Abstract
The lack of early renal function recovery among geriatric patients with acute kidney injury (AKI) in the intensive care unit (ICU) is a commonly observed and acknowledged poor prognostic factor, especially for older adults. However, no reliable prognostic biomarker is available for identifying individuals at risk of renal non-recovery or mortality in older adults. In this prospective observational cohort study, we enrolled critically ill older adults (aged ≥ 60 years) with AKI from the ICU and followed their disease progression. The primary endpoint was renal non-recovery within seven days of follow-up, while the secondary endpoint was the determinants of 30-day mortality after AKI. We assessed the predictive accuracy using receiver operating characteristic curves and performed between-group comparisons using the log-rank test. Among 209 older adults, 117 (56.0%) experienced renal recovery. Multiple regression analysis revealed that urine levels of tissue inhibitor of metalloproteinase-2 (TIMP-2) multiplied by insulin-like growth factor-binding protein 7 (IGFBP7) ([TIMP-2]*[IGFBP7]), AKI stages 2–3, and the Acute Physiology and Chronic Health Evaluation (APACHE II) score were independently associated with renal non-recovery. The regression model incorporating [TIMP-2]*[IGFBP7] demonstrated a fair predictive value (AUC 0.774, p < 0.001), with the optimal threshold set at 0.81 (ng/mL)2/1000. When [TIMP-2]*[IGFBP7] was combined with AKI severity and the APACHE score, the AUC increased to 0.851. In conclusion, urine [TIMP-2]*[IGFBP7] is a reliable biomarker associated with renal non-recovery in critically ill older adults, and its predictive efficacy can be further enhanced when combined with AKI severity and the APACHE score.
Introduction
The past century has witnessed significant increases in human longevity, leading to a substantial rise in the elderly population over the next two decades [Citation1]. Consequently, there is an increasing prevalence of elderly patients in intensive care units (ICUs) [Citation2]. In the Asian-Pacific region, the World Health Organization (WHO) defines older adults as individuals above the age of 60, further subdivided into the old (60–74 years) and very old (75 years and older) populations [Citation3]. Currently, China has the highest number of older adults worldwide, with statistical data indicating that the proportion of individuals aged ≥60 was 18.1% by the end of 2019 [Citation4]. This number is projected to continue expanding.
Acute kidney injury (AKI) is more common in older adults than in younger individuals. Several studies demonstrated that older adults (aged ≥60 years) have a higher incidence of AKI than their younger counterparts [Citation5–9]. The increased risk of AKI development in this population can be attributed to multiple comorbidities, polypharmacy, and age-related changes in kidney function (‘kidney aging’) [Citation10]. Older adults are more prone to structural and functional degeneration [Citation11], resulting in a poorer prognosis [Citation12,Citation13]. Despite the emergence of therapeutics for AKI, cases of renal non-recovery, poor prognosis, and a high risk of chronic kidney disease (CKD), end-stage renal disease (ESRD) requiring renal replacement therapy (RRT), and death continue to occur [Citation14–17]. This failure-to-recover phenomenon, known as renal non-recovery, is widespread among older adults. Previous studies have shown that approximately one-third of older adults with AKI fail to recover [Citation18], with even lower recovery rates among those in the ICU. Consequently, early intervention for renal non-recovery can potentially improve AKI prognosis [Citation19]. Similarly, renal recovery and patient survival are crucial considerations for short- and long-term prognosis in AKI patients. Given that the long-term prognosis of older adults with multiple chronic diseases and AKI can be poor and influenced by numerous factors [Citation20,Citation21], short-term events such as renal recovery and 7- or 30-day mortality may provide more practical insights. An instrumental factor contributing to the high mortality rate among patients with AKI is the inability to determine renal recovery possibility during clinical practice. This can lead to delayed provision of appropriate care, and irreversible kidney damage may have occurred. Therefore, accurate prediction of renal function recovery in the early stages of AKI is essential, as it informs us the window during which effective interventions against AKI can be administered, including optimizing fluid management and hemodynamic support, avoiding nephrotoxic drugs, and controlling hyperglycemia.
