Abstract
Background and purpose: Acute kidney injury (AKI) is a common serious complication in sepsis patients with a high mortality rate. This study aimed to develop and validate a predictive model for sepsis associated acute kidney injury (SA-AKI). Methods: In our study, we retrospectively constructed a development cohort comprising 733 septic patients admitted to eight Grade-A tertiary hospitals in Shanghai from January 2021 to October 2022. Additionally, we established an external validation cohort consisting of 336 septic patients admitted to our hospital from January 2017 to December 2019. Risk predictors were selected by LASSO regression, and a corresponding nomogram was constructed. We evaluated the model’s discrimination, precision and clinical benefit through receiver operating characteristic (ROC) curves, calibration plots, decision curve analysis (DCA) and clinical impact curves (CIC) in both internal and external validation. Results: AKI incidence was 53.2% in the development cohort and 48.2% in the external validation cohort. The model included five independent indicators: chronic kidney disease stages 1 to 3, blood urea nitrogen, procalcitonin, D-dimer and creatine kinase isoenzyme. The AUC of the model in the development and validation cohorts was 0.914 (95% CI, 0.894–0.934) and 0.923 (95% CI, 0.895–0.952), respectively. The calibration plot, DCA, and CIC demonstrated the model’s favorable clinical applicability. Conclusion: We developed and validated a robust nomogram model, which might identify patients at risk of SA-AKI and promising for clinical applications.
1. Introduction
Sepsis is a life-threatening condition characterized by organ dysfunction resulting from a dysregulated host response to infection, which represents the leading cause of death for patients in the intensive care unit (ICU) [Citation1]. Acute kidney injury (AKI) is the most prevalent organ dysfunction in sepsis, accounting for more than 50% of AKI cases in ICU [Citation2], which not only extends the length of ICU stays and hospitalizations but also imposes a significant financial burden while exacerbating patients’ prognosis [Citation3,Citation4]. Fortunately, AKI can be reversed when identified and treated promptly during its early stages, ultimately reducing AKI-related mortality [Citation5]. Therefore, early identification of patients with high risk of sepsis-associated acute kidney injury (SA-AKI) is of crucial importance for the management of critical patients.
The prediction of AKI in septic patients remains a prominent topic in critical care medicine. Numerous efforts have been made to predict AKI in sepsis patients over the past decades. On the one hand, several novel biomarkers had been developed to improve diagnostic sensitivity and specificity of SA-AKI. Frustratingly, these biomarkers mostly are prohibitively expensive for widespread clinical use [Citation6]. On the other hand, some researchers have established predictive models for AKI in septic patients. However, these models have not been widely applied, they are liable to overfitting due to their reliance on small sample sizes or lack of external validation [Citation7–10]. Consequently, there are still no universally accepted criteria for predicting SA-AKI. Thus, we utilized readily available, inexpensive, and commonly collected variables from several major hospitals in Shanghai to establish a nomogram model, which might propose a valuable clinical tool for early identification of SA-AKI.
2. Patients and methods
2.1. Development and external validation cohorts
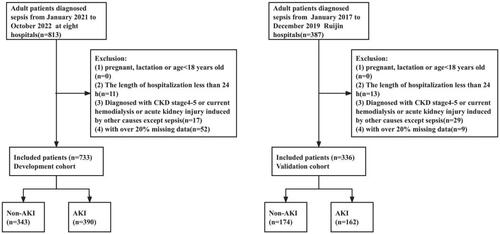
This is a multicenter retrospective clinical study (ChiCTR2000036252) which included a total of 1200 septic patients. In the development cohort, we screened 813 septic patients admitted to emergency department (ED) or ICU across eight Grade-A tertiary hospitals in Shanghai from January 2021 to October 2022. This cohort consisted of patients from eight various hospitals, including Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (341 patients), Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (133 patients), Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (82 patients), Shanghai Tenth People’s Hospital Affiliated to Tongji University School of Medicine (68 patients), Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (67 patients), Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (50 patients), Zhongshan Hospital Affiliated to Fudan University (46 patients) and Shanghai First People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (26 patients). In validation cohort, we screened 387 septic patients admitted to ED or ICU of Ruijin Hospital from January 2017 to December 2019. The data from these two cohorts were entirely independent of each other.
2.2. Participants
Inclusive criteria were as follows: Patients had to be diagnosed with sepsis according to sepsis 3.0 and were admitted to ED or ICU. Exclusion criteria were as follows: (1) with pregnancy, lactation or age less than 18 years old; (2) the length of hospitalization less than 24 h; (3) diagnosed of chronic kidney disease (CKD) stage4-5 with estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73m3 or had undergone regular dialysis or had kidney transplant or acute kidney injury induced by other causes except sepsis; (4) with over 20% missing data. Both the development cohort and validation cohort adhered to the same inclusive and exclusion criteria.
