Abstract
Background
Although handgrip strength is associated with all-cause mortality in patients with chronic kidney disease (CKD), whether this relationship is dose-related is unknown. Therefore, we examined dose-response relationships between handgrip strength and all-cause mortality in CKD patients based on previous studies by meta-analysis.
Methods
Data sources included three electronic databases (PubMed, Web of Science, and Embase) from inception through October 2023. The included cohort was a CKD population not limited to disease stage, and their handgrip strength was objectively measured. Two researchers independently screened studies, extracted data, and assessed the risk of bias. We utilized estimates of handgrip strength categories using robust-error meta-regression (REMR), pooled study-specific estimates, and established dose-response relationships. Outcomes of interest included only all-cause mortality.
Results
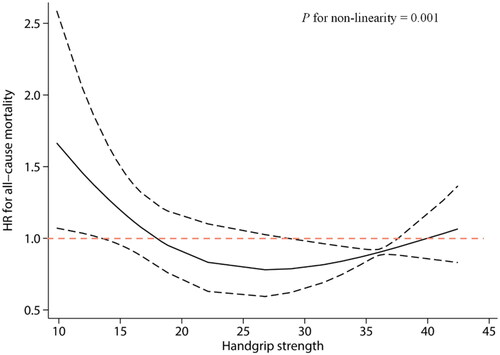
A total of 18 studies with 4810 participants (aged 47–71 years) were included. REMR modeling showed a U-shaped trend of association between handgrip strength and all-cause mortality in patients with CKD. Higher handgrip strength values, from 10 kg to approximately 28 kg, were associated with lower mortality risk. After that, the risk of death increased slightly.
Conclusion
A U-shaped association exists between handgrip strength and all-cause mortality risk in CKD patients. Future studies with quantitative measurements for each CKD stage will help to determine precise relative risk estimates between handgrip strength and mortality risk in patients with different stages of CKD.
Introduction
Handgrip strength is a reliable proxy for individual upper limb muscle strength [Citation1]. In chronic kidney disease (CKD) patients, handgrip strength has been reported to be lower than in healthy controls and to decline with worsening kidney function [Citation2]. Handgrip strength has also been proposed as a potential marker of frailty and sarcopenia in CKD patients, which are common and severe complications [Citation3]. Recently, there has been increasing evidence that increasing muscle strength may reduce the risk of all-cause mortality in CKD [Citation4,Citation5].
However, some studies differ in sample size, ethnicity, and other characteristics, leading to inconsistencies in their interpretation [Citation6,Citation7]. In addition, there are minimal reports on optimal handgrip strength levels for preventing all-cause mortality in CKD despite the availability of reference handgrip strength values for different populations.
Therefore, we hypothesized that the relationship between handgrip strength and the risk of all-cause mortality in CKD is not purely linear. This study is a further dose-response meta-analysis of our published systematic review [Citation8] to obtain quantitative estimates of the association between handgrip strength and the risk of all-cause mortality in CKD to determine the ‘optimal dose’, aim to explore the potential clinical implications of using handgrip strength as a prognostic and therapeutic tool in CKD management.
Methodology
This secondary systematic review and dose-response meta-analysis was reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [Citation9] and Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklists (Table S1) [Citation10], and the original systematic review was registered (PROSPERO: CRD42022308810).
Search strategy
Initially, we systematically searched PubMed, Web of Science, and Embase databases from inception to July 2022, updated in October 2023 [Citation8]. The specific search strategy is shown in the Tables S2–S4. Moreover, we reviewed conference abstracts from the International Society of Nephrology (https://www.theisn.org/), European Renal Association (https://www.era-online.org/en/), and American Society of Nephrology (https://www.asn-online.org/) for the past five years to look for other potential studies. We also searched ClinicalTrials.gov for studies registered as completed but not yet published. Finally, we manually investigated a relevant reference of systematic reviews to search for additional potential studies.
