?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Recent individual studies have indicated that ultra-processed food (UPF) consumption may be associated with the incidence of chronic kidney disease (CKD). We conducted a systematic review and meta-analysis based on those longitudinal studies evaluating the relationship between UPF consumption and the risk of incident CKD, and synthesizing the results.
Method
PubMed, Embase, The Cochrane Library, Web of Science, and Scopus were searched from inception through 22 March 2023. Any longitudinal studies evaluating the relationship between UPF consumption and the risk of incident CKD were included. Two researchers independently conducted the literature screening and data extraction. RR and its 95% CI were regarded as the effect size. The Newcastle–Ottawa Scale (NOS) was applied to assess the quality of the studies included, and the effect of UPF consumption on the risk of incident CKD was analyzed with STATA version 15.1. This study’s protocol was registered in PROSPERO (CRD42023411951).
Results
Four cohort studies with a total of 219,132 participants were included after screening. The results of the meta-analysis suggested that the highest UPF intake was associated with an increased risk of incident CKD (RR = 1.25; 95% CI: 1.18–1.33).
Conclusions
High-dose UPF intake was associated with an increased risk of incident CKD. However, the underlying mechanisms remain unknown. Thus, more standardized clinical studies and further exploration of the mechanisms are needed in the future.
1. Introduction
Chronic kidney disease (CKD) is a progressive disease with characteristics of proliferating and irreversible decline in kidney function [Citation1]. It has become one of the most severe global health problems [Citation2]. It has been estimated that the global prevalence of CKD is as high as 9.1%, with 697.5 million CKD patients worldwide, and its prevalence and mortality rate have been increasing in recent years [Citation3]. Some patients with CKD will eventually develop end-stage kidney disease (ESKD), requiring kidney replacement therapy to maintain basic physiological functions. However, the treatment incurs tremendous costs on global medical and economic systems [Citation4]. Therefore, there is an urgent need to identify and target factors that may promote the onset and progression of incident CKD, of which diet is one modifiable lifestyle factor [Citation5].
NOVA (which is not an acronym), is a food classification system presented by scientists at the University of São Paulo which classified foods into four groups according to their degree of processing: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods (UPFs) [Citation6]. UPFs are extracted from natural foods and processed through a series of complex industrial procedures, which add considerable flavor, color, preservatives, antioxidants, etc. Hamburgers, pizza, chocolate, cookies, ice cream, and candy are common UPFs in daily life. These foods are generally tasty and convenient. However, they are mostly high in calories, sugar, saturated fat and trans fat, and salt. Moreover, they are deficient in dietary fiber, micronutrients, and other bioactive compounds, and especially low in protein [Citation7].
As global economies have developed, UPF consumption has been on the rise worldwide, especially in middle and high-income countries [Citation8]. It has been reported that the United States and the United Kingdom have the highest levels of UPF consumption, with UPF accounting for about 60% of daily caloric intake [Citation9]. However, the proliferation of UPF consumption and its potential effects on human health have caused much concern. Studies have shown that UPF consumption is associated with various chronic non-communicable diseases, including inflammatory bowel disease, obesity, diabetes, and hypertension [Citation10], yet the results of many studies have been conflicting. For example, some studies reported a significant association between a higher intake of UPFs and increased risk of obesity and type 2 diabetes compared to lower intake of UPFs [Citation11–13]. Additionally, Adams J. and White and Son J. et al. found no significant association between UPF consumption and the risk of obesity or type 2 diabetes [Citation14,Citation15]. A cross-sectional study in South Korea found that CKD prevalence was higher among those with the highest UPF consumption, and that UPF consumption was negatively associated with estimated glomerular filtration rate (eGFR) [Citation16]. In a Brazilian cohort study, Rey-García et al. discovered that UPF intake increased the risk of declining kidney function, and UPF consumption may be directly associated with impaired kidney function [Citation17]. However, multiple observational studies are often needed to provide more convincing conclusions. Yet, some confounding factors are inevitable in observational studies, and the limitations of the area and the population will inevitably affect the rigor and generalizability of the conclusions. Meta-analysis, which integrates multiple studies to enlarge a sample size, cannot only improve the accuracy and precision of the results, but also facilitate the analysis of whether the inconsistent results across studies are due to the studies’ heterogeneity. To date, there has been a lack of meta-analysis on the relationship between UPF and CKD. Therefore, we performed meta-analysis to comprehensively synthesize the results from the longitudinal observational studies on the relationship between UPF consumption and the risk of incident CKD.
