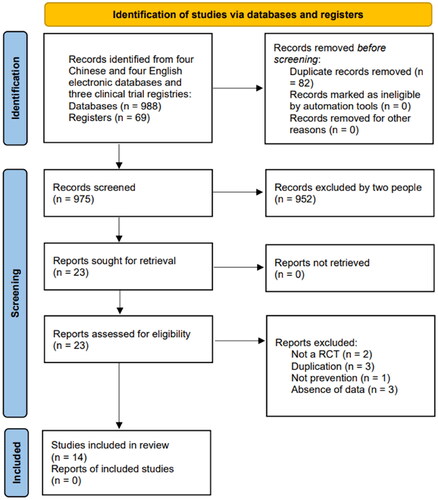
A total of 14 RCTs [Citation38–51] were included in this study, and details of the literature search are shown in . Records screened and exclusion reasons are shown in Supplementary Appendix A2. A total of 1271 participants received intravascular injections of contrast agents (688 in the experimental group and 583 in the control group), all from China. Eleven clinical trials [Citation41–51] were published in Chinese and other three [Citation38–40] in English, which were published between 2011 and 2021.
3.2. Characteristics of included studies
Participants in nine studies [Citation38–40,Citation42,Citation43,Citation45,Citation47,Citation48,Citation50] received the percutaneous coronary intervention (PCI) and two studies [Citation41,Citation46] received coronary angiography, both with an arterial injection of contrast. In addition, two studies [Citation44,Citation51] received coronary CT angiography (CTA), with an intravenous injection of contrast, and one study [Citation44] did not mention the type of examination or injection method of contrast agents. Nine studies [Citation38,Citation39,Citation41–44,Citation48,Citation49,Citation51] used low-osmolar nonionic iodinated contrast agents, and three studies [Citation40,Citation47,Citation50] used iso-osmolar nonionic iodinated contrast agents, with no statistical difference in the doses of contrast agents used in either the experimental or control groups. Besides, one study [Citation46] only mentioned the contrast agent containing iodine and one study [Citation45] did not report the type of contrast agent, none of which mentioned the osmotic pressure. The baseline Scr levels were not statistically different between the experimental and control groups in all studies. Eight studies [Citation38,Citation40,Citation42,Citation43,Citation47–49,Citation51] showed no statistical difference in eGFR between the two groups at baseline, and the mean eGFR was >30 mL/min·1.73 m2 in all studies. Seven of the included trials reported detailed funding sources or conflicts of interest, while the other seven did not report funding sources.
A total of three FOSM products were involved in the included studies: Bailing capsule (six trials) [Citation38–40], Jinshuibao capsule (seven trials) [Citation41,Citation44–48,Citation50], and Zhiling capsule (one trial) [Citation51]. The treatment groups of 12 trials used FOSM products in addition to the control group, the remaining two [Citation44,Citation45] used FOSM products alone. The control group includes saline hydration and alprostadil, so we summarized it as routine preventive procedure (RPP). The baseline Scr levels were not statistically different between the experimental and control groups in all studies. The basic information is shown in , other detailed information (publish language, study aim, study setting, funding, the definition of CA-AKI, contrast medium injection type, participants sex, complications, baseline creatinine, and baseline eGFR) is shown in Supplementary Table.
Table 1. Basic information of the included studies.
3.3. Risk of bias in included studies
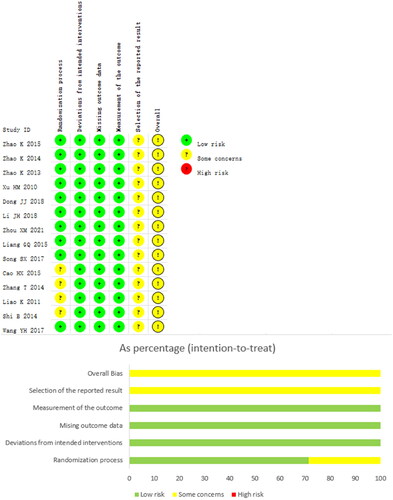
All 14 trials [Citation38–51] showed ‘some concerns’ in terms of overall risk of bias. Regarding the randomization process, eight trials [Citation38–40,Citation42,Citation43,Citation47,Citation48,Citation51] used a random number table, two trials [Citation44,Citation49] used a simple random assignment method, one trial [Citation50] used an incorrect randomization method, and the remaining three trials [Citation41,Citation45,Citation46] only mentioned randomization and did not describe the detailed randomization method. All trials did not provide allocation concealment information. No blinded information was mentioned in any of the trials. None of the included trials reported any missed visits. The risk of bias due to selective reporting of outcomes was assessed as some concerns for all trials, as none of the trial protocols were available for review. shows the methodological quality of the included trials.