Eligibility criteria
The following articles were considered eligible for inclusion: (1) patients: adult patients with a confirmed diagnosis of CKD, including pre-dialysis, peritoneal dialysis, hemodialysis, and kidney transplant recipients; (2) exposures: handgrip strength measured using a dynamometer and reported at least two handgrip strength categories; (3) outcomes: all-cause mortality; (4) study design: cohort studies, both prospective and retrospective; and (5) full-text or non-full-text (e.g., conference abstracts and research letters), published in English with sufficient analyzable data. Studies were excluded if they (1) did not provide sufficient data, if the authors were contacted and did not respond, and (2) were case-control studies, cross-sectional studies, and randomized controlled trials. In the dose-response meta-analysis, homogeneity of measurement units was required; therefore, studies that could not isolate the effect of handgrip strength or studies reporting a mortality risk per 1 unit of elevated handgrip strength were excluded. Reviews, as well as animal studies, were not considered.
Quality assessment
The quality of the included studies was assessed according to the Newcastle-Ottawa Scale (NOS) [Citation11]. Two independent authors (LYH and YB) completed the quality assessment, and a third author (FZ) was consulted if disagreement was encountered.
Literature screening and data extraction
Two independent authors (LYH and YB) first read the title and abstract and, after excluding irrelevant literature, further read the full text to determine inclusion. Data extraction was done independently by two reviewers (LYH and YB). In disagreement, the third author could be consulted (FZ). Characteristics extracted from each study included first author, population country, disease stage, age, number of participants and cases, handgrip strength thresholds, reported effect estimates, and corresponding 95% confidence intervals (95% CI).
For the dose-response meta-analysis, the required data were re-extracted by two authors and reviewed by a third author. For the open-ended highest category of handgrip strength (i.e., >X kg) or the lowest category (i.e., <X kg), we obtained doses by *1.5 or/1.5 [Citation12]. When a study reported a risk estimate relative to a reference category other than the highest category of handgrip strength, we performed centering to make it the lowest category as a reference. Handgrip strength was used to describe effect sizes, and studies that reported a risk ratio was considered to approximate the hazard ratio (HR) [Citation13].
Since most studies used handgrip strength thresholds based on gender classification, we used their mean values as available thresholds. For example, Isoyama et al. [Citation14] reported low muscle strength, defined as handgrip strength below 30 kg for men and 20 kg for women, and then we used 25 as the shared threshold. Where handgrip strength intervals were not reported, they were estimated according to the method of Lauretani et al. [Citation15].
Statistical analysis
A dose-response relationship was established by treating handgrip strength as an independent continuous variable and regressing it against all-cause mortality. Considering the dose-response relationship could likely be a non-linear pattern, we used the restricted cubic spline function to fit the dose-response curve [Citation16]. This was done by setting three fixed knots at the 5th, 50th, and 95th quartiles of handgrip strength distribution (By setting three random knots on the quartiles of handgrip strength distribution) [Citation17]. The Wald test was used to test the non-linearity with the null hypothesis, as the coefficients of the non-linear terms were equal to zero. The robust-error meta-regression (REMR) model synthesized the dose-response relationship across available studies [Citation18]. This is a one-stage procedure that treats each study as a cluster and weighs the effects of each study by its inverse variance while employing the robust variance to address the potential correlation of within-study impacts. In addition, the robust variance has been proven to be a good solution in case of outliers [Citation18]. Results are expressed as an HR and a 95% confidence interval (95% CI). We used funnel plots to assess the risk of publication bias and performed Egger’s tests for the examined. All statistical analyses were performed using Stata 17.0, and p values were two-sided with a significance level of .05.
Quality of evidence
The quality of evidence for the dose-response association of handgrip strength with all-cause mortality was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) deterministic ratings [Citation19]. Quality of evidence was rated as high, moderate, low, or very low based on risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Results
Based on the screening results of the original published articles [Citation8], we reviewed the newly retrieved literature and read the potential full text. Therefore, 18 articles [Citation6,Citation14,Citation20–35] were included in the analysis (). lists the main characteristics and ‘dose’ information of the included studies. The methodological assessment of the included articles is shown in Table S5.
Table 1. Characteristics of included studies.
A total of 4810 patients with CKD were included in the dose-response meta-analysis. One, two, 11, and one studies had pre-dialysis, peritoneal dialysis, hemodialysis, and kidney transplant recipients, respectively (). Two other studies recruited dialysis patients. The mean age of the participants was between 46.61 and 71.
