Abstract
Background
Sepsis-associated acute kidney injury (S-AKI) is a critical illness and is often associated with high morbidity and mortality rates. The soluble urokinase-type plasminogen activator receptor (suPAR) is an important immune mediator and is involved in kidney injury. However, its diagnostic value in S-AKI patients remains unclear. Therefore, we assessed the early predictive value of suPAR for S-AKI patients.
Methods
We prospectively enrolled adult patients, immediately after fulfilling the sepsis-3 criteria. Plasma suPAR levels at 0-, 12-, 24-, and 48-h post-sepsis diagnosis were measured. S-AKI development was the primary outcome. S-AKI risk factors were analyzed using logistic regression, and the value of plasma suPAR for early S-AKI diagnosis was assessed using receiver operating characteristic (ROC) curves.
Results
Of 179 sepsis patients, 63 (35.2%) developed AKI during hospitalization. At 12-, 24-, and 48-h post-sepsis diagnosis, plasma suPAR levels were significantly higher in patients with S-AKI than in patients without S-AKI (p < 0.05). The plasma suPAR had the highest area under the ROC curve of 0.700 (95% confidence interval (CI), 0.621–0.779) at 24-h post-sepsis diagnosis, at which the best discrimination ability for S-AKI was achieved with suPAR of ≥6.31 ng/mL (sensitivity 61.9% and specificity 71.6%). Logistic regression analysis showed that suPAR at 24-h post-sepsis diagnosis remained an independent S-AKI risk factor after adjusting for mechanical ventilation, blood urea nitrogen, and pH.
Conclusions
The findings suggest that plasma suPAR may be a potential biomarker for early S-AKI diagnosis.
Introduction
Acute kidney injury (AKI) is a frequent complication in hospitalized adults and its incidence has increased among inpatients from 1.9% to 10–15% [Citation1,Citation2], while the incidence of AKI in intensive care units (ICUs) exceeds 50% [Citation1,Citation3]. Although creatinine levels in most patients with AKI gradually return to normal at later stages, several studies have suggested that AKI raises the risk of mortality, cardiovascular events, and chronic kidney disease (CKD) [Citation4–7].
Sepsis is a potentially fatal infection-related disease that causes organ dysfunction [Citation8]. Sepsis-associated AKI (S-AKI) is the most common type of AKI in the ICU [Citation9], with a reported incidence of up to 59% [Citation10]. S-AKI has been linked to poor clinical outcomes, including a higher risk of in-hospital death and longer hospital stays than other types of AKI [Citation11]. Many clinical and basic studies have conducted in-depth discussions on S-AKI; however, effective measures to prevent and treat S-AKI remain lacking. Therefore, identifying biomarkers for S-AKI as early as possible is important to guide clinical treatment and improve patient outcomes.
The soluble urokinase-type plasminogen activator receptor (suPAR) is a signaling glycoprotein comprising three domains [Citation12]. It is the soluble form of uPAR that can be detected in the blood, urine, and other body fluids [Citation12–14]. Several investigations have revealed that suPAR is connected to a number of kidney diseases, and that high levels of suPAR can directly affect the kidney by activating αVβ3 integrin in podocytes, leading to proteinuria [Citation15–17]. Elevated suPAR levels in CKD patients are linked with worsening renal function and poor prognosis [Citation18]. A recent study found that suPAR may play a role in the etiology of AKI by altering the mitochondrial energy metabolism of the proximal renal tubules and increasing oxidative stress, making the proximal tubules more sensitive to injury [Citation19]. Additionally, several studies have suggested that suPAR is valuable in the early diagnosis and prognosis of sepsis [Citation20–22], but its role in S-AKI remains unclear.
We hypothesized that suPAR could be used to predict the course of S-AKI. To test this hypothesis, we assessed the early predictive value of suPAR for S-AKI patients using blood samples collected at different time points after the diagnosis of sepsis, and observed dynamic changes in plasma suPAR levels in these patients.