2. Materials and methods
2.1. Design and registration
This systematic review and meta-analysis were conducted following the 2020 PRISMA guidelines [Citation18], and have been submitted and approved in the International Prospective Register of Systematic Reviews (PROSPERO). The registration number is: CRD42023411951.
2.2. Literature search
We conducted an extensive search on PubMed, Embase, The Cochrane Library, Web of Science, and Scopus from inception until 22 March 2023, without any limitation of country or article type. The search terms were a combination of Medical Subject Headings (MeSH) and free-text keywords related to UPF and incident CKD, including ‘Ultra-Processed Food’, ‘Food, Processed’, ‘Fast Food’, ‘NOVA’, ‘Renal Insufficiency, Chronic’, and ‘Chronic Kidney Disease’ (see Supplementary Table S1 for search strategies).
2.3. Inclusion criteria
Included studies had to meet the following criteria: Longitudinal design was conducted in adults (≥18 years) examining the relationship between UPF consumption and the risk of incident CKD. The results also had to report effect estimates as a hazard ratio (HR), relative ratio (RR), or odds ratio (OR) with a 95% confidence interval value that assessed the relationship between UPF consumption and the risk of incident CKD. If none of these were reported, it had to at least provide the raw data for the observations.
2.4. Exclusion criteria
Conference papers and reviews were excluded. Any studies with missing data or obvious data errors were excluded. When there were studies with duplicate data, the most recently published study was selected. For original studies that did not report results as OR, RR, or HR, we tried to contact the authors for data before exclusion.
2.5. Data extraction
Two researchers (B.X. and J.H.) conducted the literature screening independently, and when there was any disagreement, they would discuss and deliberate with the third researcher (Y.W.). Then two researchers (B.X. and J.H.) carefully read the literature included and extracted additional primary details separately, including the name of the study, the author’s name, year of publication, country of the study, sample size, definition of the outcome, number of positive outcomes, exposure assessment modes, effect size, follow-up time, baseline population profile, and adjusted covariates. If there was any disagreement between the two researchers regarding the literature, they would discuss and deliberate first. If it could not be resolved, then the third researcher (Y.W.) would make a decision after discussion.
2.6. Quality assessment
Two researchers (B.X. and J.H.) independently evaluated the quality of the enrolled literature by applying the Newcastle–Ottawa Scale (NOS). The NOS is designed to assess the quality of nonrandomized studies in meta-analysis. The assessment is scored in three main areas – the selection of study subjects (4 points), the comparability of the study groups (2 points), and the assessment of study outcomes (3 points). A perfect score is 9 points, with a score higher than six being considered good quality.
2.7. Statistical analyses
We performed the statistical analysis with Stata version 15.1 (Stata Corp, College Station, TX). The type of analytical model used depended on the heterogeneity test. The heterogeneity was considered significant where p < 0.1 and I2 > 50%, and in these instances, we adopted a random-effects model. Otherwise, we applied a fixed-effects model. RR and its 95% CI were regarded as the effect size. HR was considered to be approximately equal to RR [Citation19]. The following formula was performed to convert OR to RR [Citation20]:
P0 refers to the incidence in the control group. Publication bias was initially judged by funnel plots. Further assessment was conducted with Egger’s regression asymmetry and Begg’s rank correlation, and publication bias was identified as significant if p < 0.05.