There was a clear U-shaped relationship between handgrip strength and all-cause mortality risk in CKD with a significant non-linear association (). Higher handgrip strength values were associated with a lower risk of death from 10 kg to approximately 28 kg. After that, the risk of death increased slightly. In addition, we noted that handgrip strength between 18 and 40 kg was associated with a low risk of all-cause mortality. Exceeding 40 kg appears to be associated with an increased mortality risk. The HR and 95% CI for each ‘dose’ are available in Table S6.
Regarding the assessment of publication bias, severe asymmetry was found in our funnel plot (Figure S1). Egger’s test also confirmed a significant risk of publication bias (Figure S2). Therefore, the quality of evidence was rated as very low due to inconsistency and publication bias (Table S7).
Discussion
Our dose-response meta-analysis allowed the estimation of handgrip strength values associated with a reduced risk of all-cause mortality in CKD. This finding is particularly interesting as it provides a range of reference values for preventing all-cause mortality in patients with CKD, which may be particularly useful in clinical settings and inform future guidelines and public health recommendations.
Low handgrip strength is a reliable marker for morbidity and mortality outcomes in chronic diseases, including CKD. Multiple findings report a strong association between lower handgrip strength and premature all-cause and cardiovascular mortality [Citation36–39]. Similarly, our prior meta-analysis observed a consistent inverse relationship between higher upper extremity muscle strength and all-cause mortality [Citation8]. A recent cohort study evaluating the association between handgrip strength index and mortality in kidney transplant recipients yielded similar results [Citation40]. Despite the availability of handgrip strength reference values for different populations, there are few reports on the optimal handgrip strength for preventing all-cause mortality in CKD. Most published studies assume a linear relationship between handgrip strength and mortality outcomes, although some evidence suggests otherwise.
The evidence for dose-response relationship between handgrip strength and all-cause mortality risk in CKD was analyzed with low certainty due to high heterogeneity and lack of precision. Rücker et al. [Citation41] proposed three sources of heterogeneity in meta-analyses, including clinical heterogeneity (e.g., differences between sample characteristics), statistical heterogeneity, and other sources of heterogeneity (e.g., design-related heterogeneity). Testing individual handgrip strength using different assessment tools was one of the sources that led to the observed heterogeneity. On the other hand, there were differences in disease stage of participants recruited into the included studies.
Age, baseline nutritional status, and physical activity level are key variables that may influence handgrip strength [Citation42–44]. In addition, previous studies have shown that dialysis treatment leads to muscle atrophy, which reduces handgrip strength [Citation45], whereas transplantation may have beneficial effects on muscle strength in patients with end-stage renal disease [Citation46]. These characteristics may have various implications for heterogeneous samples, thus making the external validity of this meta-analysis challenging for generalizability.
The main novelty of this study is estimating the range of handgrip strength associated with a low risk of all-cause mortality in CKD. Notably, we found that the relationship between handgrip strength and all-cause mortality in CKD showed a U-shaped dose-response relationship rather than a higher handgrip strength. Importantly, handgrip strength cannot be assumed to be a proxy for overall muscle strength [Citation47], and an excessively high handgrip strength may suggest that patients have other health problems, such as obesity, which may explain this observation.
It is well known that because handgrip strength decreases with declining renal function in pre-dialysis CKD patients, patients with end-stage renal disease gain some restoration of physical function, including handgrip strength, after receiving a kidney transplant. However, a significant decrease in muscle strength due to rapid loss of muscle protein in dialysis-dependent CKD patients may be sufficient to reduce their risk of mortality even if it is increased modestly or maintained at acceptable levels [Citation48]. Thus, treatment at different CKD stages, lifestyle-related factors, and how it affects muscle strength may explain a broader range of handgrip strength thresholds. Future research is needed to elucidate the optimal levels of handgrip strength values for different periods of CKD to reduce the mortality risk associated with different CKD stages.