The incidence of AKI is significantly higher in older critically ill patients, and their prognosis tends to be worse than younger ones [Citation22]. However, limited researches exist regarding the validity and utility of AKI biomarkers in this population. There is an urgent need to identify accurate biomarkers for detecting renal recovery in older adults with AKI. We designed the current study focusing on renal recovery in older adults to address this research gap.
Urinary tissue inhibitors of metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) are markers of renal tubular cell injury that increase in response to AKI. They can assist in detecting kidney injury independent of serum creatinine changes. Prior studies have demonstrated that [TIMP-2]*[IGFBP7] (product of TIMP2 and IGFBP7) levels are significantly elevated in patients with AKI, and their predictive ability outperforms that of individual biomarkers alone [Citation23]. In addition, others have demonstrated the potential of [TIMP-2]*[IGFBP7] in assessing renal recovery status [Citation24,Citation25]. Therefore, the current study aimed to investigate whether [TIMP-2]*[IGFBP7] is a reliable predictor of renal non-recovery in older adults with AKI over seven days. We also aimed to analyze the risk factors associated with renal non-recovery and 30-day mortality following AKI in these patients.
Materials and methods
Study design
We conducted this prospective cohort study between 1 July 2019, and 31 December 2021, in the ICUs of Beijing Chao-yang Hospital and Beijing Lu-he Hospital. This study was approved by the Human Ethics Committee of Beijing Chao-yang Hospital from Capital Medical University, Beijing, China (ethics No. 2018-117). All participants provided informed consent.
Study population
This study was conducted in two intensive care units (ICUs) in different tertiary hospitals in China. Older adults admitted to these ICUs were screened for inclusion between October 2020 and May 2022. Only patients who developed AKI during their ICU stay were prospectively and consecutively enrolled. Exclusion criteria included: (1) patients below 60 years old; (2) those who already had AKI before being admitted to ICUs; (3) those with preexisting CKD prior to ICU admission; (4) those with an ICU stay of less than or equal to 24 h; and (5) those unable to provide sufficient urine samples for testing.
Measurements and data collection
We collected urine specimens at AKI occurrence, which were then centrifugated at a speed of 3000 revolutions per minute for 10 min. After centrifugation, the supernatant was obtained and stored at −80 °C for further analysis. TIMP-2 and IGFBP7 levels were detected using the NephroCheck® Assay (Astute Medical, San Diego, CA). NephroCheck® is a commercially available product that combines two urinary biomarkers, [TIMP-2] and [IGFBP7], expressed as [TIMP-2]*[IGFBP7] in ng/mL2/1000.
We also collected serum creatinine (Scr) during patients’ ICU admission, every 12 h after that, up to 7 days after AKI, as well as their hourly urine output (UO) during their ICU stay. We obtained patients’ disease severity scores, including Acute Physiology and Chronic Health Evaluation (APACHE II) and sequential organ failure assessment (SOFA) scores during the first 24 h of ICU admission. We further recorded data regarding their comorbidities, the cause of AKI, their baseline renal function, RRT in ICU, the Scr upon leaving ICU, the length of ICU and hospital stay, and Scr at discharge. In addition, we followed up with them to document their 30-day mortality rate after AKI occurrence.
Definitions
The diagnosis of AKI was made based on the changes in Scr levels or urine output, as suggested by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines. AKI was diagnosed if any of the following criteria were met: (1) increase in Scr levels by 0.3 mg/dL (26.5 µmol/L) or more within 48 h; (2) increase in Scr levels to 1.5 times or more of their baseline level, known or suspected to have occurred in the past 7 days; (3) urine output less than 0.5 mL/kg/h for more than 6 h [Citation26]. Baseline creatinine levels were determined as follows: if more than 5 values were available within 6 months to 7 days prior to admission, the median of all available values was used. Otherwise, the lowest value obtained 7 days before admission was used. If the baseline glomerular filtration rate (GFR) was assumed to be 75 mL/min/1.73 m2, the missing baseline creatinine was estimated using the Modification of Diet in Renal Disease formula [Citation27]. CKD was defined as an estimated GFR (eGFR) of less than 60 mL/min/1.73 m2 for at least three months, according to the National Kidney Foundation [Citation28].
