?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Arteriovenous fistula (AVF) dysfunction is a common complication in patients undergoing maintenance hemodialysis (MHD). Elevated serum levels of fibroblast growth factor 21 (FGF21) are associated with atherosclerosis and cardiovascular mortality. However, its association with vascular access outcomes remains elusive. The present study evaluated the relationship of serum FGF21 levels with AVF dysfunction and all-cause mortality in patients undergoing MHD.
Methods
We included patients undergoing MHD using AVF from January 2018 to December 2019. FGF21 concentration was detected using enzyme-linked immunosorbent assay. Patients were followed up to record two clinical outcomes, AVF functional patency loss and all-cause mortality. The follow-up period ended on April 30, 2022.
Results
Among 147 patients, the mean age was 58.49 ± 14.41 years, and the median serum level of FGF21 was 150.15 (70.57–318.01) pg/mL. During the median follow-up period of 40.83 months, the serum level of FGF21 was an independent risk factor for AVF functional patency loss (per 1 pg/mL increase, HR 1.002 [95% CI: 1.001–1.003, p = 0.003]). Patients with higher serum levels of FGF21 were more likely to suffer from all-cause mortality (per 1 pg/mL increase, HR 1.002 [95% CI: 1.000–1.003, p = 0.014]). The optimal cutoffs for FGF21 to predict AVF functional patency loss and all-cause mortality in patients undergoing MHD were 149.98 pg/mL and 146.43 pg/mL, with AUCs of 0.701 (95% CI: 0.606–0.796, p < 0.001) and 0.677 (95% CI: 0.595–0.752, p = 0.002), respectively.
Conclusions
Serum FGF21 levels were an independent risk factor and predictor for AVF functional patency loss and all-cause mortality in patients undergoing MHD.
Introduction
Maintenance hemodialysis (MHD) is the main treatment modality for end-stage kidney disease (ESKD), and well-functioning dialysis access is the primary requirement for hemodialysis treatment [Citation1]. The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines recommend an autologous arteriovenous fistula (AVF) as the preferred vascular access for hemodialysis [Citation2]. AVF dysfunction is a common complication associated with increased hospitalizations and mortality in patients undergoing MHD [Citation3]. The main cause of AVF dysfunction is intimal hyperplasia (IH), and its mechanisms include uremia, inflammation, shear stress and hypoxia [Citation4]. Diabetes, obesity, cardiovascular disease, peripheral vascular disease, and vascular calcification are also risk factors for AVF dysfunction [Citation5,Citation6]. However, there are no effective biomarkers for predicting AVF dysfunction.
Fibroblast growth factor 21 (FGF21) is a secreted protein that is involved in a variety of physiological processes, such as metabolic homeostasis, oxidative stress, inflammation, and atherosclerosis [Citation7]. Elevated levels of FGF21 are associated with coronary artery disease [Citation8], carotid atherosclerosis [Citation9], and inflammatory responses (sepsis and ARDS) [Citation10,Citation11]. Our previous studies indicated that FGF21 cooperated with parathyroid hormone to promote the trans-differentiation of endothelial cells into osteoblasts, which promoted vascular calcification (VC) in patients undergoing MHD [Citation12]. Due to the effects of FGF21 on oxidative stress, inflammation, calcification, and its pleiotropic metabolism, FGF21 may be an effective biomarker of AVF dysfunction. However, the relationship between serum FGF21 levels and AVF dysfunction in patients undergoing MHD has not been clarified.
Therefore, the present study investigated the predictive value of FGF21 for AVF dysfunction and all-cause mortality in patients undergoing MHD.
Methods
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Zhongda Hospital Affiliated to Southeast University (Ethical approval ID: 2019ZDKYSB191). The informed consent was waived because of the retrospective design of the study.
Study design and population
This study was a retrospective cohort study of patients undergoing MHD who started treatment in the Blood Purification Center of Zhongda Hospital Affiliated to Southeast University from January 1, 2018, to December 31, 2019. The following inclusion criteria were used for all participants: (1) aged 18 years or older; (2) ESKD, stable dialysis time ≥ 3 months. All selected patients were dialyzed 3 times per week for 4 h each session using bicarbonate dialysate; and (3) received hemodialysis via radiocephalic AVF. The following exclusion criteria were used: (1) coagulopathy or known ipsilateral central vein stenosis; (2) the presence of malignant disease; and (3) in the acute phase of infections or other acute illnesses.