Materials and methods
Study design and patients
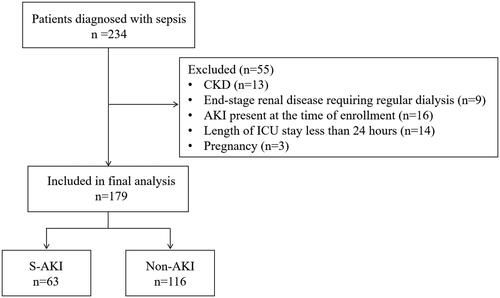
This prospective observational study was conducted at the ICU of the Henan Provincial People’s Hospital. The patients were consecutively selected between January 2022 and March 2023. Patients were included in the study if they fulfilled the criteria for sepsis-3[8] and were ≥18 years of age. The exclusion criteria were as follows: CKD, end-stage renal disease requiring regular dialysis, AKI present at the time of enrollment, expected ICU length of stay of 24 h or less, or pregnancy. The study protocol was approved by the Ethics Committee of Henan Provincial People’s Hospital (approval number: 2023-Lunshen-47) and informed consent was obtained from the patient or the patient’s representative. The clinical registration number is ChiCTR2300072045.
Control group
To compare the levels of suPAR in patients with and without infection or renal failure, a control group consisting of healthy individuals who underwent physical examinations at our hospital was included in the study. The suPAR levels in blood samples collected from medical examination centers were measured in the control group.
Clinical endpoint and definitions
The primary endpoint was the development of AKI within 7 days of sepsis diagnosis. AKI was defined, according to the Kidney Disease: Improving Global Outcomes Working Group criteria, as an absolute increase in the creatinine level of at least 0.3 mg/dL (26.5 μmol/L) within the first 48 h after sepsis diagnosis, a relative increase of at least 50% in the creatinine level within the first 7 d after sepsis diagnosis, or urine volume <0.5 mL/kg/h for at least 6 h [Citation23]. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (defined as Sequential Organ Failure Assessment [SOFA] equal to or greater than two scores)[Citation8].
Clinical and laboratory data collection
Demographic and clinical data were collected from all patients on the day of sepsis diagnosis. Acute Physiology and Chronic Health Evaluation II (APACHE II) and SOFA scores were calculated according to the worst vital signs and biochemical indices of the patients within 24 h after sepsis diagnosis.
Sample collection and measurement of suPAR values
Blood samples were collected from the patients at the time of sepsis diagnosis and at 12, 24, and 48 h thereafter. Plasma was immediately separated by centrifugation at 1000 × g for 10 min. The supernatants were transferred to Eppendorf tubes and stored at −80 °C for further analysis. Plasma suPAR concentrations were measured using a commercially available enzyme-linked immunosorbent assay kit (Elabscience, Wuhan, China) according to the manufacturer’s protocol.
Statistical analysis
Statistical analyses were performed using SPSS Statistics version 20 (IBM Corp, Armonk, NY). Continuous variables are presented as means (±standard deviation) or as medians (25th–75th interquartile range) for normally and non-normally distributed data, respectively. Categorical variables are presented as proportions (%). To compare patients with and without S-AKI, we used an independent sample t-test or Mann–Whitney U-test for continuous variables and the Pearson Chi-square test or Fisher exact test for categorical variables. The predictive accuracy of suPAR was tested using receiver operating characteristic (ROC) curve analysis by calculating the area under the curve (AUC) and 95% confidence interval (CI). Youden’s statistic was used to select the optimum suPAR cutoff point for the diagnosis of S-AKI. Multiple logistic regression analysis was used to analyze factors influencing S-AKI. Statistical significance was set at p < 0.05.