A recent study of the beneficial association between handgrip strength and cancer mortality did not find a minimum threshold, i.e., a low level of handgrip strength (i.e., a threshold of 16 kg for women and 22 kg for men), to be beneficial in reducing mortality risk in cancer patients [Citation38]. In contrast, our findings on all-cause mortality showed a U-shaped association, and because kidney disease accelerates the loss of muscle strength, implementing targeted strategies to increase handgrip strength or maintain it at acceptable levels in patients with CKD may be able to reduce their risk of death. However, as noted above, the relationship between handgrip strength and all-cause mortality was not the same for participants with different baseline characteristics, and large, long-follow-up cohorts will be needed in the future to clarify the optimal level of handgrip strength.
Strength and limitations
In this review, we found 18 articles (22 studies) that collected available evidence on the dose-response association between handgrip strength and the risk of all-cause mortality in CKD. Limitations of this study include, first, that the handgrip strength thresholds determined for all-cause mortality in CKD are limited by the estimates obtained from the included studies, and individuals with higher handgrip strength than observed in this study may also benefit from the risk of all-cause mortality. A related limitation is that at the higher end of the exposure spectrum, the rise in the dose-response curve may represent a lack of data rather than an actual lack of association. The inversion of the right part of the dose-response curve in this study may reflect a scarcity of data/events rather than a genuine lack of beneficial association at higher levels of handgrip strength. Second, low handgrip strength thresholds range widely between men and women, and direct averaging may introduce some degree of measurement bias into the results. Also, due to the few studies that reported effects for gender separately, subgroup analyses could not be performed in this study to clarify handgrip strength thresholds between men and women. Nonetheless, the credibility of our analysis is moderate due to the inclusion of studies with a high number of participants and high quality. Third, because the participants in this study were predominantly hemodialysis-dependent patients, it is impossible to generalize it to full-spectrum CKD patients, as noted above. Fourth, although we used estimates from the most adjusted models in each cohort, this does not necessarily imply that choosing covariates was appropriate [Citation1]. Sixth, a certain degree of measurement bias may exist due to the significant heterogeneity in the follow-up period and different covariate-adjusted lists across studies. On the other hand, we reduced the quality of evidence by potential publication bias. Therefore, the certainty of evidence from existing studies is very low and has limited ability to inform clinical care or policy, and future studies may provide different results.
Despite these limitations, our study provides some guidance for clinical practice. Diminished handgrip strength is a significant predictor of mortality risk in patients with CKD. Health caregivers can assess muscle strength in patients with CKD by the simple method of measuring handgrip strength and take appropriate measures, such as recommending proper exercise and nutritional support, so that this population’s handgrip strength can be improved or maintained within a specific range to improve overall health and reduce the mortality risk.
Conclusion
In patients with CKD, handgrip strength is associated with all-cause mortality in a U-shaped curve. Until further studies determine the optimal handgrip strength ‘dose’, handgrip strength management in CKD patients should be enhanced and maintained at least above 18 kg and possibly limited to less than 40 kg. This study provides two important directions for future research, including (1) determining the optimal range of handgrip strength for different CKD stages and (2) reporting the effect of handgrip strength and mortality risk between genders with the data obtained.
Author contributions
Study conception and design, data acquisition: FZ and YFZ; data interpretation and statistical analysis: FZ, YB, and LYH; manuscript writing: HC, FZ and YB; revision of the manuscript: HC, YL, and YFZ.
Supplemental Material
Download PDF (519.5 KB)Acknowledgments
We thank Prof. Chang Xu for selflessly disclosing the Stata code of REMR.
Additional information
Funding
References
- Wilkinson TJ, Gabrys I, Lightfoot CJ, et al. A systematic review of handgrip strength measurement in clinical and epidemiological studies of kidney disease: toward a standardized approach. J Ren Nutr. 2022;32(4):1–8. doi: 10.1053/j.jrn.2021.06.005.
- Dahl H, Sandblost SRT, Welland NL, et al. Medication prescription, common side-effects, and nutritional status are associated in patients with chronic kidney disease. J Ren Nutr. 2022;32(5):520–528. doi: 10.1053/j.jrn.2021.10.008.
- Otobe Y, Rhee CM, Nguyen M, et al. Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr Opin Nephrol Hypertens. 2022;31(1):109–128. doi: 10.1097/MNH.0000000000000763.