3.4. Primary outcomes
3.4.1. Incidence of contrast-associated acute kidney injury
Eleven trials compared FOSM products plus RPP versus RPP for the incidence of CA-AKI. The pooled results showed that the Cordyceps group was superior to the routine group in reducing the incidence of CA-AKI (5.35% and 14.62%, respectively; RD = −0.06, 95% CI −0.09 to −0.03, 11 trials [Citation38–44,Citation46–48,Citation50,Citation51], 1111 participants; low certainty) ().
Figure 3. Forest plot of incidence of contrast-associated acute kidney injury (A), serum creatinine level (B), and epidermal growth factor receptor level (C) for Fermented O. sinensis mycelia products plus routine preventive procedure versus routine preventive procedure.
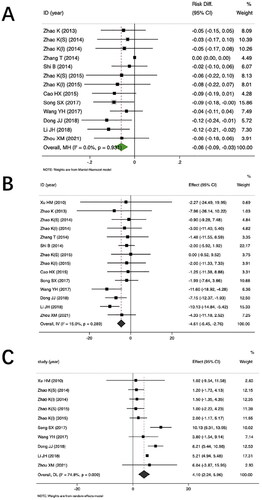
The results of subgroup analysis based on the type of FOSM products showed that the bailing plus routine prevention (5.30% and 14.10%, respectively, p < .05) or Jinshuibao plus routine prevention (5.6% and 15.6%, respectively, p < .05) demonstrated lower incidence of CA-AKI compared to routine prevention. However, there was no statistical difference in Zhiling (3.7% and 10.34%, respectively, p > .05).
Subgroup analysis based on the moment of intervention of FOSM products showed that, FOSM products taken before injection of the contrast, the Cordyceps group was superior to the routine group in reducing the incidence of CA-AKI (RD = −0.06, p < .05). When FOSM products were taken after injection of the contrast, there was no statistical difference between the FOSM group and the routine group (RD = −0.02, p > .05) ().
Table 2. Subgroup analysis on the incidence of CA-AKI.
Besides, one trial compared FOSM products alone with normal saline, including 80 participants. The results showed that the FOSM products alone were superior to normal saline in reducing the incidence of CA-AKI (RR = 0.063, 95% CI 0.025–0.100, p = .001, one trial [Citation44], 80 participants).
3.5. Secondary outcomes
3.5.1. Serum creatinine level
Thirteen trials compared FOSM products plus RPP versus RPP for Scr. The pooled results showed that the FOSM product group was superior to the routine group in reducing Scr (MD = −4.61 μmol/L, 95% CI −6.45 to −2.76, I2 = 15%, 12 trials [Citation38–43,Citation46–51], 1151 participants; very low certainty) ().
3.5.2. Epidermal growth factor receptor level
Eight trials comparing FOSM products plus RPP versus RPP reported eGFR. The pooled results showed that the FOSM product group was superior to the routine group in increasing eGFR (MD = 4.10 mL/min-1.73 m2, 95% CI 2.24–5.96, I2 = 74.8%, eight trials [Citation38,Citation40,Citation42,Citation43,Citation47–49,Citation51], 848 participants) ().
3.5.3. Kidney injury molecule-1 level
Three trials comparing FOSM products plus RPP versus RPP reported KIM-1. The pooled results showed that the Cordyceps group was superior to the routine group in reducing KIM-1 (MD = −2.18 ng/mL, 95% CI −3.35 to −1.01, I2 = 99.7%, three trials [Citation38–40], 373 participants) (Supplementary Appendix B1).
3.5.4. Need for dialysis
Ten trials comparing FOSM products plus RPP versus RPP reported the need for dialysis. The pooled results showed no difference in the reduction of need for dialysis between the Cordyceps and the routine groups (RD = −0.00, 95% CI −0.01 to 0.01, I2 = 0%, 10 trials [Citation38,Citation40–43,Citation45,Citation47,Citation48,Citation50,Citation51], 968 participants) (Supplementary Appendix B2).