Clinical and biochemical data collection
The general clinical data of the patients were collected, including age, sex, dialysis vintage, blood pressure before dialysis, height and dry weight, fractional clearance index for urea (Kt/V), concomitant diseases (e.g. diabetes, hypertension, and CVD) and current medications (e.g., calcium, cinacalcet, ACEI or ARB drugs). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automatic sphygmomanometer (Omron, Japan). Body mass index (BMI) was calculated using the following formula: BMI = (weight after dialysis)/height [Citation2]. Predialysis hemoglobin, albumin, uric acid, triglycerides, cholesterol, high-density lipoprotein, low-density lipoprotein, high-sensitivity C-reactive protein, serum calcium, serum phosphorus, parathyroid hormone, and brain natriuretic peptide were measured using routine laboratory methods.
Serum FGF21 measurement
A commercial ELISA kit was used for FGF21 determination (Neobioscience, China). Assays were performed as previously described, following the manufacturer’s instructions [Citation12]. The inter-assay and intra-assay coefficients of variation for FGF21 were less than 10%.
Follow-up and outcomes
All subjects were followed-up after baseline assessments until death or withdrawal from hemodialysis or to the end of the follow-up in this study (April 30, 2022). The primary endpoint event was the first episode of AVF functional patency loss. Functional patency was defined as the duration from access placement to the occurrence of thrombosis or any intervention needed to facilitate, maintain, or reestablish patency [Citation2,Citation13]. The secondary outcome was all-cause mortality. All patients finished the follow-up with complete baseline data.
Statistical analyses
Measurement data with a normal distribution are represented by ± s, and comparisons between two groups were performed using Student’s t-test. Non-normally distributed measurement data are represented as medians (interquartile range, IQR), and the Mann–Whitney U-test was used for comparisons between groups. Enumeration data are expressed as the number of cases and percentages, and comparisons between two groups were performed using the χ2 test. Kaplan–Meier survival curves and log-rank tests were used to compare differences in survival rates between the two groups. The Cox regression model was used to analyze the risk factors for AVF failure and all-cause death in patients with maintenance hemodialysis. Variables with p < 0.2 in the univariate Cox regression analysis were included in the multivariate Cox regression analysis. The receiver operating characteristic (ROC) curve was used to analyze the predictive ability of FGF21 on the risk of AVF failure and all-cause death in MHD patients. The area under the curve (AUC) and 95% CI were calculated. The optimal cutoff value for FGF21 was derived from the maximum Oden index (J), which was calculated as J = sensitivity + specificity − 1. A two-tailed test was used, and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 26.0, IBM Corp., Armonk, NY), MedCalc (version 15.0, Ostend, Belgium) and R (version 4.2.0, R Development Core Team, 2022) software.
Results
1. Clinical characteristics
A total of 147 patients undergoing MHD were included in this study. The demographic, clinical, and laboratory characteristics of the participants stratified according to median serum FGF21 level (150.15 pg/mL) are shown in . The mean age was 58.49 ± 14.41 years, and 58.50% were male. The median dialysis time was 3.30 (1.20–8.00) years. The prevalence of diabetes, hypertension, and cardiovascular disease was 31.97%, 93.88%, and 27.21%, respectively. The participants included in the analyses did not differ significantly between the low FGF21 (≤150.15 pg/mL, n = 74) group and the high FGF21 (>150.15 pg/mL, n = 73) group in sex, blood pressure level, BMI, hemoglobin, albumin, uric acid, triglyceride, cholesterol, high-density lipoprotein, low-density lipoprotein, high-sensitivity C-reactive protein, serum calcium, serum phosphorus, parathyroid hormone, BNP levels, or history of medication use. The mean age of the low FGF21 group was 55.04 ± 15.50 years, and the mean age of the high FGF21 group was 59.86 ± 13.44 years (p = 0.046).