Renal recovery was defined as the absence of any stage of AKI according to either Scr or urine output criteria [Citation29]. For instance, patients classified as renal recovery needed to demonstrate a decrease in Scr to below 150% of their baseline and without signs of oliguria (urine output less than 0.5 mL/kg/h) for more than 6 h. Patients requiring RRT on day 7 or who passed away within 7 days after developing AKI were classified as non-recovery cases.
Comorbidities, such as hypertension, diabetes, cerebrovascular disease, cardiovascular disease (myocardial infarct or congestive heart failure), chronic obstructive pulmonary disease (COPD), or chronic liver disease, were identified based on the criteria proposed by Charlson and colleagues [Citation30,Citation31].
Study endpoints
The primary endpoint of this study was a failure to recover from AKI within 7 days. The secondary endpoints were the length of stay in ICU and during hospitalization, in-hospital and 30-day mortality.
Statistical analysis
Continuous variables were presented in medians with 25th and 75th percentiles reported. Categorical variables were described in numbers with percentages. Mann–Whitney U-tests were used to compare two groups if data were non-normally distributed. Pearson’s chi-squared or Fisher’s exact tests were used for categorical variables. We used logistic regression analyses to identify independent factors associated with renal non-recovery among older adults with AKI in ICUs. Variables with statistically significant differences between groups were subsequently included in multiple regression analyses, including [TIMP-2]*[IGFBP7], APACHE II score, and the presence of stages AKI 2 to 3. We further used receiver-operating characteristics (ROC) curves to evaluate model predictive ability, with the Youden’s test harnessed to determine the optimal threshold for analysis, followed by obtaining the associated sensitivity and specificity values. We also compared survival curves using the Mantel (or log-rank) test. p-Values lower than 0.05 indicated statistical significance. We performed all analyses using SPSS statistics version 24 (IBM, Chicago, IL) and MedCalc® software version 17.9.7 (Ostend, Belgium).
Results
Participants’ clinical characteristics and outcomes
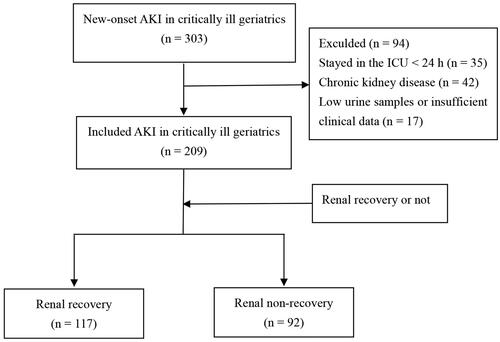
The flow chart of the study is presented in . Among the 303 older adults (aged 60 years or older) with AKI admitted to ICUs between 1 July 2019 and 1 December 2021, a total of 209 individuals who stayed in the ICU for more than 24 h met the inclusion criteria. Of these participants, 92 (44.0%) experienced renal non-recovery. Those with renal non-recovery had a higher likelihood of requiring vasopressor treatment (non-recovery vs. recovery, 39.1% vs. 21.4%, p = 0.005) and RRT in ICUs (28.3% vs. 9.4%, p < 0.05). Additionally, the non-recovery group exhibited higher disease severities but lower mean arterial pressure (MAP) (both p < 0.05) (). We also compared clinical features between participants aged ≥75 and <75, and no significant differences were observed between the groups (supplemental Table S1).
Table 1. Clinical characteristics of older adults with acute kidney injury in intensive care units.