3. Results
3.1. Study characteristics
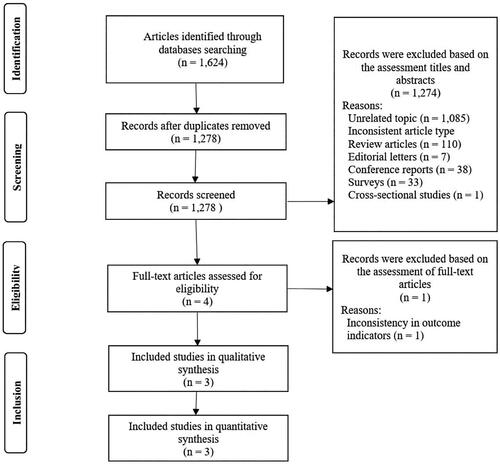
A total of 1624 publications were searched, including 756 in PubMed, 243 in Scopus, 214 in Embase, 168 in Web of Science, and 243 in the Cochrane Library. Based on the titles and abstracts, 344 studies were removed because they were duplicates, and 1274 studies with inconsistent study types or irrelevant subjects were excluded. One study with inconsistent outcome indicators was excluded after reading the full-text. Ultimately, we included 3 studies. Among them, Gu et al.’s study included a cohort study conducted in China, and another from the United Kingdom. Therefore, we ended up with a total of 4 longitudinal observational studies exploring the relationship between the UPF intake and the risk of incident CKD ().
Each of these four studies was a prospective cohort study. They were conducted in four countries (the United States, the Netherlands, China, and the United Kingdom), with follow-up periods ranging from 3.6 to 24 years. A total of 219,132 healthy participants (with no incident CKD at baseline) were enrolled in the trials, of whom males accounted for an average of 44.4%. All studies characterized the classification of foods according to the NOVA, and assessed daily UPF intake via the food frequency questionnaire (FFQ) or the 24-h dietary assessment. Both FFQ and 24-h dietary assessment had been validated previously. Within NOVA category, these four studies used different methods to measure participants’ daily UPF intake: the Atherosclerosis Risk in Communities (ARIC) cohort (United States, n = 14,679) used the number of servings per day (in servings/d) to estimate participants’ daily UPF intake; the Lifelines cohort (Netherlands, n = 78,346) calculated the proportion of the weight of UPF consumed per day to the total weight of all food consumed (in gram/d); the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort (China, n = 23,775) and the UK Biobank cohort (United Kingdom, n = 102,332) reported using the weight of energy-adjusted food providing 1000 kcal of energy per day (in g/1000 kcal/d) to represent participants’ daily UPF intake. Despite the inconsistency in the units for assessing daily UPF consumption in these four studies, they were all analyzed in subgroups according to the UPF intake divided into the lowest intake group (Q1), the lower intake group (Q2), the higher intake group (Q3), and the highest intake group (Q4) ().
Table 1. Characteristics of the included studies.
3.2. Quality assessment results
Based on the results of the quality assessment by applying the NOS, both the ARIC cohort (United States, n = 14,679) and the UK Biobank cohort (United Kingdom, n = 102,332) scored 8. Both the Lifelines cohort (Netherlands, n = 78,346), and the TCLSIH cohort (China, n = 23,775) scored 7 ().
Table 2. Quality assessment of the included studies.