2.3. Definition of sepsis and AKI
In our study, we conformed with Sepsis 3.0 definition [Citation1] and the Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria [Citation11] on diagnosis of SA-AKI. We evaluated the diagnosis of SA-AKI after 7 days of admission. Sepsis diagnosed as an infection and Sequential Organ Failure Assessment score (SOFA) ≥2 which encompassed the respiratory function, cardiovascular function, platelet count, Glasgow Coma Scale score, bilirubin concentration and kidney function. AKI was diagnosed as serum creatinine (Scr) ≥ 26.4 μmol/L within 48 h or Scr ≥50% within 7 days or urine output volume less than 0.5 mL/kg/hour for at least 6 h. AKI severity was categorized as AKI I, II, or III according to varying degrees of creatinine elevation and whether renal replacement therapy (RRT) was administered. Notably, ascertaining baseline Scr values can be challenging, making it difficult to identify true changes in Scr and confirm AKI presence. There is still no consensus method to define baseline Scr. In our study, we defined baseline Scr as the average of all Scr values in the past 1 year [Citation12]. If not available and in the absence of prior renal disease, we used Modification of Diet in Renal Disease (MDRD) Back-Estimated Baseline Scr: Creatinine (µmol/L) =88.4×(0.74 − 0.2 + 0.003 × age)in females, and Creatinine (µmol/L) =88.4×(0.74 + 0.003 × age)in males [Citation13]. For those suspected CKD patients, we defined the baseline SCr as the first measured at admission [Citation14]. CKD is defined as abnormalities of kidney structure or function, eGFR less than 60 mL/min/1·73 m2, or abnormalities of laboratory testing or imaging, or kidney damage markers, such as albuminuria, hematuria and that are present for at least 3 months. In our study, the CKD stage1-3 refers to the level of eGFR above 30 mL/min/1.73m3 for more than 3 months according to GFR category (G1–G5) [Citation15].
2.4. Data collection
In our study, we collected data with worst value observed within the initial 24 h following admission to the emergency department (ED) or intensive care unit (ICU). These data encompassed a wide range of factors, including demographics, vital signs, medical history, laboratory test results, scoring systems, therapeutic interventions, and outcome measurements. Demographic included age and gender. Vital signs included body temperature (T), heart rate (HR), respiratory rate (RR) and mean arterial pressure (MAP). Medical history included the admission diagnosis, main site of the septic infection, blood culture positivity, antibiotic exposure within one month before admission, and the presence of chronic medical conditions such as hypertension, diabetes, CKD stage1-3, malignancies and immunosuppressive conditions. Laboratory data included white blood cell (WBC), neutrophil percentage (NEUT%), C-reactive protein (CRP), serum procalcitonin (PCT), platelet (PLT), lactic acid (Lac), oxygenation index, hemoglobin (HGB), activated partial thromboplastin time (APTT),prothrombin time (PT), fibrinogen (FIB), fibrinogen degradation products (FDP), D-dimer (D-D), albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), blood urea nitrogen (BUN), serum creatinine (Scr), potassium, sodium, calcium, phosphorus, cardiac troponin I (cTnI), myoglobin (MB) and creatine kinase isoenzyme (CK-MB). Scoring systems included acute physiology and chronic health assessment (APACHE II score), SOFA score, GCS score, Charlson score. Therapeutic managements included operation, hemodialysis, vasopressor use, anticoagulants use and albumin administration. Outcome measurements included incidence and classification of AKI, length of ICU stay, length of hospital stay and in-hospital mortality.
2.5. Statistical analysis
Variables with more than 20% missing data were excluded from study, and variables with less than 20% missing data were filled with multiple imputation. All statistical analysis were performed by SPSS 26.0(p < 0.05 was considered statistically significant) and the R software version 4.3.2. Continuous variables were expressed as means ± standard deviation (SD) and non-normally distributed variables were expressed as medians with interquartile ranges (IQR). The differences between continuous variables groups were compared by t-test. Categorical variables were expressed as frequencies (percentages) or absolute values and the differences were compared by the χ2 test or Fisher’s exact test. The development cohort was used to construct the prediction model and the bootstrapping with 1000 resamples was used for internal validation. In addition, the external validation cohort was used to verify the accuracy of the model. Owing to large numbers of variables in our study, the independent risk factors were screened by the least absolute shrinkage and selection operator (LASSO) regression. Subsequently, multivariate logistic regression was carried out for independent risk factors and the nomogram was constructed. Throughout these processes, we complied with TRIPOD (transparent reporting of a multivariable prediction model for individual prognosis or diagnosis) guidance for performing the prediction model [Citation16].