Results
During the 15-month study period, 179 patients were enrolled (). S-AKI developed in 63 patients (35.2%). Mild S-AKI occurred in 35 (55.6%), moderate S-AKI in 19 (30.2%), and severe AKI in 9 (14.3%) patients. The baseline characteristics of the 179 patients are presented in . In the S-AKI group, patients had higher APACHE II and SOFA scores, higher 28-day mortality, a higher proportion of required mechanical ventilation, a higher proportion of blood culture positivity, higher blood urea nitrogen and prothrombin time, and lower pH levels than did those who did not develop S-AKI (p < 0.05).
Table 1. Baseline characteristics.
Additionally, blood samples were collected from 50 healthy control individuals for comparison with the results of patients with sepsis (Figure S1). The mean age in the control group was 57.86 ± 10.88 years (24–84 years), and males accounted for 68%. The study and control groups were comparable, with a non-significant difference between them regarding age (p = 0.179) and sex (p = 0.693). The plasma suPAR concentrations in the sepsis group at 0, 12, 24, and 48 h were 5.70 (3.78, 7.84), 5.76 ± 2.26, 5.76 ± 2.36, and 5.31 (3.68, 7.23) ng/mL, respectively, while the plasma suPAR concentrations in the health examination group were 1.13 (0.84, 1.63) ng/mL (Table S1). The suPAR levels in all patients with sepsis in this study were significantly higher than those in the control group at all time-points (Figure S1).
SuPAR level between S-AKI and non-AKI groups
Patients with S-AKI showed significantly higher suPAR levels at 12, 24, and 48 h than patients without S-AKI(6.69 ± 2.18 vs. 5.26 ± 2.15, p < 0.001; 6.72 (5.11, 8.89) vs. 4.83 (3.55, 6.55), p = 0.004; 6.22 (4.73, 8.51) vs. 4.68 (3.08, 6.57), p = 0.029), but there was no significant difference in suPAR levels between the two groups at 0 h(5.91 (4.14, 8.57) vs. 5.55 (3.78, 7.43), p = 0.688) (). Within 48 h after the confirmation of sepsis, the suPAR levels in the S-AKI group increased and then decreased, while suPAR levels remained low in the non-AKI group (Figure S2). We also compared the suPAR levels of different AKI stages at different time points (Figure S3). At 12 h, there was only a significant difference in suPAR levels between the non-AKI group and AKI 3 stage patients. At 24 h, there was a significant difference in suPAR levels between the non-AKI group and AKI 1, AKI 2, and AKI 3 stage patients. At 48 h, there was a significant difference in suPAR levels between the non-AKI group and AKI 2 and AKI 3 stage patients.
Table 2. Comparison of suPAR levels between S-AKI and non-AKI groups at four time-points after sepsis diagnosis.
Predictive value of the suPAR levels for AKI occurrence in patients with sepsis
illustrates the performance of suPAR in predicting S-AKI at different time-points. SuPAR at 24 h after sepsis diagnosis showed the highest AUC of 0.700 (95% CI, 0.621–0.779) (). The optimal cutoff value for the suPAR level at 24 h was 6.31 ng/mL, with a sensitivity of 61.9% and specificity of 71.6% ().
Figure 2. The receiver operating characteristic curve of suPAR for predicting sepsis-associated acute kidney injury (S-AKI) in patients with sepsis.
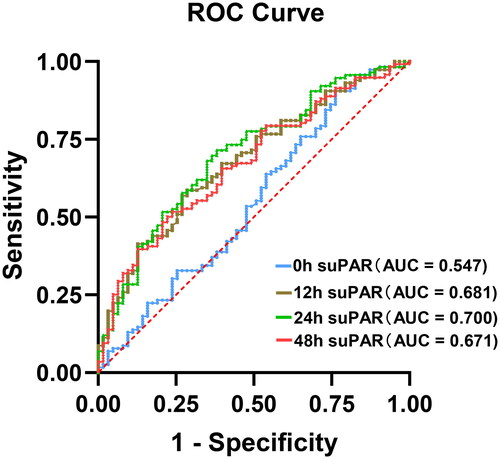
Table 3. Receiver operating characteristic analysis for predicting S-AKI based on the suPAR level.