3.3. UPF and the risk of incident CKD
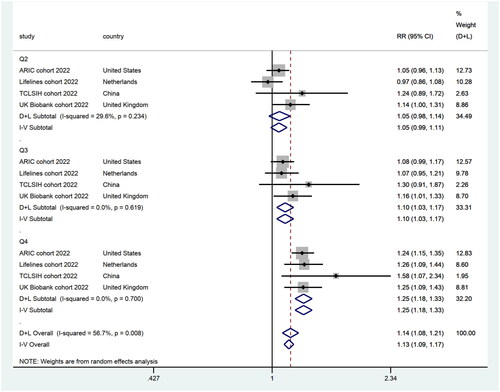
We divided the individuals into four subgroups based on the UPF intake levels: the lowest intake group (Q1, n = 54,785), the lower intake group (Q2, n = 54,784), the higher intake group (Q3, n = 54,779), and the highest intake group (Q4, n = 54,784), and integrated them with their maximally adjusted RR. According to the results of the heterogeneity test (I2 = 56.7%, p = 0.008), we conducted meta-analysis of the four studies with a random effects model. It revealed that there was no statistical significance between Q1 (n = 54,785) and Q2 (RR = 1.05; 95% CI: 0.99–1.11). This implied that Q2 (n = 54,784) was not associated with an increased risk of incident CKD, compared to Q1 (n = 54,785). However, compared to Q1 (n = 54,785), there was a 10% increased risk of incident CKD in Q3 (n = 54,779) (RR = 1.10; 95% CI: 1.03–1.17), and a 25% increased risk of incident CKD in Q4 (n = 54,784) (RR = 1.25; 95% CI: 1.18–1.33) ().
Figure 2. Forest plots demonstrating RR and 95% CI for the pooled results from the random-effects models to evaluate the relationship between UPF consumption and risk of incident CKD.
A tabular summary of the secondary findings indicated that an additional serving of UPF per day in the ARIC cohort (United States, n = 14,679) was associated with a 5% increase in the risk of incident CKD. In contrast, each substitution of minimally processed foods for UPFs was associated with a 6% reduction in the risk of incident CKD. In the Lifelines cohort (Netherlands, n = 78,346), participants in the highest quartile of UPF consumption had a more rapid decline in eGFR, compared to those in the lowest quartile (β = −0.17; 95% CI: −0.23 to −0.11). Also, dose-response analysis suggested that every 10% increase in dietary UPF intake was associated with an 11% increase in the risk of incident CKD (OR = 1.11; 95% CI: 1.06–1.17) ().
Table 3. Secondary findings from the included studies.
3.4. Sensitivity analysis
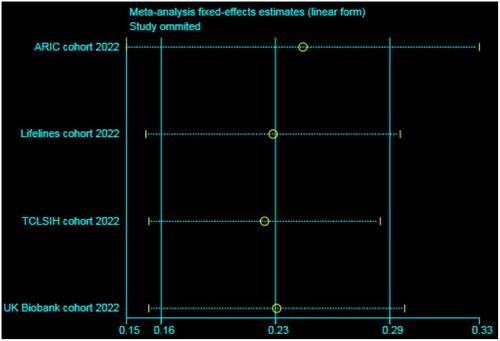
Sensitivity analysis suggested that the results were not moderated by any single study, implying that the integrated data were relatively stable ().
3.5. Publication bias
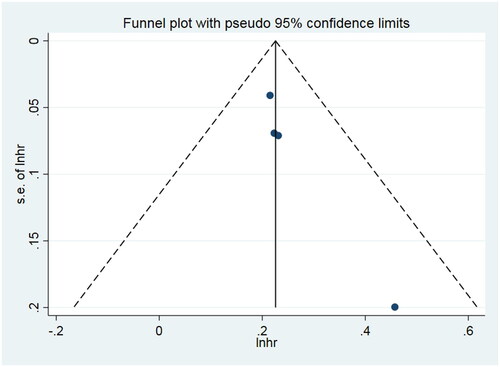
Subjectively, the funnel plot was asymmetric, indicating a publication bias. We also examined publication bias in the four included studies with Egger’s regression asymmetry (p = 0.024 < 0.05). However, Begg’s rank correlation showed no publication bias (p = 0.089 > 0.05) ().