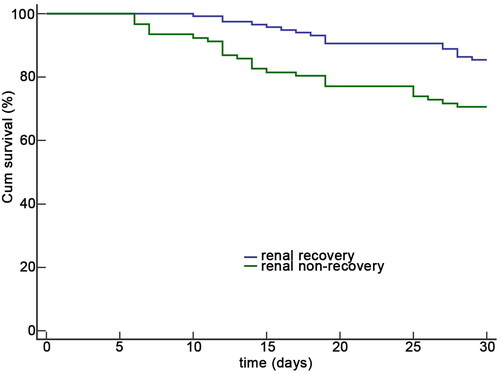
Regarding outcome comparison, we found that older adults with renal non-recovery had longer ICU stays (8 vs. 6 days, p = 0.029) and hospitalizations (23 vs. 19 days, p = 0.041) than those with renal recovery. Furthermore, participants with renal non-recovery experienced higher in-hospital mortality (27.2% vs. 14.5%, p = 0.024) and 30-day mortality (29.3% vs. 14.5%, p = 0.009) compared to those with recovery (). Kaplan-Meier analysis also demonstrated lower survival among participants with renal non-recovery (p = 0.005) ().
Analysis of the risk of renal non-recovery
We conducted further analysis to identify factors associated with renal non-recovery among older adults with AKI in ICUs. Univariate logistic regression analysis revealed significant differences between groups in various variables, including urinary [TIMP-2]*[IGFBP7], APACHE II score, nonrenal SOFA score, MAP, serum lactate, presence of AKI stages 2–3, requirement of vasopressor treatment, and PaO2/FiO2 ratio. Subsequently, multiple logistic regression analysis was performed to assess the risk factors for renal non-recovery. The results indicated that higher urinary [TIMP-2]*[IGFBP7] (odds ratio [OR] 1.747, 95% confidence interval [CI] 1.350–2.261, p < 0.001), higher APACHE II score (OR 1.056, 95% CI 1.007–1.108, p = 0.028), and the presence of stages 2 to 3 AKI (OR 5.954, 95% CI 2.827–12.544, p < 0.001) were associated with an increased risk of renal non-recovery ().
Table 2. Risk factors associated with renal non-recovery among participants.
Improving the risk estimation for renal non-recovery from AKI
During the logistic regression analysis incorporating urinary [TIMP-2]*[IGFBP7], we found that the associated area under ROC curves (AUCs) was 0.774 (95% CI 0.711–0.829, p < 0.001), with an optimal threshold value determined at 0.81 (ng/mL)2/1000. The AUC for predicting renal non-recovery increased to 0.851 (95% CI 0.795–0.896, p < 0.001) if we combined [TIMP-2]*[IGFBP7] with APACHE II score and the presence of AKI stages 2–3 (p < 0.05 by Delong’s test) (supplemental Table S2). We also presented the assessment results involving the predicted value of urinary biomarkers in and the associated ROC curves in . Multiple logistic regression revealed that higher urinary [TIMP-2]*[IGFBP7], higher APACHE II score, and the presence of AKI stages 2–3 were independent risk factors of renal non-recovery.
Figure 3. The predictive value of urinary biomarkers and the associated model. The ROC curves of urinary [TIMP-2]*[IGFBP7] and the associated model for predicting renal non-recovery from AKI. The AUC of using urinary [TIMP-2]*[IGFBP7] only, clinical prediction model only, and the [TIMP-2]*[IGFBP7] combined with clinical prediction model. ROC: receiver operating characteristic; AUC: area under the ROC.
![Figure 3. The predictive value of urinary biomarkers and the associated model. The ROC curves of urinary [TIMP-2]*[IGFBP7] and the associated model for predicting renal non-recovery from AKI. The AUC of using urinary [TIMP-2]*[IGFBP7] only, clinical prediction model only, and the [TIMP-2]*[IGFBP7] combined with clinical prediction model. ROC: receiver operating characteristic; AUC: area under the ROC.](/cms/asset/5f6be0b9-8d17-4721-8369-11e824bf5aee/irnf_a_2304099_f0003_c.jpg)
Table 3. Biomarkers and combination models for predicting non-recovery from AKI.
A sub-analysis based on age stratum was also performed. Among those with age between 60 and 74 years, the model incorporating urinary [TIMP-2]*[IGFBP7] had an AUC of 0.784 (95% CI 0.700–0.854, p < 0.01), with an optimal threshold at 0.34 (ng/mL)2/1000. Among those with age 75 years and older, the model incorporating urinary [TIMP-2]*[IGFBP7] similarly had an AUC of 0.763 (95% CI 0.660–0.847, p < 0.01), with an optimum threshold at 0.86 (ng/mL)2/1000. The relevant results and ROC curves are shown in supplemental Table S3, respectively.