Table 1. Baseline characteristics of patients undergoing maintenance hemodialysis according to median serum FGF21 level (150.15 pg/mL).
2. Relationship between serum FGF21 levels and AVF functional patency loss
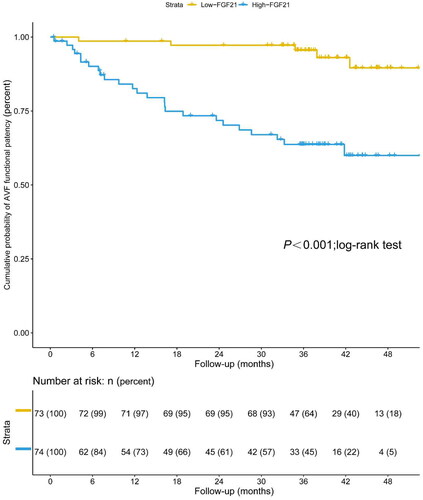
During a median follow-up of 40.83 months, 30 (20.41%) of the 147 participants using AVF experienced an access complication that required an intervention or replacement. Kaplan-Meier survival analysis demonstrated that patients undergoing MHD with serum FGF21 levels lower than 150.15 pg/mL (median value) had significantly higher AVF functional patency compared to serum FGF21 levels over 150.15 pg/mL (p < 0.001; log-rank test) (). The Cox multivariate analysis () showed an independent association between FGF21 and the occurrence of AVF functional patency loss. Each 1 pg/ml increase in FGF21 had a 1.002-fold higher risk of AVF functional patency loss (95% CI: 1.001–1.003, p = 0.003). Furthermore, the Cox multivariate analysis revealed that male gender (HR = 0.416, 95%CI: 0.196–0.884, p = 0.022) and calcium (HR = 0.378, 95%CI: 0.182–0.788, p = 0.009) were also independent predictors of AVF functional patency loss in patients undergoing MHD.
Figure 1. Kaplan–Meier estimate of AVF functional patency in patients undergoing maintenance hemodialysis with low levels of serum FGF21 (≤150.15 pg/mL) and high levels of serum FGF21 (>150.15 pg/mL) (p < 0.001; log-rank test).
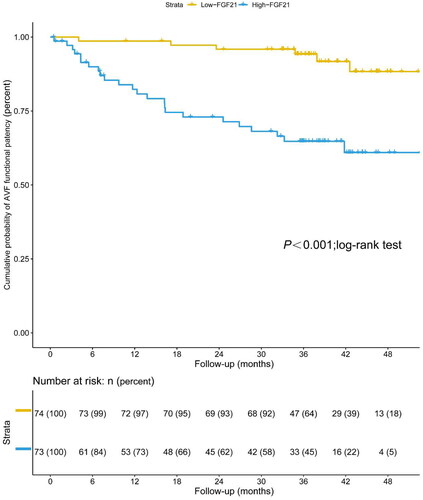
Table 2. Cox proportional hazard models on the risk of arteriovenous fistula failure in patients undergoing maintenance hemodialysis.
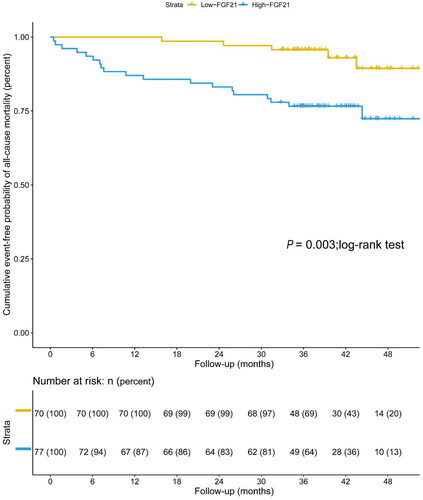
3. Relationship between serum FGF21 levels and all-cause mortality
The total incidence of all-cause mortality was 16.33% (24 of 147 participants), including 12 patients with cardiac death and 8 patients with infectious death during follow-up. Kaplan-Meier survival analysis demonstrated that patients undergoing MHD with serum FGF21 levels lower than 150.15 pg/mL (median value) had significantly higher overall survival compared to serum FGF21 levels over 150.15 pg/mL (p = 0.016; log-rank test) (). The Cox multivariate analysis () showed an independent association between FGF21 and all-cause mortality. Each 1 pg/ml increase in FGF21 had a 1.002-fold higher risk of all-cause mortality (95% CI: 1.000–1.003, p = 0.014). Furthermore, the Cox multivariate analysis revealed that diastolic blood pressure (HR = 0.946, 95%CI: 0.909–0.985, p = 0.006), total cholesterol (HR = 0.346, 95%CI: 0.172–0.695, p = 0.003), and BNP (HR = 1.000, 95%CI: 1.000–1.001, p = 0.017) were also independent predictors of all-cause mortality in patients undergoing MHD.