4. Discussion
Our results showed a positive association between UPF intake and the risk of incident CKD. Participants in the highest UPF intake quartile had a higher risk of incident CKD than those in the lowest quartile in the four longitudinal cohort studies. Respectively, participants in the highest UPF intake quartile had a 24%, 50%, and 25% higher risk of incident CKD compared with those in the lowest quartile after adjusting for various variables in the ARIC, TCLSIH, and UK Biobank cohorts. Furthermore, dose-response analysis in the Lifelines cohort showed that a 10% increase in UPF intake was associated with an 11% increased risk of incident CKD. Further evidence suggested that replacing UPF with minimally processed foods may reduce the risk of incident CKD. These four studies, conducted in the United States, the Netherlands, China, and the United Kingdom, each proposed that higher UPF intake increased the risk of incident CKD, suggesting that the association between UPF consumption and the increased risk of incident CKD was not limited to specific countries.
Traditional food categorization focuses on single nutrient content, thus some food combinations are challenging to categorize. NOVA classifies foods according to their degree of processing, offering new insights into dietary categorization and providing a supplement to traditional dietary guidelines [Citation24]. In these four studies included, the food lists of UPF were obtained after the researchers sorted the foods consumed by the participants according to the food categorization criteria of NOVA. However, categorization bias may occur due to the limitations of the dietary assessment tools and the inconsistent degree of processing of some similar food groups. For example, the FFQ used in the Lifelines cohort did not distinguish between spreadable and nonspreadable cheeses, and most cheeses on the market contain more than five ingredients. The authors considered the cheeses as UPF uniformly in the study [Citation22].
The four studies assessed participants’ UPF food intake through self-reporting such as the FFQ or 24-h dietary assessment. The TCLSIH cohort used the FFQ containing 100 food items (FFQ-100) to assess participants’ food intake over the past month, and 150 participants randomly selected from the cohort were evaluated for reproducibility and validity of the questionnaire [Citation23,Citation25]. The Lifelines cohort used the FFQ containing 110 food items (FFQ-110) which evaluated the the frequency and amount of food consumed by participants in the past month. The ARIC cohort used a 66-item modified semiquantitative Willett FFQ (FFQ-66). All FFQs mentioned above have been validated [Citation25–30]. While in the UKB cohort, a 24-h web-based dietary questionnaire was used to assess participants’ dietary intake over the past 24 h, which mainly consisted of questions on the consumption of about 200 common foods and beverages. The relative validity and repeatability of this questionnaire have also been demonstrated [Citation31,Citation32]. However, neither the FFQ nor the 24-h web-based dietary questionnaire was specifically designed to measure UPF intake. The FFQ is commonly used to assess long-term intake, but suffers from shortcomings, such as long-term recall bias. The 24-h dietary recall provides relatively accurate, detailed, open-ended data on dietary intake, and does not rely on long-term memory, but also suffers from the possibility of short-term recall errors, omissions, and incorrect estimation of food portion sizes. Therefore, the ascertainment of the exposure (UPF food intake) might not be accurate enough. These limitations may affect the final study results. Future studies should be based on standardized and validated food assessment tools to minimize the interference of relevant confounding factors. For example, objective biomarkers related to UPF intake can be considered identifying and utilizing.
Though the four cohort studies included in this study had NOS scores over 6, demonstrating the overall high quality of these included studies and the sensitivity analysis suggested that the combined results were robust, this meta-analysis also has some other limitations: (a) Since the included studies were all observational studies with some confounders that could not be ignored, the results of the meta-analysis could only suggest an association, rather than a causal relationship, between the UPF consumption and the risk of incident CKD. (b) The results may be subject to a reasonable degree of bias due to heterogeneity in participants’ ethnicities and the varying methods of UPF intake assessment and estimation. Also, because there were no uniform grouping criteria among the studies, there was no way to obtain a critical intake value for UPF intake associated with the risk of incident CKD in this study. (c) These four studies had follow-up periods ranging from 3.6 to 24 years, with a large disparity in follow-up time across studies. Thus, there may have been some differences in the results indicators. (d) As the secondary endpoints and subgroup analyses were different in each study, further combined analyses could not be performed, and instead they are only presented in textual and tabular form. Furthermore, due to the inconsistency of the dose units among the studies, dose-response analyses could not be performed to comprehensively further synthesize a more specific dose relationship between UPF and the risk of incident CKD. (e) The small amount of literature included in this study may have led to unstable and/or inaccurate results in the assessment of publication bias. Thus, the results should be treated with caution.