Logistic regression analysis of risk factors for S-AKI
Univariate logistic regression analysis showed that the APACHE II score, SOFA score, mechanical ventilation, BUN, pH value, and 24-h suPAR level post-sepsis diagnosis were risk factors for S-AKI. Multivariate logistic regression analysis showed that, after adjusting for mechanical ventilation, BUN, and pH values, suPAR at 24-h post-sepsis diagnosis remained an independent risk factor for S-AKI ().
Table 4. Logistic regression analysis of the risk factors for S-AKI.
Discussion
As far as we are aware, no prior research has evaluated the diagnostic efficacy of suPAR for predicting S-AKI. In this research, we observed that patients suffering from sepsis had significantly increased blood suPAR levels, with the highest concentrations found in patients with S-AKI. We also found that the diagnostic performance of suPAR values improved over time, with the best discrimination ability for S-AKI achieved at 24 h after sepsis diagnosis. The optimal suPAR cutoff point for predicting S-AKI was established at 6.31 ng/mL, with an AUC of 0.700 (sensitivity 0.619, specificity 0.716). Multivariate logistic regression analysis showed that suPAR at 24 h after sepsis diagnosis was an independent risk factor for S-AKI.
Previous studies examined the performance of suPAR in diagnosing AKI in patients in various settings [Citation24–26]. Mossanen et al. [Citation24] evaluated the predictive ability of suPAR in 107 patients undergoing cardiac surgery, 21 of whom developed AKI (AUC, 0.647; sensitivity, 73.0%; specificity, 57%). Walls et al. [Citation25] analyzed the diagnostic value of suPAR in older patients in the emergency department, obtaining an AUC of 0.69 (95% CI, 0.60–0.77; sensitivity, 94%; specificity, 40%). In a study of 399 patients receiving percutaneous coronary intervention treatment, Qin et al. [Citation26] found that suPAR predicted the occurrence of AKI with an AUC of 0.765, a sensitivity of 63.1%, and a specificity of 82.3%. However, the diagnostic value of suPAR in patients with sepsis in the ICU was uncertain. Thus, we evaluated the performance of suPAR in the early prediction of S-AKI and found that the suPAR level was a moderate marker for predicting AKI occurrence in patients with sepsis.
The suPAR is a circulating protein that tends to be present at low levels in the blood of healthy individuals [Citation12,Citation27]. Our research likewise found that suPAR levels in healthy individuals were significantly lower than those in patients with sepsis. It is now agreed that high levels of suPAR reflect the inflammatory state of the organism [Citation28]. During inflammation, the innate immune receptors of immune cells induce uPAR expression by activating transcription factors. This mRNA is translated to a protein that localizes to the surface of the cell membrane by specific anchors. uPAR can be cleaved by various proteases or their ligands to produce suPAR. As a result, suPAR levels in vivo frequently reflect the body’s level of inflammation and immune activation [Citation12]. Inflammatory responses play an important role in S-AKI pathogenesis [Citation29]. Therefore, elevated suPAR levels in S-AKI patients may be associated with inflammatory stimulation.
The pathogenesis of S-AKI is multifaceted, involving inflammatory damage, cell-cycle disruption, microcirculatory dysfunction, mitochondrial dysfunction, and metabolic reprogramming [Citation29]. In our study, suPAR was found to be predictive of S-AKI. However, the underlying mechanism was not investigated and may be related to abnormal mitochondrial energy metabolism and oxidative stress. Hayek et al. found that suPAR-treated human proximal renal tubular epithelial cells (HK-2 cells) had higher energy requirements and that bioenergetic changes in HK-2 cells returned to normal after suPAR was inhibited via a monoclonal antibody [Citation19]. In transgenic mice expressing high levels of suPAR, AKI was more severe, as evidenced by higher serum creatinine levels and more severe renal disease, than in control mice after iohexol injection [Citation19]. Therefore, suPAR may be directly involved in AKI pathogenesis by modulating cellular bioenergetics and increasing oxidative stress, rendering the proximal tubules of the kidney more sensitive to injury. Notably, in our study, suPAR levels in patients with S-AKI were significantly higher than those in patients without S-AKI at 12, 24, and 48 h after sepsis diagnosis, whereas suPAR levels at 0 h were comparable between groups. This suggests that patients who will develop S-AKI are not initially exposed to high levels of suPAR; however, suPAR levels gradually increase during progression to AKI, and that high levels of suPAR may further contribute to the course of AKI.