3.5.5. Neutrophil gelatinase-associated lipocalin level
Two trials that reported on NGAL compared FOSM products plus RPP with RPP. The pooled results showed that the Cordyceps group was superior to the routine group in reducing NGAL (MD = −17.17 ng/mL, 95% CI −25.94 to −8.41, I2 = 91.4%, two trials [Citation38,Citation40], 302 participants) (Supplementary Appendix B3).
3.5.6. Blood urea nitrogen level
Nine trials that reported on BUN compared FOSM products plus RPP with RPP. The pooled results showed that the Cordyceps group was superior to the routine group in reducing BUN (MD = −1.30 mmol/L, 95% CI −2.33 to −0.28, I2 = 97.1%, nine trials [Citation41–43,Citation45–48,Citation50,Citation51], 778 participants) (Supplementary Appendix B4).
3.5.7. Cystatin C level
Three trials reporting Cys C compared FOSM products plus RPP with RPP. The pooled results showed that the Cordyceps group was superior to the routine group in reducing Cys C (MD = −0.23 mg/L, 95% CI −0.38 to −0.07, I2 = 75%, three trials [Citation41,Citation50,Citation51], 176 participants) (Supplementary Appendix B5).
3.5.8. Malondialdehyde level
Three trials that reported MDA compared FOSM products plus RPP with RPP. The pooled results showed that the Cordyceps group was superior to the routine group in reducing MDA (SMD = −1.58, 95% CI −1.89 to −1.28, I2 = 0%, three trials [Citation41,Citation43,Citation50], 222 participants) (Supplementary Appendix B6).
3.5.9. Superoxide dismutase level
Four trials reporting SOD compared FOSM products plus RPP with RPP. Pooled results showed that the Cordyceps group was superior to the routine group in increasing SOD (SMD = 2.82, 95% CI 2.19–3.45, I2 = 73%, four trials [Citation41–43,Citation50], 302 participants) (Supplementary Appendix B7).
3.5.10. Interleukin 18 level
Three trials reporting IL-18 compared FOSM products plus RPP with RPP. The pooled results showed that the Cordyceps group was superior to the routine group in lowering IL-18 (MD = −14.23 ng/L, 95% CI −18.58 to −9.89, I2 = 96.3%, three trials [Citation38–40], 373 participants) (Supplementary Appendix B8).
3.6. Adverse events
Adverse events were reported in nine studies, and all studies compared FOSM products plus RPP with RPP. Seven of these studies [Citation38,Citation40,Citation41,Citation47,Citation48,Citation50,Citation51] claimed no adverse events in either group. The pooled data from the other two studies [Citation42,Citation43] showed no statistical difference between the Cordyceps and the routine groups in terms of adverse events ().
Table 3. Adverse events reported in included studies.
3.7. Sensitivity analysis
We performed a sensitivity analysis for the incidence of CA-AKI, the levels of eGFR, Scr, KIM-1, NGAL, BUN, Cys C, and IL-18, and all results except BUN level were not reversed, and the results were robust and reliable. Nine trials were included for the BUN level outcome indicator, and after excluding Liao K or Shi B, none of the remaining trials (eight) had statistically significant combined results that were inconsistent with the original combined results (MD = −1.30 mmol/L, 95% CI −2.33 to −0.28).
3.8. Publication bias
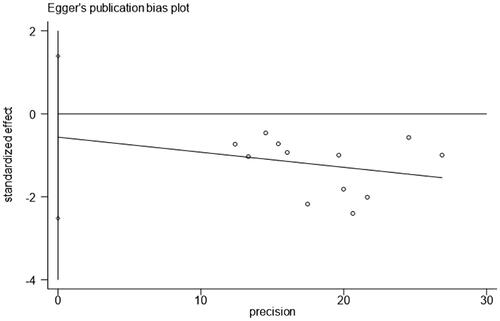
Egger’s test showed no publication bias for the incidence of CA-AKI () (t = −0.64, p value = .536).
3.9. Certainty of evidence
The GRADE tool was applied to assess the certainty of the evidence for incidence of CA-AKI, Scr level, and eGFR level. Most of the evidence were assessed as low or very low certainty ().
Table 4. Evidence summary of CA-AKI: FOSM products + RPP versus RPP.