Despite the interesting results of this meta-analysis, the potential mechanism between the UPF intake and kidney injury has yet to be conclusively identified. UPF production involves splitting the food into smaller portions, chemical reactions, adding various additives, and complex packaging [Citation33]. Each of these processes may be a relevant factor in incident CKD. Some studies have shown that advanced glycosylation end-products (AGEs) produced during food processing due to the Maillard reaction, could promote glomerulosclerosis, thickening of the basement membrane, and tubulointerstitial fibrosis through multiple mechanisms, resulting in kidney damage [Citation34–36]. Sodium benzoate (SB), potassium sorbate (PS), monosodium glutamate (MSG), and butylated hydroxytoluene (BHT) are common food preservatives used in UPF. They are widely used in food processing to extend food’s shelf life and prevent bacterial infections [Citation37]. In an animal study, serum urea, creatinine, and uric acid have been shown to significantly increase in rats exposed to PS and SB. In addition, pathological changes, such as tubular cell granular degeneration, glomerular constriction, intertubular vascular congestion, and interstitial edema have been found in rats’ kidney sections [Citation38].
Compared to minimally processed foods, UPF contains excess energy, saturated and trans fats, sugars, and sodium [Citation39]. While excessive energy and sugar intake may promote obesity, which is considered one of the main drivers of CKD [Citation40]. Excessive fructose consumption could led to damage in the form of glomerular interstitial and vascular arteriopathy [Citation41,Citation42]. Higher fructose consumption has been shown to raise uric acid levels, which are pro-inflammatory and pro-oxidant to the kidneys, leading to damage to the kidney microvasculature and glomerulus [Citation43–47]. At the same time, more and more evidence emphasizes that higher sodium intake may have toxic effects on the vasculature by modulating oxidative stress, inflammatory reactions, and endothelial dysfunction, and lead to hypertension, increasing the risk of cardiovascular disease [Citation48].
In conducting this study, we found that despite the current growing interest in UPF, there remains a lack of high-quality research. Therefore, ultimately only four cohort studies were included in this study. Also, most of the studies had examined the relationship between UPF intake and incident CKD in the healthy population using incident CKD as the primary outcome. At the time we conducted the meta-analysis, there were no articles examining the relationship between UPF intake and CKD prognosis with CKD patients as the study population. More standardized clinical studies are needed in the future to explore and validate the relationship between UPF intake and the risk of incident CKD, the impact of UPF intake on the prognosis of patients with CKD and to explain the mechanism of action.
5. Conclusions
The results of our analysis showed an association between high-dose UPF intake and increased risk of incident CKD. However, there was no standardized criterion regarding the definition of high-dose. Moreover, the underlying mechanisms remain unknown and more standardized clinical studies and exploration of the mechanisms are needed in the future.
Author contributions
B.X. and J.H. designed and conceptualized the research. J.L. and H.C. analyzed the data. B.X., J.H., L.C., and Y.L. wrote the manuscript, and L.F., F.T., W.O., and Y.W. revised it. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download PDF (120.8 KB)Disclosure statement
The authors report that there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Y.W.], upon reasonable request.
Additional information
Funding
References
- Bábíčková J, Klinkhammer BM, Buhl EM, et al. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017;91(1):1–10. doi: 10.1016/j.kint.2016.07.038.
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, et al. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–288. doi: 10.1038/s41581-019-0248-y.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3.