In this study, we observed dynamic changes in plasma suPAR levels after patients were diagnosed with sepsis. The suPAR levels showed a gradual decrease over time in both the S-AKI and non-AKI groups, which may be related to antibiotics use. A previous study found a significant reduction in suPAR concentrations in patients with community-acquired pneumonia treated with antibiotics [Citation30]. Our research did not compare suPAR concentrations before and after antibiotic use, but effective anti-infective treatment helped to reduce the inflammatory response in vivo. As suPAR reflects the level of inflammation, a reduced inflammatory response may decrease suPAR levels.
This study has some limitations. First, this was a single-center study with a small sample size. Therefore, the diagnostic thresholds and trends presented in this study require further validation. Second, this study revealed only the predictive capability of suPAR for AKI. The function of suPAR and its regulatory mechanisms in critically ill patients remain unknown. Therefore, further prospective, large-scale studies are necessary to validate our findings. Finally, our study did not further track changes in renal function in patients with S-AKI. The relationship between plasma suPAR and long-term renal function outcome remains to be confirmed by further clinical studies.
In conclusion, this study showed that suPAR at 24 h after sepsis diagnosis was a moderate predictor of AKI. Our findings may have major clinical significance for the treatment and prognosis of patients with sepsis who are at a risk of AKI.
Ethics approval
The study protocol was approved by the Ethics Committee of Henan Provincial People’s Hospital (approval number: 2023-Lunshen-47) and informed consent was obtained from the patient or the patient’s representative.
Authors’ contributions
W.Z., F.S., and Y.G. conceived and designed the study. W.Z. and J.Z. performed the experiment. Sample collection, data collection, and analysis are performed by W.Z., J.W. X.Z., X.D., H.L.,L.Y., and X.J. Manuscript preparation and revision was done by W.Z.,Y.G. and F.S. All authors revised and approved the final manuscript and are accountable for the accuracy and integrity of the work.
Supplemental Material
Download PDF (46.7 KB)Acknowledgments
We want to thank all the co-authors of this study. This research was not published as a preprint. A portion of the study’s findings were presented at the 60th ERA Congress Abstracts in June 2023 with the title ‘Clinical Value of Soluble Urokinase-Type Plasminogen Activator Receptor in Diagnosis and Prognosis of Sepsis-Associated Acute Kidney Injury’, Link: ttps://doi.org/10.1093/ndt/gfad063c_3644.
Disclosure statement
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1–7. doi: 10.1016/S0140-6736(19)32563-2.
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2.
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7.
- Ikizler TA, Parikh CR, Himmelfarb J, et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021;99(2):456–465. doi: 10.1016/j.kint.2020.06.032.
- Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–387. doi: 10.1681/ASN.2016010105.
- Nagata K, Horino T, Hatakeyama Y, et al. Effects of transient acute kidney injury, persistent acute kidney injury and acute kidney disease on the long-term renal prognosis after an initial acute kidney injury event. Nephrology (Carlton). 2021;26(4):312–318. doi: 10.1111/nep.13831.
- Soto K, Campos P, Pinto I, et al. The risk of chronic kidney disease and mortality are increased after community-acquired acute kidney injury. Kidney Int. 2016;90(5):1090–1099. doi: 10.1016/j.kint.2016.07.018.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217–230. doi: 10.1038/nrneph.2017.184.