Figure 2. Kaplan–Meier estimate of overall survival in patients undergoing maintenance hemodialysis with low levels of serum FGF21 (≤150.15 pg/mL) and high levels of serum FGF21 (>150.15 pg/mL) (p = 0.016; log-rank test).
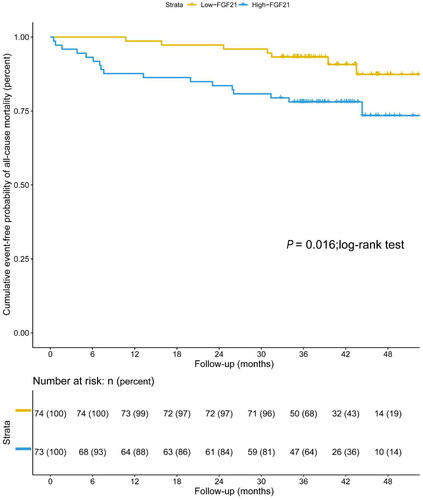
Table 3. Cox proportional hazard models on the risk of overall survival in patients undergoing maintenance hemodialysis.
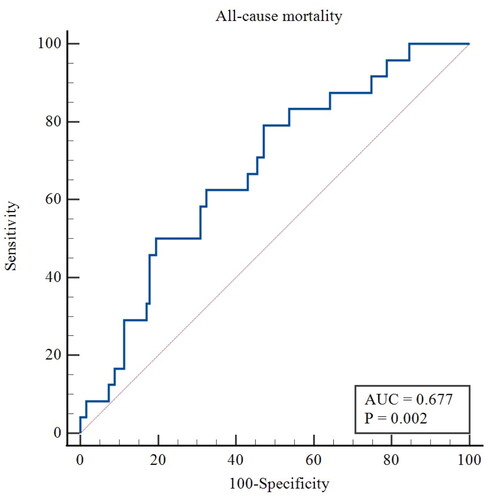
4. ROC curve analysis of the serum FGF21 levels in predicting AVF functional patency loss and all-cause mortality
For the primary outcome, the ROC curve showed that the AUC of FGF21 was 0.701 (95% CI 0.606–0.796, p < 0.001), with a sensitivity of 83.33% and a specificity of 58.12% for predicting functional patency in patients undergoing MHD. The optimal cutoff was 149.98 pg/mL (). Kaplan-Meier estimates showed significantly higher AVF functional patency for patients with serum FGF21 levels lower than 149.98 pg/mL compared to FGF21 concentrations over 149.98 pg/mL (p < 0.001; log-rank test) ().
Figure 3. ROC curve of the predictive value of FGF21 for AVF functional patency loss. AUC, area under the curve.
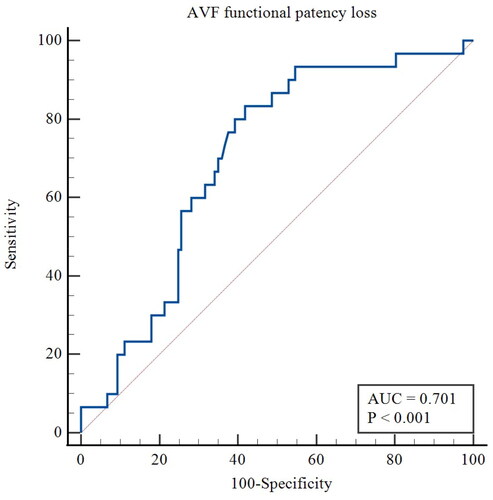
Figure 4. Kaplan–Meier estimate of AVF functional patency in patients undergoing maintenance hemodialysis with low levels of serum FGF21 (≤149.98 pg/mL) and high levels of serum FGF21 (>149.98 pg/mL) (p < 0.001; log-rank test).