- Wang J, Zhang L, Tang SCW, et al. Disease burden and challenges of chronic kidney disease in North and East Asia. Kidney Int. 2018;94(1):22–25. doi: 10.1016/j.kint.2017.12.022.
- He LQ, Wu XH, Huang YQ, et al. Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies. Nutr J. 2021;20(1):4. doi: 10.1186/s12937-020-00661-6.
- Moubarac JC, Parra DC, Cannon G, et al. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. 2014;3(2):256–272. doi: 10.1007/s13679-014-0092-0.
- Monteiro CA, Cannon G, Moubarac JC, et al. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234.
- Baker P, Machado P, Santos T, et al. Ultra-Processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21(12):e13126. doi: 10.1111/obr.13126.
- Marino M, Puppo F, Del Bo’ C, et al. A systematic review of worldwide consumption of ultra-processed foods: findings and criticisms. Nutrients. 2021;13(8):2778. doi: 10.3390/nu13082778.
- Mm L, Ja D, S B, et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev J Int Assoc Study Obes. 2021;22(3):e13146.
- Rauber F, Steele EM, Louzada MLdC, et al. Ultra-Processed food consumption and indicators of obesity in the United Kingdom population (2008-2016). PloS One. 2020;15(5):e0232676. doi: 10.1371/journal.pone.0232676.
- Nardocci M, Leclerc BS, Louzada ML, et al. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019;110(1):4–14. doi: 10.17269/s41997-018-0130-x.
- Llavero-Valero M, Escalada-San Martín J, Martínez-González MA, et al. Ultra-Processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin Nutr. 2021;40(5):2817–2824. doi: 10.1016/j.clnu.2021.03.039.
- Adams J, White M. Characterisation of UK diets according to degree of food processing and associations with Socio-Demographics and obesity: cross-Sectional analysis of UK national diet and nutrition survey (2008-12). Int J Behav Nutr Phys Act. 2015;12(1):160 doi: 10.1186/s12966-015-0317-y.
- Son J, Lee Y, Park K. Effects of processed red meat consumption on the risk of type 2 diabetes and cardiovascular diseases among Korean adults: the Korean genome and epidemiology study. Eur J Nutr. 2019;58(6):2477–2484. doi: 10.1007/s00394-018-1799-6.
- Kityo A, Lee S-A. The intake of ultra-processed foods and prevalence of chronic kidney disease: the health examinees study. Nutrients. 2022;14(17):3548. doi: 10.3390/nu14173548.
- Rey-García J, Donat-Vargas C, Sandoval-Insausti H, et al. Ultra-Processed food consumption is associated with renal function decline in older adults: a prospective cohort study. Nutrients. 2021;13(2):428. doi: 10.3390/nu13020428.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Delpino FM, Figueiredo LM, Bielemann RM, et al. Ultra-Processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol. 2022;51(4):1120–1141. doi: 10.1093/ije/dyab247.
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690.
- Du S, Kim H, Crews DC, et al. Association between ultraprocessed food consumption and risk of incident CKD: a prospective cohort study. Am J Kidney Dis. 2022;80(5):589–598.e1. doi: 10.1053/j.ajkd.2022.03.016.
- Cai Q, Duan MJ, Dekker LH, et al. Ultraprocessed food consumption and kidney function decline in a population-based cohort in The Netherlands. Am J Clin Nutr. 2022;116(1):263–273. doi: 10.1093/ajcn/nqac073.
- Gu Y, Li H, Ma H, et al. Consumption of ultraprocessed food and development of chronic kidney disease: the Tianjin chronic Low-Grade systemic inflammation and health and UK biobank cohort studies. Am J Clin Nutr. 2023;117(2):373–382. doi: 10.1016/j.ajcnut.2022.11.005.
- Nazmi A, Tseng M, Robinson D, et al. A nutrition education intervention using NOVA is more effective than MyPlate alone: a proof-of-Concept randomized controlled trial. Nutrients. 2019;11(12):2965. doi: 10.3390/nu11122965.