Predictors of 30-day mortality
We further illustrated the ROC curve using urinary [TIMP-2]*[IGFBP7] to predict 30-day mortality among participants (). The model incorporating urinary [TIMP-2]*[IGFBP7] had an AUC < 0.7. According to the threshold value derived above, participants were divided into a high ([TIMP-2]*[IGFBP7] ≥ 0.81) and a low ([TIMP-2]*[IGFBP7] < 0.81) group, and each group was further subdivided according to APACHE II score and the presence of AKI stages 2 to 3 (high vs. low). Kaplan-Meier curves revealed that the high urinary [TIMP-2]*[IGFBP7] levels (p = 0.013) and the high urinary [TIMP-2]*[IGFBP7] with high APACHE II + AKI stages 2 to 3 (p < 0.001) groups had significantly lower survival compared to those with low urinary [TIMP-2]*[IGFBP7] levels and with low urinary [TIMP-2]*[IGFBP7] as well as low APACHE II + AKI stages 2–3, respectively. The associated ROC curves are shown in .
Figure 4. The predictive value of biomarkers for 30-day mortality. The ROC curves of urinary [TIMP-2]*[IGFBP7] for predicting 30-day mortality after AKI. ROC: receiver operating characteristic; AUC: area under the ROC.
![Figure 4. The predictive value of biomarkers for 30-day mortality. The ROC curves of urinary [TIMP-2]*[IGFBP7] for predicting 30-day mortality after AKI. ROC: receiver operating characteristic; AUC: area under the ROC.](/cms/asset/726fee0c-a0d9-44f8-a1fe-d5feb260f792/irnf_a_2304099_f0004_c.jpg)
Figure 5. Kaplan-Meier survival curves according to the cutoff value. Survival analysis regarding 30-day mortality using the Kaplan-Meier technique according to the cutoff value. (a) Survival curves of participants with urinary [TIMP-2]*[IGFBP7] ≥ 0.81 and those with < 0.81. (b) Survival curves of participants with high urinary [TIMP-2]*[IGFBP7] and high clinical risk model (≥ 0.50) and those with low clinical risk (< 0.50).
![Figure 5. Kaplan-Meier survival curves according to the cutoff value. Survival analysis regarding 30-day mortality using the Kaplan-Meier technique according to the cutoff value. (a) Survival curves of participants with urinary [TIMP-2]*[IGFBP7] ≥ 0.81 and those with < 0.81. (b) Survival curves of participants with high urinary [TIMP-2]*[IGFBP7] and high clinical risk model (≥ 0.50) and those with low clinical risk (< 0.50).](/cms/asset/82458d81-7715-4962-b8fe-7ac4830af627/irnf_a_2304099_f0005_c.jpg)
Discussion
Previous studies have highlighted the heightened risk of AKI in older adults compared to the general population in China. Wei et al. demonstrated that more than half of hospitalized AKI patients were 60 years or older [Citation32]. Despite advancements in treatment, the prognosis for older adults with AKI remains poor. Moreover, there is a lack of comprehensive information regarding renal non-recovery in older adults with AKI. Hence, this study aimed to identify significant factors associated with renal non-recovery in older patients with AKI. Another reason underscoring the importance of this study is the time frame allowed by the KDIGO criteria for AKI, which permits a 7-day window for establishing the diagnosis. Identifying comorbidities and patients requiring prolonged mechanical ventilation can reduce hospital stay length. Notably, delayed recognition of renal non-recovery at hospital discharge can diminish the opportunity for optimal renal care. Therefore, we defined renal non-recovery within 7 days of AKI onset. Our findings revealed that urinary [TIMP-2]*[IGFBP7], APACHE II score, and the presence of AKI stages 2–3 were independent predictive factors for renal non-recovery. Furthermore, the predictive efficacy was enhanced when urinary [TIMP-2]*[IGFBP7] was combined with the APACHE II score and the presence of AKI stages 2–3. Additionally, the 30-day mortality rate was higher in the renal non-recovery group compared to the recovery group. Notably, when urinary [TIMP-2]*[IGFBP7] exceeded 0.81 in combination with a high APACHE II score and the presence of AKI stages 2–3, patients exhibited a higher 30-day mortality. These findings suggest that renal non-recovery status could be an important endpoint for future research to improve short-term outcomes in critically ill older adults.