- Hwang SH, Lee DH, Min J, et al. Handgrip strength as a predictor of all-cause mortality in patients with chronic kidney disease undergoing dialysis: a meta-analysis of prospective cohort studies. J Ren Nutr. 2019;29(6):471–479. doi: 10.1053/j.jrn.2019.01.002.
- Ribeiro HS, Neri SGR, Oliveira JS, et al. Association between sarcopenia and clinical outcomes in chronic kidney disease patients: a systematic review and meta-analysis. Clin Nutr. 2022;41(5):1131–1140. doi: 10.1016/j.clnu.2022.03.025.
- Giglio J, Kamimura MA, Lamarca F, et al. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis. J Ren Nutr. 2018;28(3):197–207. doi: 10.1053/j.jrn.2017.12.003.
- Stenvinkel P, Barany P, Chung SH, et al. A comparative analysis of nutritional parameters as predictors of outcome in male and female ESRD patients. Nephrol Dial Transplant. 2002;17(7):1266–1274. doi: 10.1093/ndt/17.7.1266.
- Zhang F, Wang H, Bai Y, et al. Handgrip strength and all-cause mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis of cohort studies. Int Urol Nephrol. 2023;55(11):2857–2865. doi: 10.1007/s11255-023-03603-3.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- University of Ottawa. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Canada. [cited 2023 Jan 1]. Available from: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp
- Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24(2):301–308. doi: 10.1093/annonc/mds337.
- Wang D, Li W, Cui X, et al. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;219:231–239. doi: 10.1016/j.ijcard.2016.06.027.
- Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–1728. doi: 10.2215/CJN.10261013.
- Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003.
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504.
- Xu C, Liu Y, Jia PL, et al. The methodological quality of dose-response meta-analyses needed substantial improvement: a cross-sectional survey and proposed recommendations. J Clin Epidemiol. 2019;107:1–11. doi: 10.1016/j.jclinepi.2018.11.007.
- Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. 2018;16(3):138–144. doi: 10.1097/XEB.0000000000000132.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD.
- Chan W, Chin SH, Whittaker AC, et al. The associations of muscle strength, muscle mass, and adiposity with clinical outcomes and quality of life in prevalent kidney transplant recipients. J Ren Nutr. 2019;29(6):536–547. doi: 10.1053/j.jrn.2019.06.009.
- Chiou TTY, Lee YT, Ng HY, et al. Handgrip strength predicts survival in chronic hemodialysis patients. Nephrol Dial Transplant. 2014;29:iii292.
- Dantas MA, Resende LL, Silva LF, et al. Handgrip strength as a predictor of death in incident hemodialysis patients of different age groups: a 6-year cohort study. Nephrol Dial Transplant. 2014;29:iii300.
- Kim JK, Kim SG, Oh JE, et al. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J Intern Med. 2019;34(3):599–607. doi: 10.3904/kjim.2017.083.
- Lee YH, Kim JS, Jung SW, et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020;21(1):166. doi: 10.1186/s12882-020-01831-8.
- Lopes AA, Silva LF, Matos CM, et al. Associations of handgrip strength with mortality risk and health-related quality of life in maintenance haemodialysis patients: a 6-year cohort study. Nephrol Dial Transplant. 2012;27:ii390.
- Lopes MB, Silva LF, Dantas MA, et al. Sex-age-specific handgrip strength and mortality in an incident hemodialysis cohort: the risk explained by nutrition and comorbidities. Int J Artif Organs. 2018;41(12):825–832. doi: 10.1177/0391398818793088.
- Matos CM, Silva LF, Santana LD, et al. Handgrip strength at baseline and mortality risk in a cohort of women and men on hemodialysis: a 4-year study. J Ren Nutr. 2014;24(3):157–162. doi: 10.1053/j.jrn.2013.12.005.
- Peng X, Jia Y, Xia J, et al. Lower handgrip strength predicts six year’ mortality of hemodialysis patients. Nephrol Dial Transplant. 2015;30(suppl_3):iii340. doi: 10.1093/ndt/gfv184.23.
- Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi: 10.1681/ASN.2012070702.
- Valenzuela PL, Cobo F, Diez-Vega I, et al. Physical performance, plasma S-klotho, and all-cause mortality in elderly dialysis patients: a prospective cohort study. Exp Gerontol. 2019;122:123–128. doi: 10.1016/j.exger.2019.05.003.