- Xia Y, Xiang Q, Gu Y, et al. A dietary pattern rich in animal organ, seafood and processed meat products is associated with newly diagnosed hyperuricaemia in Chinese adults: a propensity score-matched case-control study. Br J Nutr. 2018;119(10):1177–1184. doi: 10.1017/S0007114518000867.
- Siebelink E, Geelen A, de Vries JH. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr. 2011;106(2):274–281. doi: 10.1017/S0007114511000067.
- Streppel MT, de Vries JH, Meijboom S, et al. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden longevity study. Nutr J. 2013;12(1):75. doi: 10.1186/1475-2891-12-75.
- Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086.
- Du S, Kim H, Rebholz CM. Higher ultra-processed food consumption is associated with increased risk of incident coronary artery disease in the atherosclerosis risk in communities study. J Nutr. 2021;151(12):3746–3754. doi: 10.1093/jn/nxab285.
- Stevens J, Metcalf PA, Dennis BH, et al. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr Res. 1996;16(5):735–745. doi: 10.1016/0271-5317(96)00064-4.
- Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14(11):1998–2005. doi: 10.1017/S1368980011000942.
- Bradbury KE, Young HJ, Guo W, et al. Dietary assessment in UK biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66.
- Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762.
- Nie C, Li Y, Qian H, et al. Advanced glycation end products in food and their effects on intestinal tract. Crit Rev Food Sci Nutr. 2022;62(11):3103–3115. doi: 10.1080/10408398.2020.1863904.
- Feng JX, Hou FF, Liang M, et al. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007;71(9):901–911. doi: 10.1038/sj.ki.5002162.
- Fotheringham AK, Gallo LA, Borg DJ, et al. Advanced glycation end products (AGEs) and chronic kidney disease: does the modern diet AGE the kidney? Nutrients. 2022;14(13):2675. doi: 10.3390/nu14132675.
- Martínez-Pineda M, Vercet A, Yagüe-Ruiz C. Are food additives a really problematic hidden source of potassium for chronic kidney disease patients? Nutrients. 2021;13(10):3569. doi: 10.3390/nu13103569.
- Abd-Elhakim YM, Behairy A, Hashem MMM, et al. Toll-like receptors and nuclear factor kappa B signaling pathway involvement in hepatorenal oxidative damage induced by some food preservatives in rats. Sci Rep. 2023;13(1):5938. doi: 10.1038/s41598-023-32887-9.
- Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health-processing or nutrient content? Curr Obes Rep. 2017;6(4):420–431. doi: 10.1007/s13679-017-0285-4.
- Chen Y, Dabbas W, Gangemi A, et al. Obesity management and chronic kidney disease. Semin Nephrol. 2021;41(4):392–402. doi: 10.1016/j.semnephrol.2021.06.010.
- Sánchez-Lozada LG, Tapia E, Jiménez A, et al. Fructose-Induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292(1):F423–429. doi: 10.1152/ajprenal.00124.2006.
- Zheng Z, Harman JL, Coresh J, et al. The dietary fructose: vitamin C intake ratio is associated with hyperuricemia in African-American adults. J Nutr. 2018;148(3):419–426. doi: 10.1093/jn/nxx054.
- Asselman M, Verkoelen CF. Fructose intake as a risk factor for kidney stone disease. Kidney Int. 2008;73(2):139–140. doi: 10.1038/sj.ki.5002700.
- Johnson RJ, Nakagawa T, Jalal D, et al. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28(9):2221–2228. doi: 10.1093/ndt/gft029.
- Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, et al. Uric acid-Induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3-4):e71-78–e78. doi: 10.1159/000345509.
- Sánchez-Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–1141. doi: 10.1152/ajprenal.00104.2008.
- Sánchez-Lozada LG, Tapia E, Santamaría J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x.
- Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. doi: 10.1155/2014/406960.