For the secondary outcome, the ROC curve showed that the AUC of FGF21 was 0.677 (95% CI 0.595–0.752, p = 0.002), with a sensitivity of 79.17% and a specificity of 52.85% for predicting all-cause mortality in patients undergoing MHD. The optimal cutoff was 146.43 pg/mL (). Kaplan-Meier estimates showed a significantly higher overall survival for patients with serum FGF21 levels lower than 146.43 pg/mL compared to FGF21 concentrations over 146.43 pg/mL (p = 0.003; log-rank test) ().
Discussion
The present study indicated that higher serum FGF21 was an independent risk factor and predictor of AVF functional patency loss in patients undergoing MHD. Our study also confirmed an association between FGF21 and all-cause mortality in patients undergoing MHD. To the best of our knowledge, we are the first study to analyze the relationship of serum FGF21 levels with AVF functional patency in patients undergoing MHD.
Intimal hyperplasia is a key event in AVF dysfunction [Citation14]. Previous studies showed that pathogenic factors, such as inflammation, oxidative stress, shear stress, and puncture injury, induced an inflammatory cascade reaction, promoted the proliferation of smooth muscle cells, and caused these cells to migrate to the vascular intima, which led to neointimal hyperplasia (NIH) in AVF tissue [Citation15,Citation16]. Other potential factors, such as diabetes mellitus, cardiovascular disease, vascular calcification, and peripheral vascular disease, may also contribute to AVF dysfunction [Citation17]. FGF21 belongs to the superfamily of fibroblast growth factors (FGFs), and it is a stress-inducible endocrine hormone that plays a crucial role in oxidative stress and the inflammatory response [Citation18–20]. Previous study suggests that serum FGF21 may be a strong predictive factor for the progression of CKD [Citation21]. In addition, there is increasing evidence that FGF21 plays a role in a variety of vascular pathological processes in arterial diseases. The TNT study reported a 24% increase in the risk of cardiovascular (CV) events for every 1 standard deviation increase in FGF21 in patients treated with atorvastatin [Citation22]. Wu et al. [Citation23] found that baseline FGF21 was an independent predictor of atherosclerotic cardiovascular disease via a survey of patients with atherosclerosis, and the risk of atherosclerotic cardiovascular disease was three times higher in patients with high FGF21 levels compared to patients with low FGF21 levels. Our preliminary results confirmed that serum FGF21 was a potential predictor and promoter of VC in patients undergoing MHD [Citation12]. Because of the association of FGF21 with oxidative stress, inflammation, vascular calcification and atherosclerosis, it was reasonable to hypothesize that FGF21 was involved in the development of NIH and closely related to AVF functional patency loss.
Our current study, patients undergoing MHD with higher FGF21 levels had a relatively higher incidence of AVF dysfunction events. Univariate and multivariate Cox regression analyses revealed that FGF21 was the most important independent predictor of AVF functional patency loss. Our results suggest the potential application of FGF21 as a biomarker for predicting the occurrence of AVF dysfunction events in patients undergoing MHD. Therefore, early monitoring and intervention of FGF21 levels may be an important target in the treatment of AVF patency loss. Except for the role of FGF21 in AVF functional patency loss, the importance of gender in predicting access patency has been well-established, with females being more prone to patency loss [Citation24]. However, there is currently controversy regarding the relationship between serum calcium and AVF patency [Citation25,Citation26].