3. Results
3.1. Comparison of baseline characteristics between the development and validation cohorts
Out of the initial 1200 patients diagnosed with sepsis, 131 patients were subsequently excluded based on these criteria, resulting in a total of 1069 patients included in our study. The inclusion and exclusion process of development and validation cohort are shown in . The detailed baseline characteristics of the total 1069 patients in both development and validation cohort are illustrated in . Baseline characteristics are not exactly balanced between the two cohorts because they derive from completely different data source. For the development cohort, the patients (477 males and 256 females) had a median age of 70 (range, 60 to 79) years. In our study, the prevalence of AKI was 53.21% and categorized into stage 1 (172 patients, 23.47%), stage 2 (92 patients, 12.55%) and stage 3 (126 patients, 17.19%). For the external validation cohort, the patients (227 males and 109 females) had a median age of 66.5(range, 48.0 to 76.8) years, of which the prevalence of AKI was 48.21%. All these AKI patients were categorized into stage 1 (70 patients, 20.83%), stage 2 (43 patients, 12.80%) and stage 3 (49 patients, 14.58%) respectively according to KIDGO criteria. Moreover, whether in the development or validation cohort, septic infections mostly derive from the respiratory system and gastrointestinal tract. Causative pathogens were predominantly gram-negative bacteria, followed by fungi and gram-positive bacteria. However, in some septic patients, the exact causative organism could not be identified.
Table 1. Summarized baseline and outcome characteristics of the patients with sepsis in development and validation cohort.
3.2. Comparison of baseline characteristics between the non-AKI and AKI in development cohort
The differences of characteristics between AKI and non-AKI groups in development cohort are described in supplementary table 1. The results showed that patients in the AKI group had higher mortality and higher incidence of decreased MAP (<65mmHg), septic shock, chronic kidney disease stage 1-3, increased PCT (>25ng/ml), increased lactate (>2mmol/L), urinary tract resource infection, fungi infection, decreased PaO2/FiO2 (<300mmHg) and sodium abnormalities. The values of heart rate, SOFA score, APACHE II score, GCS score, Charlson Score, WBC, NEUT%, CRP, BUN, PT, APTT, D-D, CK-MB, MB and troponin were much higher, but Hct and albumin were lower in AKI group compared with those without AKI (p < 0.05). AKI patients were more likely to require anticoagulants and vasopressor administration compared with those non-AKI patients.
3.3. Screening for predictive factors by LASSO and multivariate logistic regression
We screened these predictive factors by LASSO regression, which may eliminate the multicollinearity in variables [Citation17]. In our model, LASSO regularization algorithm with 10-fold cross-validation was performed to explore the optimal parameter. Eventually, the resulting 6 variables with non-zero coefficients were screened based on the log λ (1-se) value (). These potential predictors consist of CKD1-3 stage, D-dimer, BUN, PCT, CK-MB and vasopressors administration. Then multivariate logistic regression analysis was used to further identification. Lastly, CKD1-3 stage (OR: 3.893, 95% CI: 1.710–8.866, p = 0.001), BUN (OR: 1.263, 95% CI: 1.210–1.319, p < 0.001), PCT more than 25 ng/ml (OR: 2.690, 95% CI: 1.594–4.542, p < 0.001), D-dimer (OR: 1.044, 95% CI: 1.022–1.066, p < 0.001) and CK-MB (OR: 1.018, 95% CI: 1.005–1.032, p = 0.008) as independent risk factors by multivariate logistic regression in the development cohort ().
Figure 2. Feature selection using the LASSO regression method in the development cohort.
(A) Two dotted vertical lines denote the optimal parameter λ (1-se) values based respectively. Lastly, 6 variables with nonzero coefficients were selected by verifying the optimal λ (1-se) in the LASSO model.
(B) LASSO coefficient profiles of the 54 variables. The coefficient profile plot was produced against the log λ (1-se) sequence.
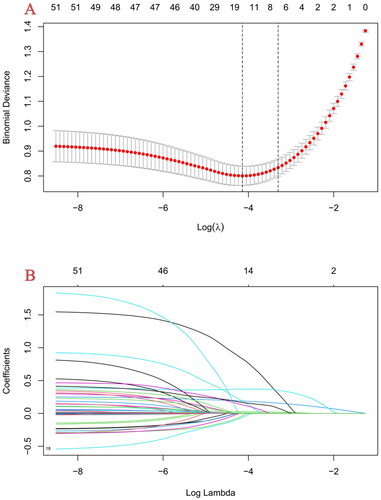
Table 2. Multivariate logistic regression analysis for risk factors of SA-AKI.