TIMP-2 and IGFBP7 are cell cycle arrest markers in the G1 phase, and their expression levels increase in renal tubular cells under stress. These biomarkers were recently discovered, and their utility was confirmed in a multicenter study, demonstrating their ability to predict the likelihood of developing AKI stages 2 or 3 within 12 h among high-risk patients [Citation27]. Kane-Gill et al. [Citation33] also reported that urinary [TIMP-2]*[IGFBP7] can help identify the risk of AKI. Hence, we hypothesized that urinary [TIMP-2]*[IGFBP7] might be associated with AKI outcomes in older adults. Dewitte et al. revealed that [TIMP-2][IGFBP7] at ICU admission predicted rapid recovery from AKI among 56 critically ill patients, with an AUC of 0.71 (0.57–0.82) [Citation34]. Our findings in critically ill older adults with AKI align with their study, with an AUC of 0.774. Zhao et al.’s study indicated that the optimal cutoff age for non-recovery of renal function after AKI was 63 years old. That study also found that the rate of renal non-recovery in critically ill AKI patients aged 63 years and older was higher than in those younger than 63 years [Citation35]. We also observed a high rate of renal non-recovery (44.0%) in critically ill older adults with AKI. In our age stratification analysis, we noted that urinary [TIMP-2][IGFBP7] could predict renal non-recovery in both age groups (60–74 years and 75 years and older). However, the cutoff value of urinary [TIMP-2]*[IGFBP7] was 0.34 for participants aged 60–74, which was lower than that for participants aged 75 years and older. This discrepancy may be due to the relatively common occurrence of stage 1 AKI among patients aged 60–74. Nevertheless, further research is needed to investigate whether age affects the secretion of [TIMP-2]*[IGFBP7].
We further investigated risk factors for renal non-recovery by multiple regression analysis. The results showed that urinary [TIMP-2]*[IGFBP7], APACHE II score, and the presence of AKI stages 2–3 were independent risk factors for renal non-recovery in older adults with AKI at ICUs. If we used the APACHE II score and the presence of AKI stages 2–3 for predicting renal non-recovery, the AUC was 0.771, whereas the addition of urinary [TIMP-2]*[IGFBP7] further improved the AUC to 0.851. Generally speaking, older adults have a more prominent decline in their glomerular filtration rate, and the susceptibility to AKI may increase gradually with age. Age-associated decrease in functional renal reserve can be responsible for older adults’ tendency to have renal non-recovery relative to younger ones. In our study, the rate of renal non-recovery was comparable between participants of different age strata. This may result from our sample size not being large enough to detect between-group differences.
Several studies have identified AKI as an important risk factor for morbidity and mortality. However, most of the existing data come from critically ill patients in ICUs. On the other hand, mortality rates of older adults with AKI are quite variable, with estimates between 31% and 80% [Citation12]. Our study determined that participants with renal non-recovery had a higher in-hospital or 30-day mortality than recovered ones. However, we revealed that urinary [TIMP-2]*[IGFBP7] could not predict 30-day mortality in older adults with AKI in ICUs. A possible reason is that these older adults may have increased mortality due to other factors such as advanced age, hospitalization-related complications, and varying disease severity. Nonetheless, the mortality of the renal non-recovery group was still higher. The 30-day mortality differed between those with urinary [TIMP-2]*[IGFBP7] ≥ 0.81((ng/mL)2/1000) and those with values <0.81 ((ng/mL)2/1000). Moreover, higher urinary [TIMP-2]*[IGFBP7] combined with higher APACHE II scores and AKI stage 2–3 in older adults increased the risk of poor renal prognosis.