Our survival analysis showed that higher serum FGF21 levels correlated with higher all-cause mortality. The association between FGF21 and all-cause mortality in patients undergoing MHD remains controversial. Li et al. [Citation27] found that FGF21 was an independent risk factor for all-cause mortality in ESKD patients. Our study grouped by median and calculated the optimal cutoff value of FGF21 and analyzed its prognosis. Our results demonstrated that FGF21 was an independent predictor of all-cause mortality in patients undergoing hemodialysis. One recent study showed that FGF21 levels were not predictive of mortality in patients undergoing chronic hemodialysis [Citation28]. The reason for the discrepancy in results may be the different baseline data of samples in different studies. In our study, patients exhibiting elevated FGF21 levels were, on average, five years older than their counterparts with lower FGF21 levels. This age difference could potentially account for the observed increased mortality rate. More large-scale studies are needed to clarify the association between FGF21 and all-cause mortality and compare the prognostic value of FGF21 with other biomarkers. In addition, consistent with previous studies, lower diastolic blood pressure, lower total cholesterol, and BNP were associated with an increased risk of all-cause mortality in patients undergoing MHD [Citation29–31].
The observational and associated human data contrast sharply with the animal and cell culture experimental data. Previous in vitro and in vivo preclinical studies reported that FGF21 improved endothelial dysfunction [Citation32] and suppressed the proliferation of smooth muscle cells [Citation33]. Therefore, it remains unclear whether elevated serum FGF21 in patients undergoing MHD is a protective or deleterious response. There are several possible reasons to explain the increase in FGF21. First, several factors in CKD lead to dysregulation of FGF21 receptor signaling, which results in the downregulation of β-klotho or FGFR1c expression in target tissues to cause FGF21 resistance in target organs [Citation34]. Second, elevation of FGF21 is an adaptive response to pathological conditions, but it cannot counteract the damage caused by multiple noxious stimuli, such as uremia. Third, FGF21 may be a potential pathogenic agent that aggravates AVF failure and accelerates mortality because it is associated with calcification and CKD-mineral and bone disorders [Citation12,Citation35].
The study has the following limitations. (1) Our study was a single-center study. Therefore, the sample size was relatively insufficient, which limited our stratified analyses. (2) Due to the presence of inevitable clinical confounding factors, the discriminatory ability, as measured by the area under the curve (AUC), of FGF21 in predicting all-cause mortality was relatively limited. (3) Given the retrospective nature of this clinical study, the precise causal role of serum FGF21 in the observed outcome remained elusive. (4) FGF21 measurements were only taken once, and we were unable to capture its dynamic changes over time. Consequently, it is imperative to conduct multicenter studies involving larger sample sizes and longer follow-up periods to comprehensively evaluate the viability of FGF21 as a prognostic indicator for AVF functional patency loss and all-cause mortality in patients undergoing MHD. At the same time, considering the variability in serum FGF21 levels, individual dynamic monitoring of FGF21 in patients can be conducted in the future. Moreover, these investigations are crucial in elucidating the therapeutic potential of targeting FGF21 and its associated signaling pathways, thereby determining the feasibility of such interventions in providing clinical benefits.
Conclusion
Our study revealed that a higher serum level of FGF21 in patients undergoing MHD was associated with a higher risk of AVF functional patency loss and all-cause mortality. Therefore, FGF21 may serve as a promising novel biomarker for risk stratification and guide for patient management.
Acknowledgement
This project has already been published in Abstract format. The paper titled “Fibroblast Growth Factor 21 Predicts Arteriovenous Fistula Functional Patency Loss and Mortality in Patients Undergoing Maintenance Hemodialysis (https://academic.oup.com/ndt/article/38/Supplement_1/gfad063a_5528/7195376)” is a conference abstract presented on the 60th ERA Congress (Milan and Virtual, Italy, 15/06/2023-18/06/2023).
Author contributions
X-HH, HL and BW were responsible for the design and concept. X-HH developed the first draft of the manuscript. HD made modifications and improvements to the manuscript. QW, R-XC, W-TZ, L-QJ, JW, H-FL and J-YC were responsible for the statistical analysis. HL and BW were responsible for the guidance and review of the thesis. All authors revised the final manuscript and made suggestions.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Zhongda Hospital Affiliated to Southeast University (Ethical approval ID: 2019ZDKYSB191). The informed consent was waived because of the retrospective design of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Mousavi SF, Sepehri MM, Khasha R, et al. Improving vascular access creation among hemodialysis patients: an agent-based modeling and simulation approach. Artif Intell Med. 2022;126:1. doi: 10.1016/j.artmed.2022.102253.
- Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4):S1–10. doi: 10.1053/j.ajkd.2019.12.001.
- Lawson JH, Niklason LE, Roy-Chaudhury P. Challenges and novel therapies for vascular access in haemodialysis. Nat Rev Nephrol. 2020;16(10):586–602. doi: 10.1038/s41581-020-0333-2.
- Barcena AJR, Perez JVD, Liu O, et al. Localized perivascular therapeutic approaches to inhibit venous neointimal hyperplasia in arteriovenous fistula access for hemodialysis use. Biomolecules. 2022;12(10):1367. doi: 10.3390/biom12101367.
- Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the achilles’ heel. Nat Rev Nephrol. 2013;9(6):348–357. doi: 10.1038/nrneph.2013.76.
- Vazquez-Padron RI, Allon M. New insights into dialysis vascular access: impact of preexisting arterial and venous pathology on AVF and AVG outcomes. Clin J Am Soc Nephrol. 2016;11(8):1495–1503. doi: 10.2215/CJN.01860216.
- Yang M, Liu C, Jiang N, et al. Fibroblast growth factor 21 in metabolic syndrome. Front Endocrinol (Lausanne). 2023;14:1220426. doi: 10.3389/fendo.2023.1220426.
- Li Q, Zhang Y, Ding D, et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J Clin Endocrinol Metab. 2016;101(12):4886–4894. doi: 10.1210/jc.2016-2308.
- Tan H, Yue T, Chen Z, et al. Targeting FGF21 in cardiovascular and metabolic diseases: from mechanism to medicine. Int J Biol Sci. 2023;19(1):66–88. doi: 10.7150/ijbs.73936.
- Yan F, Yuan L, Yang F, et al. Emerging roles of fibroblast growth factor 21 in critical disease. Front Cardiovasc Med. 2022;9:1053997. doi: 10.3389/fcvm.2022.1053997.
- Li X, Shen H, Zhou T, et al. Does an increase in serum FGF21 level predict 28-day mortality of critical patients with sepsis and ARDS? Respir Res. 2021;22(1):182. doi: 10.1186/s12931-021-01778-w.
- Jiang L, Yin Q, Yang M, et al. Fibroblast growth factor 21 predicts and promotes vascular calcification in haemodialysis patients. Kidney Dis (Basel). 2021;7(3):227–240. doi: 10.1159/000512750.
- Aitken E, Kearns R, Gaianu L, et al. Long-Term functional patency and cost-effectiveness of arteriovenous fistula creation under regional anesthesia: a randomized controlled trial. J Am Soc Nephrol. 2020;31(8):1871–1882. doi: 10.1681/ASN.2019111209.
- Feng S, Peden EK, Guo Q, et al. Downregulation of the endothelial histone demethylase JMJD3 is associated with neointimal hyperplasia of arteriovenous fistulas in kidney failure. J Biol Chem. 2022;298(5):101816. doi: 10.1016/j.jbc.2022.101816.
- Chang CJ, Lai YJ, Tung YC, et al. Osteopontin mediation of disturbed flow-induced endothelial mesenchymal transition through CD44 is a novel mechanism of neointimal hyperplasia in arteriovenous fistulae for hemodialysis access. Kidney Int. 2023;103(4):702–718. doi: 10.1016/j.kint.2022.12.022.
- Ruan L, Yao X, Li W, et al. Effect of galectin-3 in the pathogenesis of arteriovenous fistula stenosis formation. Ren Fail. 2021;43(1):566–576. doi: 10.1080/0886022X.2021.1902822.
- Baek J, Lee H, Yang T, et al. Plasma interleukin-6 level predicts the risk of arteriovenous fistula dysfunction in patients undergoing maintenance hemodialysis. J Pers Med. 2023;13(1):151. doi: 10.3390/jpm13010151.
- Chen Z, Yang L, Liu Y, et al. The potential function and clinical application of FGF21 in metabolic diseases. Front Pharmacol. 2022;13:1089214. doi: 10.3389/fphar.2022.1089214.