- Vogt BP, Borges MCC, Goés CR, et al. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr. 2016;35(6):1429–1433. doi: 10.1016/j.clnu.2016.03.020.
- Do JY, Kang SH. Association between low handgrip strength and obesity with mortality in peritoneal dialysis patients. Sci Rep. 2023;13(1):1852. doi: 10.1038/s41598-023-28708-8.
- Rodrigues J, Santin F, Brito F, et al. Association between nutritional markers and mortality in elderly on hemodialysis. Nephrol Dial Transplant. 2017;32(suppl_3):iii363–iii364. doi: 10.1093/ndt/gfx155.
- Kittiskulnam P, Chertow GM, Carrero JJ, et al. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92(1):238–247. doi: 10.1016/j.kint.2017.01.024.
- Xu X, Yang Z, Ma T, et al. The cut-off values of handgrip strength and lean mass index for sarcopenia among patients on peritoneal dialysis. Nutr Metab. 2020;17(1):84. doi: 10.1186/s12986-020-00506-3.
- Wei L, Zeng J, Fan M, et al. Associations between handgrip strength and skeletal muscle mass with all-cause mortality and cardiovascular mortality in people with type 2 diabetes: a prospective cohort study of the UK biobank. J Diabetes. Published online August 22, 2023. doi: 10.1111/1753-0407.13464.
- Zhang C, Li J, Shi H, et al. Independent and combined associations of upper and lower limb strength with all-cause mortality in community-based older adults: findings from the Chinese longitudinal healthy longevity survey. Public Health. 2023;220:57–64. doi: 10.1016/j.puhe.2023.04.023.
- López-Bueno R, Andersen LL, Koyanagi A, et al. Thresholds of handgrip strength for all-cause, cancer, and cardiovascular mortality: a systematic review with dose-response meta-analysis. Ageing Res Rev. 2022;82:101778. doi: 10.1016/j.arr.2022.101778.
- Mey R, Calatayud J, Casaña J, et al. Handgrip strength and respiratory disease mortality: longitudinal analyses from SHARE. Pulmonology. Published online October 21, 2022. doi: 10.1016/j.pulmoe.2022.09.007.
- van Vliet IMY, Post A, Kremer D, et al. Muscle mass, muscle strength and mortality in kidney transplant recipients: results of the TransplantLines Biobank and cohort study. J Cachexia Sarcopenia Muscle. 2022;13(6):2932–2943. doi: 10.1002/jcsm.13070.
- Rücker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8(1):79. doi: 10.1186/1471-2288-8-79.
- Do JY, Kang SH. Sex difference in the association among nutrition, muscle mass, and strength in peritoneal dialysis patients. Sci Rep. 2022;12(1):17900. doi: 10.1038/s41598-022-22722-y.
- Gulcicek S, Seyahi N. Factors associated with sarcopenia in patients with chronic kidney disease: a cross-sectional single-center study. Med Sci Monit. 2023;29:e939457. doi: 10.12659/MSM.939457.
- Yoshioka M, Kosaki K, Matsui M, et al. Physical activity, sedentary behavior, and skeletal muscle strength in patients with chronic kidney disease: an isotemporal substitution approach. Phys Ther. 2021;101(7):pzab101. doi: 10.1093/ptj/pzab101.
- Yang Y, Da J, Yuan J, et al. One-year change in sarcopenia was associated with cognitive impairment among haemodialysis patients. J Cachexia Sarcopenia Muscle. 2023;14(5):2264–2274. doi: 10.1002/jcsm.13311.
- Dienemann T, Ziolkowski SL, Bender S, et al. Changes in body composition, muscle strength, and fat distribution following kidney transplantation. Am J Kidney Dis. 2021;78(6):816–825. doi: 10.1053/j.ajkd.2020.11.032.
- Yeung SSY, Reijnierse EM, Trappenburg MC, et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc. 2018;19(8):703–709. doi: 10.1016/j.jamda.2018.04.019.
- Carrero JJ, Johansen KL, Lindholm B, et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53–66. doi: 10.1016/j.kint.2016.02.025.