3.4. Risk prediction nomogram development and internal validation
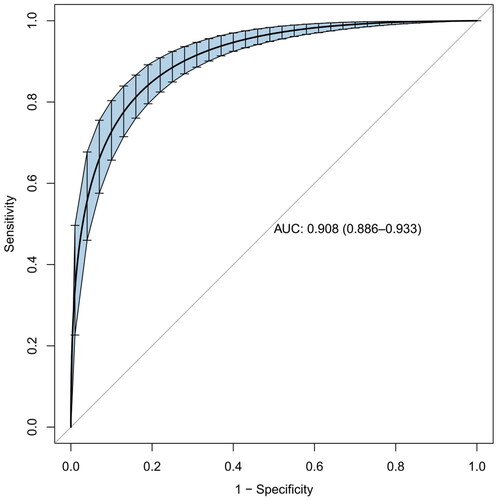
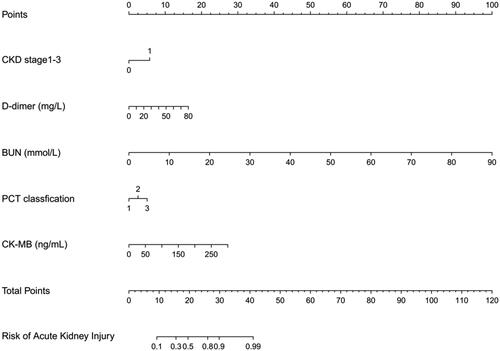
Based on the LASSO regression and multivariate logistic regression analysis, incorporating the clinical corresponding results, five predictors from the development cohort were screened in the SA-AKI risk prediction and expressed as a nomogram (). After the construction of the nomogram, we performed internal validation by bootstrapping with 1000 resamples. The resulting calculated area under the curve (AUC) value of 1000 bootstrap samples was 0.908 (95% CI, 0.886–0.933), indicating that our model has good discrimination ().
Figure 3. Nomogram to estimate the risk of S-AKI in patients with sepsis.
The nomogram illustrated that each predictor corresponding to a specific score ranging from 0 to 100. The total score is added by all predictors and presented on the ‘Total Points’ axis. The morbidity of SA-AKI corresponds to the bottom ‘prediction possibility’ axis, which illustrates the higher score, the higher risk of developing SA-AKI.
3.5. External validation, performance and clinical use of the nomogram
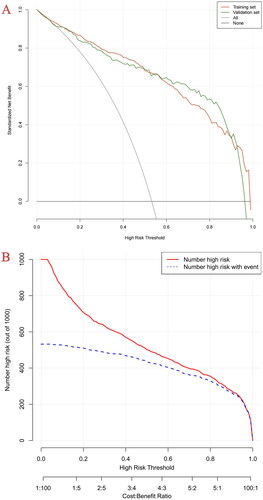
In the development cohort, the model of AUC was 0.914 (95% CI, 0.894–0.934) which is higher than SOFA score[0.749 (95% CI, 0.714–0.784)] and APACHE II score [0.735 (95% CI, 0.700–0.771)], and the calibration curve was close to the ideal diagonal line, which demonstrated good performance of the nomogram. To assess its generalizability, we validated the nomogram using clinical data from an external cohort. In this validation cohort, the AUC was 0.923 (95% CI, 0.895–0.952), and the calibration curve also showed excellent consistency with the ideal diagonal line (, ). The nomogram model had good predictive accuracy in the aspect of discrimination and calibration, but the application value in clinical practice was unknown. Therefore, we applied the decision curve analysis (DCA) and clinical impact curves (CIC) to verify the clinical applicability of the nomogram model, which is illustrated in . In the training and validation cohorts, the DCA revealed that within the threshold probability ranges of 0.01–0.99 and 0.02–0.97, the nomogram offered superior net benefits compared to ‘full intervention’ or ‘no intervention’ strategies. Clinical impact curves, another type of figure produced by DCA, visually indicated that the larger the high-risk threshold, the higher the true positives as presented in , which indicated favorable net clinical benefit [Citation18]. In both internal and external cohorts, our nomogram had excellent prediction performance, showing the nomogram has a high degree of generalizability and is conducive to clinical practice.
Figure 5. The ROC curve of the nomogram, SOFA score and APACHE II score for predicting SA-AKI in sepsis patients. The AUC of the nomogram for the prediction of AKI in septic patients was 0.914 (95% CI, 0.894–0.934) in the training set and 0.923 (95% CI, 0.895–0.952) in the validation set. The AUC of SOFA score and APACHE II score for the prediction of AKI were 0.749 (95% CI, 0.714–0.784) and 0.735 (95% CI, 0.700–0.771), respectively.