4. Discussion
4.1. Summary of main findings
This study shows that FOSM products plus RPP (saline hydration and alprostadil) was more beneficial than RPP alone in reducing the incidence of CA-AKI and Scr level, increasing eGFR and SOD level, and reducing the levels of KIM-1, NGAL, BUN (results were not robust), Cys C, MDA, and IL-18. Subgroup analysis showed that Bailing plus RPP or Jinshuibao plus RPP demonstrated lower incidence of CA-AKI compared to RPP. However, there was no statistically significant difference between Zhiling plus RPP and RPP in the incidence of CA-AKI. In addition, as for the time to injection of the contrast, only when FOSM products were taken before the injection, it was superior to RPP in reducing the incidence of CA-AKI. The overall certainty of the evidence was assessed as low or very low.
FOSM products alone can also play a positive role in the prevention of CA-AKI compared to normal saline. In terms of the need for dialysis and adverse events, FOSM products and RPP had no statistical difference.
4.2. Comparison with other studies or reviews
FOSM products as an adjunct drug in the treatment of chronic kidney disease [Citation32], diabetic nephropathy [Citation52,Citation53], nephrotic syndrome [Citation54], kidney transplant [Citation55], etc., have been systematically reviewed. These reviews showed that FOSM products were effective and had good safety profiles. For example, a Cochrane systematic review [Citation32] showed FOSM products, as an adjuvant to RPP, can reduce Scr and proteinuria, and mitigate CKD-related complications such as elevated hemoglobin and serum albumin. A pharmacological review revealed that FOSM products contain many active ingredients, among which cordycepic acid, ergosterol, nucleosides, and peptides have been extracted; they are confirmed to possess various pharmacological actions, including anti-inflammatory, antioxidant, antitumor, antiapoptosis, immunomodulatory, and nephroprotective [Citation56,Citation57].
In our study, we found that RPP plus FOSM products can further improve SOD and MDA in the prevention of CA-AKI compared with RPP alone, indicating that FOSM products have an antioxidant effect [Citation58]. A previous systematic review showed that the use of antioxidant supplements (including N-acetyl cysteine, vitamin C, and vitamin E) reduced the risk of CA-AKI, suggesting that the pharmacological effects of FOSM products in the prevention of CA-AKI may contain antioxidants [Citation59].
4.3. Strengths and limitations
As far as we know, this is the first systematic review based on RCTs to assess the effectiveness and safety of FOSM products for the prevention of CA-AKI. The relevant clinical studies were comprehensively analyzed in terms of risk factors for CA-AKI, use of FOSM products, and the preventive effect and safety of FOSM products on CA-AKI. Finally, several novel biomarkers for mechanism related to renal injury (such as the levels of NGAL, Cys C, IL-18, etc.) were included, which could more sensitively reflect the early renal injury induced by contrast media and the nephroprotective effect of FOSM products compared with the traditional renal function indicators (such as the levels of Scr, BUN, eGFR, etc.).
However, there are several limitations. First, all included studies were conducted in China, and further international studies are needed to determine whether this evidence is equally applicable to other countries outside of China. Second, based on the current evidence, it cannot be concluded that Zhiling capsule can reduce the incidence of CA-AKI and Scr level. Jinshuibao capsule, Bailing capsule, and Zhiling capsule were fermented from different FOSM strains that may have some different active ingredients and pharmacological effects [Citation60]. Furthermore, studies have also shown that the quality of exclusively produced varieties such as Bailing capsule and Jinshuibao capsule is more stable, while the quality of Zhiling capsule produced by multiple manufacturers varies greatly between manufacturers, and even between samples from different batches from the same company [Citation60,Citation61]. Finally, the included trials had differences in clinical baseline characteristics, such as inclusion criteria, contrast medium dosage, patients’ baseline renal function, etc. We performed subgroup analysis to control some of these clinical baseline differences and used random-effect models to present outcomes more conservatively; nevertheless, we must be conscious that the accuracy of the pooled results might be influenced.
4.4. Inspiration for future research
In addition to focusing on the incidence of CA-AKI, we should follow up beyond 6 months when possible, looking at hospital stay and healing in patients needing dialysis in addition to renal outcomes. Future clinical trials should preregister their protocols and make them public to facilitate assessment of reporting bias and clearly disclose funding sources and any relevant conflicts of interest.