- Peters E, Antonelli M, Wittebole X, et al. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from the intensive care over nations audit. Crit Care. 2018;22(1):188. doi: 10.1186/s13054-018-2112-z.
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106.
- Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27(3–4):157–172. doi: 10.1155/2009/504294.
- Gustafsson A, Ajeti V, Ljunggren L. Detection of suPAR in the saliva of healthy young adults: comparison with plasma levels. Biomark Insights. 2011;6:119–125. doi: 10.4137/BMI.S8326.
- Rabna P, Andersen A, Wejse C, et al. Urine suPAR levels compared with plasma suPAR levels as predictors of post-consultation mortality risk among individuals assumed to be TB-negative: a prospective cohort study. Inflammation. 2010;33(6):374–380. doi: 10.1007/s10753-010-9195-2.
- Hayek SS, Koh KH, Grams ME, et al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23(8):945–953. doi: 10.1038/nm.4362.
- Wei C, Li J, Adair BD, et al. uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest. 2019;129(5):1946–1959. doi: 10.1172/JCI124793.
- Wei C, Möller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696.
- Jhee JH, Nam BY, Lee CJ, et al. Soluble urokinase-type plasminogen activator receptor, changes of 24-Hour blood pressure, and progression of chronic kidney disease. J Am Heart Assoc. 2021;10(1):e017225. doi: 10.1161/JAHA.120.017225.
- Hayek SS, Leaf DE, Samman Tahhan A, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382(5):416–426. doi: 10.1056/NEJMoa1911481.
- Huang Q, Xiong H, Yan P, et al. The diagnostic and prognostic value of suPAR in patients with sepsis: a systematic review and Meta-Analysis. Shock. 2020;53(4):416–425. doi: 10.1097/SHK.0000000000001434.
- Nasr El-Din A, Abdel-Gawad AR, Abdelgalil W, et al. Evaluation of sTREM1 and suPAR biomarkers as diagnostic and prognostic predictors in sepsis patients. Infect Drug Resist. 2021;14:3495–3507. doi: 10.2147/IDR.S314237.
- Efat A, Shoeib SA, Arafa AF, et al. Thrombo-inflammatory biomarkers to predict sepsis outcome. Int J Immunopathol Pharmacol. 2021;35:20587384211048561. doi: 10.1177/20587384211048561.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184. doi: 10.1159/000339789.
- Mossanen JC, Pracht J, Jansen TU, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017;18(8):1662. doi: 10.3390/ijms18081662.
- Walls AB, Bengaard AK, Iversen E, et al. Utility of suPAR and NGAL for AKI risk stratification and early optimization of renal risk medications among older patients in the emergency department. Pharmaceuticals (Basel). 2021;14(9):843. doi: 10.3390/ph14090843.
- Qin Y, Qiao Y, Wang D, et al. The predictive value of soluble urokinase-type plasminogen activator receptor in Contrast-Induced acute kidney injury in patients undergoing percutaneous coronary intervention. Int J Gen Med. 2021;14:6497–6504. doi: 10.2147/IJGM.S339075.
- Chew-Harris J, Appleby S, Richards AM, et al. Analytical, biochemical and clearance considerations of soluble urokinase plasminogen activator receptor (suPAR) in healthy individuals. Clin Biochem. 2019;69:36–44. doi: 10.1016/j.clinbiochem.2019.05.010.
- Desmedt S, Desmedt V, Delanghe JR, et al. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit Rev Clin Lab Sci. 2017;54(2):117–133. doi: 10.1080/10408363.2016.1269310.
- Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026.
- Tsai PK, Tsao SM, Yang WE, et al. Plasma soluble urokinase-type plasminogen activator receptor level as a predictor of the severity of Community-Acquired pneumonia. Int J Environ Res Public Health. 2019;16(6):1035. doi: 10.3390/ijerph16061035.