This study has several limitations. First, the sample size was relatively modest. Future studies with more participants are needed to validate our findings. Second, advanced CKD is a well-known risk factor for AKI; however, we excluded patients with baseline CKD, which may introduce selection bias. Third, no significant differences were observed in clinical characteristics between different age groups, which may also contribute to selection bias. Fourth, we did not analyze the utility of urinary [TIMP-2]*[IGFBP7] in predicting the risk of RRT timing. Fifth, we did not compare the predictive value of urinary [TIMP-2]*[IGFBP7] with other AKI biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C (CYSC) for renal non-recovery, and did not compare between those aged ≥60 and <60 years. Future prospective studies should investigate this aspect with a larger sample size.
Conclusions
Our study revealed that urinary [TIMP-2]*[IGFBP7] is a reliable biomarker for predicting renal non-recovery in older adults with AKI. Combining urinary [TIMP-2]*[IGFBP7] with clinical risk factors improved the predictability of renal non-recovery. However, urinary [TIMP-2]*[IGFBP7] did not predict 30-day mortality, although patients with renal non-recovery had a higher mortality rate. We also identified cutoff values of urinary [TIMP-2]*[IGFBP7] for risk stratification. Furthermore, we demonstrated that higher urinary [TIMP-2]*[IGFBP7] levels and a combination of a high APACHE II score with AKI stages 2–3 were associated with increased 30-day mortality in older adults with AKI in ICUs. Further research is warranted to validate these findings and explore the potential of urinary [TIMP-2]*[IGFBP7] as a prognostic tool in older patients.
Authors’ contributions
LC contributed to urine collection, data interpretation, manuscript drafting, and critical revision. H-MJ, XZ, and Y-JJ contributed to urine collection and data interpretation and performed the statistical analysis. XX contributed to data collection and data interpretation. W-XL chaired the group, conceived and designed the study, performed statistical analysis, and contributed to data collection, interpretation, and critical manuscript revision. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Ethical approval
The study was approved by the Human Ethics Committee of Beijing Chao-yang Hospital, Capital Medical University (Beijing, China) (No. 2018-117). Before participation, written informed consent was obtained from patients or their next of kin.
Supplemental Material
Download PDF (144.9 KB)Acknowledgments
We thank Professor Li-Rong Liang at Beijing Lu-he Hospital for the statistical analysis. We also thank Medjaden Inc. for the scientific editing of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated and/or analyzed during this study are included in this published article.
Additional information
Funding
References
- Bolignano D, Mattace-Raso F, Sijbrands EJ, et al. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:1–9. doi:10.1016/j.arr.2014.02.003.
- Brunker LB, Boncyk CS, Rengel KF, et al. Elderly patients and management in. intensive care units (ICU): clinical challenges. Clin Interv Aging. 2023;18:93–112. doi:10.2147/CIA.S365968.
- Borges GM, Ervaltti LR, Jardim AP. Mudança Demográfica no Brasil no Início do Século XXI: subsídios Para as projeções da população. Brasil: IBGE, Instituto Brasileiro de Geografia e Estatística. 2015.
- Jia L, Quan M, Fu Y, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19(1):81–92. doi:10.1016/s1474-4422(19)30290-x.
- Chao CT, Lin YF, Tsai HB, et al. Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg. 2013;217(2):240–250. doi:10.1016/j.jamcollsurg.2013.03.024.
- Elmistekawy E, McDonald B, Hudson C, et al. Clinical impact of mild acute kidney injury after cardiac surgery. Ann Thorac Surg. 2014;98(3):815–822. doi:10.1016/j.athoracsur.2014.05.008.
- Reents W, Hilker M, Börgermann J, et al. Acute kidney injury after on-pump or off-pump coronary artery bypass grafting in elderly patients. Ann Thorac Surg. 2014;98(1):9–15. discussion 14-15. doi:10.1016/j.athoracsur.2014.01.088.
- Chao CT, Tsai HB, Wu CY, et al. The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep. 2015;5(1):13925. doi:10.1038/srep13925.
- Yokota LG, Sampaio BM, Rocha E, et al. Acute kidney injury in elderly intensive care patients from a developing country: clinical features and outcome. Int J Nephrol Renovasc Dis. 2017;10:27–33. doi:10.2147/ijnrd.s126534.