5. Conclusions
Currently, low certainty evidence suggests that preventive use of FOSM products as an adjuvant prevention was safe and might decrease the incidence of CA-AKI. Conducting trials with proper randomization, allocation concealment, double-blinding of patients and assessors, and larger sample sizes in the range of hundreds or thousands are needed to further improve the certainty of evidence.
Author contributions
Conceptualization: JP Liu and FL Pu. Methodology: FL Pu. Software: FL Pu and TL Li. Formal analysis: FL Pu. Data curation: FL Pu and TL Li. Writing – original draft: FL Pu. Writing – review and editing: JP Liu, TL Li, Q Xu, C Shen, YQ Wang, CM Tang, XW Zhang, and LJ Yan. Funding acquisition: JP Liu.
Ethical approval
This work did not require ethical approval as it does not involve any human or animal experiments.
Consent form
The author confirms that the work described has not been published before, that it is not under consideration for publication elsewhere, and that its publication has been approved by all coauthors.
Supplemental Material
Download PDF (550.8 KB)Acknowledgements
We gratefully acknowledge the assistance of Qihe Xu, Jianping Liu, and Nicola Robinson in reviewing and modifying the article.
Disclosure statement
All authors declare that there is no conflict of interest.
Data availability statement
The original data and material are available in this article/supplementary material and further enquiries can be directed to the authors.
Additional information
Funding
References
- McDonald JS, Hunt CH, Kolbe AB, et al. Acute adverse events following gadolinium-based contrast agent administration: a single-center retrospective study of 281 945 injections. Radiology. 2019;292(3):1–14. doi: 10.1148/radiol.2019182834.
- Morcos R, Kucharik M, Bansal P, et al. Contrast-induced acute kidney injury: review and practical update. Clin Med Insights Cardiol. 2019;13:1179546819878680. doi: 10.1177/1179546819878680.
- Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2016;32(2):247–255. doi: 10.1016/j.cjca.2015.05.013.
- Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9(8):1602–1613. doi: 10.1007/s003300050894.
- Cantais A, Hammouda Z, Mory O, et al. Incidence of contrast-induced acute kidney injury in a pediatric setting: a cohort study. Pediatr Nephrol. 2016;31(8):1355–1362. doi: 10.1007/s00467-016-3313-9.
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.cir.0000016043.87291.33.
- Rudnick MR, Goldfarb S, Tumlin J. Contrast-induced nephropathy: is the picture any clearer? Clin J Am Soc Nephrol. 2008;3(1):261–262. doi: 10.2215/CJN.04951107.
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275(19):1489–1494. doi: 10.1001/jama.1996.03530430033035.
- Jorgensen AL. Contrast-induced nephropathy: pathophysiology and preventive strategies. Crit Care Nurse. 2013;33(1):37–46. doi: 10.4037/ccn2013680.
- Rancic ZS. Commentary on ‘Contrast induced nephropathy and long-term renal decline after percutaneous transluminal angioplasty for symptomatic peripheral arterial disease’. Eur J Vasc Endovasc Surg. 2016;51(3):394. doi: 10.1016/j.ejvs.2015.12.013.
- Cardiology, Group of Interventional. Chinese society of cardiovascular diseases: Chinese guidelines for percutaneous coronary interventions (2016). Chin J Interv Cardiol. 2016;24(6):315.
- Hiremath S, Akbari A, Shabana W, et al. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLOS One. 2013;8(3):e60009. doi: 10.1371/journal.pone.0060009.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):C179–C184. doi: 10.1159/000339789.
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi: 10.1007/s00330-011-2225-0.
- Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389(10076):1312–1322. doi: 10.1016/S0140-6736(17)30057-0.
- Boucek P, Havrdova T, Oliyarnyk O, et al. Prevention of contrast-induced nephropathy in diabetic patients with impaired renal function: a randomized, double blind trial of sodium bicarbonate versus sodium chloride-based hydration. Diabetes Res Clin Pract. 2013;101(3):303–308. doi: 10.1016/j.diabres.2013.05.015.
- Hoste EA, De Waele JJ, Gevaert SA, et al. Sodium bicarbonate for prevention of contrast-induced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant. 2010;25(3):747–758. doi: 10.1093/ndt/gfp389.
- Meier P, Ko DT, Tamura A, et al. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009;7(1):23. doi: 10.1186/1741-7015-7-23.
- Isaka Y, Hayashi H, Aonuma K, et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol. 2020;24(1):1–44. doi: 10.1007/s10157-019-01750-5.