- Jin L, Geng L, Ying L, et al. FGF21-Sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation. 2022;146(20):1537–1557. doi: 10.1161/CIRCULATIONAHA.122.059631.
- Li X, Zhu Z, Zhou T, et al. Early increases in serum FGF21 levels predict mortality of septic patients. Cytokine. 2018;111:428–433. doi: 10.1016/j.cyto.2018.05.020.
- Yong G, Li L, Hu S. Fibroblast growth factor 21 may be a strong biomarker for renal outcomes: a meta-analysis. Ren Fail. 2023;45(1):2179336.
- Ong KL, Hui N, Januszewski AS, et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the treating to new targets [TNT] study). Metabolism. 2019;93:93–99. doi: 10.1016/j.metabol.2018.11.006.
- Wu L, Qian L, Zhang L, et al. Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events. J Am Heart Assoc. 2020;9(11):e015226. doi: 10.1161/JAHA.119.015226.
- Lee T, Qian J, Thamer M, et al. Gender disparities in vascular access surgical outcomes in elderly hemodialysis patients[J]. Am J Nephrol. 2019;49(1):11–19. doi: 10.1159/000495261.
- Gardezi AI, Karim MS, Rosenberg JE, et al. Markers of mineral metabolism and vascular access complications: the choices for healthy outcomes in caring for ESRD (CHOICE) study[J]. Hemodial Int. 2020;24(1):43–51. doi: 10.1111/hdi.12798.
- Yap Y-S, Ting K-T, Chi W-C, et al. Aortic arch calcification as a predictor of repeated arteriovenous fistula failure within 1-Year in hemodialysis patients[J]. Biomed Res Int. 2017;2017:6728437–6728439. doi: 10.1155/2017/6728437.
- Li M, Jiang LQ, Zhang MY, et al. Elevated serum FGF21 is an independent predictor for adverse events in hemodialysis patients from two large centers: a prospective cohort study. Ren Fail. 2023;45(2):2256414.
- Chiu L-T, Wang C-H, Lin Y-L, et al. Association of serum fibroblast growth factor 21 levels with skeletal muscle mass and mortality in chronic hemodialysis patients. J Formos Med Assoc. 2022;121(12):2481–2489. doi: 10.1016/j.jfma.2022.05.007.
- Khan YH, Sarriff A, Adnan AS, et al. Blood pressure and mortality in hemodialysis patients: a systematic review of an ongoing debate. Ther Apher Dial. 2016;20(5):453–461. doi: 10.1111/1744-9987.12406.
- Yap Y-S, Chi W-C, Lin C-H, et al. Association of early failure of arteriovenous fistula with mortality in hemodialysis patients. Sci Rep. 2021;11(1):5699. doi: 10.1038/s41598-021-85267-6.
- Geerse DA, Van Berkel M, Vogels S, et al. Moderate elevations of high-sensitivity cardiac troponin I and B-type natriuretic peptide in chronic hemodialysis patients are associated with mortality[J]. Clin Chem Lab Med. 2013;51(6):1321–1328. doi: 10.1515/cclm-2012-0305.
- Yao D, He Q, Sun J, et al. FGF21 attenuates hypoxia‑induced dysfunction and inflammation in HPAECs via the microRNA‑27b‑mediated PPARγ pathway. Int J Mol Med. 2021;47(6):116. doi: 10.3892/ijmm.2021.4949.
- Wei W, Li X-X, Xu M. Inhibition of vascular neointima hyperplasia by FGF21 associated with FGFR1/syk/NLRP3 inflammasome pathway in diabetic mice. Atherosclerosis. 2019;289:132–142. doi: 10.1016/j.atherosclerosis.2019.08.017.
- Suassuna P, de Paula RB, Sanders-Pinheiro H, et al. Fibroblast growth factor 21 in chronic kidney disease. J Nephrol. 2019;32(3):365–377. doi: 10.1007/s40620-018-0550-y.
- Salgado JV, Goes MA, Salgado Filho N. FGF21 and chronic kidney disease. Metabolism. 2021;118:154738. doi: 10.1016/j.metabol.2021.154738.