- Infante B, Franzin R, Madio D, et al. Molecular mechanisms of AKI in the elderly: from animal models to therapeutic intervention. J Clin Med. 2020;9(8):2574. doi:10.3390/jcm9082574.
- Liu W, Lian XJ, Chen YH, et al. Hospital-acquired acute kidney injury in older patients: clinical characteristics and drug analysis. Gerontology. 2022;68(7):763–770. doi:10.1159/000518938.
- Silveira Santos CGD, Romani RF, Benvenutti R, et al. Acute kidney injury in elderly population: a prospective observational study. Nephron. 2018;138(2):104–112. doi:10.1159/000481181.
- Ge S, Nie S, Liu Z, et al. Epidemiology and outcomes of acute kidney injury in elderly chinese patients: a subgroup analysis from the EACH study. BMC Nephrol. 2016;17(1):136. doi:10.1186/s12882-016-0351-2.
- See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. doi:10.1016/j.kint.2018.08.036.
- See EJ, Toussaint ND, Bailey M, et al. Risk factors for major adverse kidney events in the first year after acute kidney injury. Clin Kidney J. 2019;14(2):556–563. doi:10.1093/ckj/sfz169.
- Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22(1):28–38. doi:10.1681/asn.2010090934.
- Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39(3):420–428. doi:10.1007/s00134-012-2796-5.
- Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52(2):262–271. doi:10.1053/j.ajkd.2008.03.005.
- Jia HM, Cheng L, Weng YB, et al. Cell cycle arrest biomarkers for predicting renal recovery from acute kidney injury: a prospective validation study. Ann Intensive Care. 2022;12(1):14. doi:10.1186/s13613-022-00989-8.
- Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi:10.1001/jama.2009.1322.
- Pannu N, James M, Hemmelgarn B, et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi:10.2215/cjn.06480612.
- Zheng CF, Liu WY, Zeng FF, et al. Prognostic value of platelet-to-lymphocyte ratios among critically ill patients with acute kidney injury. Crit Care. 2017;21(1):238. doi:10.1186/s13054-017-1821-z.
- Fan W, Ankawi G, Zhang J, et al. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med. 2019;57(5):567–576. doi:10.1515/cclm-2018-0776.
- Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi:10.1007/s00134-017-4809-x.
- Qian BS, Jia HM, Weng YB, et al. Analysis of urinary C-C motif chemokine ligand 14 (CCL14) and first-generation urinary biomarkers for predicting renal recovery from acute kidney injury: a prospective exploratory study. J Intensive Care. 2023;11(1):11. doi:10.1186/s40560-023-00659-2.
- Levey AS. Defining AKD: the spectrum of AKI, AKD, and CKD. Nephron. 2022;146(3):302–305. doi:10.1159/000516647.
- Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi:10.1186/cc12503.
- Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi:10.1016/S0140-6736(16)32064-5.
- Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi:10.1164/rccm.201604-0799OC.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi:10.1016/0895-4356(94)90129-5.
- Wei Q, Liu H, Tu Y, et al. The characteristics and mortality risk factors for acute kidney injury in different age groups in China - a cross sectional study. Ren Fail. 2016;38(9):1413–1417. doi:10.1080/0886022x.2016.1227618.
- Kane-Gill SL, Peerapornratana S, Wong A, et al. Use of tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7 [TIMP2]•[IGFBP7] as an AKI risk screening tool to manage patients in the real-world setting. J Crit Care. 2020;57:97–101. doi:10.1016/j.jcrc.2020.02.002.
- Dewitte A, Joannès-Boyau O, Sidobre C, et al. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol. 2015;10(11):1900–1910. doi:10.2215/cjn.12651214.
- Zhao X, Li C, Lu Y, et al. Characteristics and risk factors for renal recovery after acute kidney injury in critically ill patients in cohorts of elderly and non-elderly: a multicenter retrospective cohort study. Ren Fail. 2023;45(1):2166531. doi:10.1080/0886022X.2023.2166531.