- Orlacchio A, Guastoni C, Beretta GD, et al. SIRM-SIN-AIOM: appropriateness criteria for evaluation and prevention of renal damage in the patient undergoing contrast medium examinations-consensus statements from Italian College of Radiology (SIRM), Italian College of Nephrology (SIN) and Italian Association of Medical Oncology (AIOM). Radiol Med. 2022;127(5):534–542. doi: 10.1007/s11547-022-01483-8.
- Bunel V, Qu F, Duez P, et al. Herbal medicines for acute kidney injury: evidence, gaps and frontiers. World J Tradit Chin Med. 2015;1(3):47–66. doi: 10.15806/j.issn.2311-8571.2015.0019.
- Anyu AT, Zhang WH, Xu QH. Cultivated cordyceps: a tale of two treasured mushrooms. Chin Med Cult. 2021;4(4):221–227. doi: 10.4103/CMAC.CMAC_41_21.
- Gan ZT, Shen C, Yao T. Content characteristics of trace elements in several Chinese wild cordyceps and artificial cordyceps. Int J Pharm Res. 2018;45(6):465–471.
- Han RC, Wu HT, Tao HP. 70 years of research and development of Cordyceps sinensis in China. J Appl Entomol. 2019;56(05):849–883.
- Ashraf SA, Elkhalifa A, Siddiqui AJ, et al. Cordycepin for health and wellbeing: a potent bioactive metabolite of an entomopathogenic cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules. 2020;25(12):2735. doi: 10.3390/molecules25122735.
- Liu Y, Wang J, Wang W, et al. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid Based Complement Alternat Med. 2015;2015:575063.
- Zhang J, Wen C, Duan Y, et al. Advance in Cordyceps militaris (Linn) link polysaccharides: isolation, structure, and bioactivities: a review. Int J Biol Macromol. 2019;132:906–914. doi: 10.1016/j.ijbiomac.2019.04.020.
- The Business Research Company, Cordyceps sinensis Global Market Report 2023; 2023 [cited 2023 Aug 18]. Available from: https://www.researchandmarkets.com/reports/5783073/cordyceps-sinensis-global-market-report
- Bee YO, Zoriah A. Efficacy of Cordyceps sinensis as an adjunctive treatment in hemodialysis patients: a systematic review and meta-analysis. J Tradit Chin Med. 2019;39(1):1–14.
- Hong T, Zhang M, Fan J. Cordyceps sinensis (a traditional Chinese medicine) for kidney transplant recipients. Cochrane Database Syst Rev. 2015;2015(10):CD009698.
- Liu W, Gao Y, Zhou Y, et al. Mechanism of Cordyceps sinensis and its extracts in the treatment of diabetic kidney disease: a review. Front Pharmacol. 2022;13:881835. doi: 10.3389/fphar.2022.881835.
- Zhang HW, Lin ZX, Tung YS, et al. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014;2014(12):CD008353.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160.
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from www.training.cochrane.org/handbook.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD.
- Xu C, Furuya-Kanamori L, Zorzela L, et al. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J Clin Epidemiol. 2021;135:70–78. doi: 10.1016/j.jclinepi.2021.02.012.
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from www.training.cochrane.org/handbook.
- Zhao K, Li YJ, Gao S, et al. Effect of Dongchongxiacao (cordyceps) therapy on contrast-induced nephropathy in patients with type 2 diabetes and renal insufficiency undergoing coronary angiography. J Tradit Chin Med. 2015;35(4):422–427.
- Zhao K, Li Y, Zhang H. Role of Dongchongxiacao (cordyceps) in prevention of contrast-induced nephropathy in patients with stable angina pectoris. J Tradit Chin Med. 2013;33(3):283–286. doi: 10.1016/s0254-6272(13)60165-x.
- Zhao K, Lin Y, Li YJ, et al. Efficacy of short-term Cordyceps sinensis for prevention of contrast-induced nephropathy in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Int J Clin Exp Med. 2014;7(12):5758–5764.
- Cao H, Liang G, Jia Y, et al. Clinical study on the prevention of contrast nephropathy after coronary angiography with Jin Shui Bao capsule. Chin Prim Health Care. 2015;29(10):100–101.
- Dong J, Mao Y. Effectiveness of Bailing capsules combined with prostil to prevent contrast nephropathy after coronary intervention. Int J Med Health. 2018;24(20):3153–3156.
- Li J, Zhu X, Lu Z. Analysis of the effectiveness of Bering capsules combined with prostil to prevent contrast nephropathy after coronary intervention. Inter Med. 2018;13(5):740–743.
- Liang G, Luo S, Jia Y, et al. Study on the effect of Jinshuibao capsule intervention in contrast nephropathy. Chin J Integr Chin West Med Emerg Med. 2015;22(4):422–423.
- Liao K, Yin W, Ou Q, et al. Study on the prevention of contrast nephropathy with Jinshubao capsules. J Xiangnan Coll (Med Ed). 2011;13(1):28–29.
- Shi B. Clinical study of Jinshuibao combined with hydration therapy for the prevention of contrast nephropathy in patients with renal insufficiency. J Guangzhou Med Univ. 2014;42(5):73–76.
- Song S. Effect of Jinshuibao capsule combined with prostaglandin on contrast nephropathy after coronary intervention. Pract Drugs Clin. 2017;20(11):1283–1287.
- Wang Y, Zhang S, Zheng Y, et al. Prevention of contrast nephropathy after percutaneous coronary intervention in patients with coronary artery disease by combining Renkang injection with Jinshubao capsule. Anhui Med. 2017;21(12):2288–2291.
- Xu H. Preventive and protective effects of Bailing capsules on renal damage by contrast agents. Master, Guangzhou University of Chinese Medicine; 2010.
- Zhang T, Chen S, Gong Z, et al. Clinical study on the prevention of contrast nephropathy after percutaneous coronary intervention with Jin Shui Bao capsule. Glob Chin Med. 2014;7(9):700–702.
- Zhou X. Study on the prevention and treatment of contrast nephropathy by combining kidney protective tablets with Zhi Ling Mycelium capsules. Master, Nanjing University of Traditional Chinese Medicine; 2021.
- Lu Q, Li C, Chen W, et al. Clinical efficacy of Jinshuibao capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:6806943. doi: 10.1155/2018/6806943.
- Luo Y, Yang SK, Zhou X, et al. Use of Ophiocordyceps sinensis (syn. Cordyceps sinensis) combined with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) versus ACEI/ARB alone in the treatment of diabetic kidney disease: a meta-analysis. Ren Fail. 2015;37(4):614–634. doi: 10.3109/0886022X.2015.1009820.
- Xu H, Li X, Yuan X, et al. A meta-analysis of the clinical efficacy and safety of Bailing capsules in the treatment of nephrotic syndrome. Ann Palliat Med. 2020;9(5):3170–3181. doi: 10.21037/apm-20-1252.
- Ong BY, Aziz Z. Efficacy of Cordyceps sinensis as an adjunctive treatment in kidney transplant patients: a systematic-review and meta-analysis. Complement Ther Med. 2017;30:84–92. doi: 10.1016/j.ctim.2016.12.007.
- Olatunji OJ, Tang J, Tola A, et al. The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316. doi: 10.1016/j.fitote.2018.05.010.
- Qu SL, Li SS, Li D, et al. Metabolites and their bioactivities from the genus Cordyceps. Microorganisms. 2022;10(8):1489. doi: 10.3390/microorganisms10081489.
- Abedi A, Ghobadi H, Sharghi A, et al. Effect of saffron supplementation on oxidative stress markers (MDA, TAC, TOS, GPx, SOD, and pro-oxidant/antioxidant balance): an updated systematic review and meta-analysis of randomized placebo-controlled trials. Front Med. 2023;10:1071514. doi: 10.3389/fmed.2023.1071514.
- Ali-Hasan-Al-Saegh S, Mirhosseini SJ, Ghodratipour Z, et al. Protective effects of anti-oxidant supplementations on contrast-induced nephropathy after coronary angiography: an updated and comprehensive meta-analysis and systematic review. Kardiol Pol. 2016;74(7):610–626. doi: 10.5603/KP.a2016.0007.
- Zhang P, Liu W, Zou QW, et al. Research progress on product quality evaluation and control of Cordyceps fermentum type. Chin J Pharm Sci. 2021;56(14):1118–1123.
- Zhang H, Li Y, Mi J, et al. GC-MS profiling of volatile components in different fermentation products of Cordyceps sinensis mycelia. Molecules. 2017;22(10):1800. doi: 10.3390/molecules22101800.