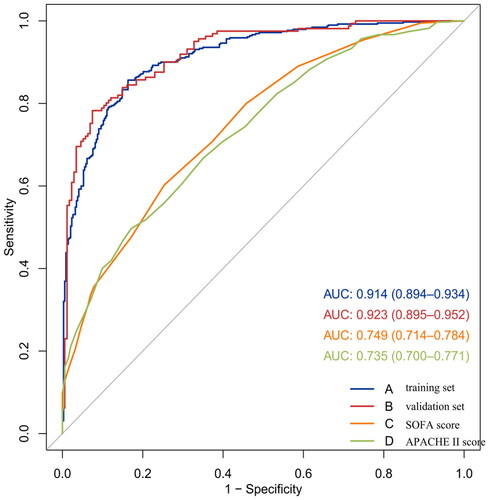
Figure 6. Calibration curves of the predictive nomogram in the training and validation set. A plot along the 45°line would show the predicted SA-AKI is identical to the actual SA-AKI. The dotted line has a close fit to the solid line, which showed good predictive accuracy of the nomogram.
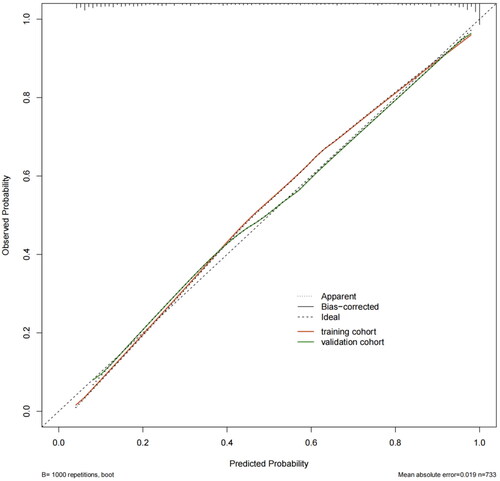
Figure 7. Decision curve analysis and clinical impact curve of the nomogram.
(A) Decision curve analysis: the x-axis displays the threshold probability. The y-axis displays standardized net benefit. The oblique grey line showed that all patients received the intervention, and the grey horizontal line showed that no patient received the intervention.
(B) Clinical impact curve: for the predictive model in the training set. The red curve shows the number of people who are identified as positive (high risk) by the model at each threshold probability. The blue curve shows the number of true positives at each threshold probability.
4. Discussion
AKI is a common serious complication in patients with sepsis and can increase patients’ mortality. In our study, the prevalence of AKI was 53.2% which is similar to previous studies (30% to 70%) [Citation19–22]. In the development cohort, the mortality of SA-AKI will be higher than septic patients without AKI (37.7% vs 17.8%, p < 0.001). Additionally, we observed that AKI occurrence was related to the severity of sepsis. Septic shock was prone to be complicated with AKI, which was consistent with the results of previous studies [Citation19,Citation22]. However, the pathogenesis of septic AKI is intricate and has not been completely identified yet. Increasing research discovered that ischemia-reperfusion injury was not the only mechanism of SA-AKI, multiple mechanisms such as inflammation, micro-circulatory dysfunction and metabolic reprogramming could result in AKI in septic patients [Citation23]. Consequently, developing a reliable prediction model is urgent for identifying AKI and providing timely interventions to improve patient prognosis. Recently, some studies developed predictive models based on logistics regression(LR) or machine learning(ML) analysis for predicting AKI in septic patients. Fan et al. [Citation7] applied LR to construct a model predicting AKI based on 15,726 septic patients, and the model showed a good predictive accuracy with AUROC 0.711. Zhang et al. [Citation24] developed an accurate ensemble model to predict AKI early by machine learning based on MIMIC-IV database in development cohort. Meanwhile, multicenter external dataset was consisting of the the eICU Collaborative Research Database, Critical Care Database and Zigong Fourth People’s Hospital Database from China. The AUROC values of this ensemble model were 0.774–0.788 and 0.756–0.813 in development and validation cohort respectively. Yue et al. [Citation21] on basis of MIMIC-IV database developed and compared several models for predicting SA-AKI by ML algorithms. Lastly, they found that urine output, mechanical ventilation, BMI, eGFR, minimum Scr, maximum PPT, and minimum BUN were mostly associated with SA-AKI and the XGBoost model showed the best predictive performance. However, these studies utilized foreign MIMIC database or included intricate indicators with an unfavorable performance. There was rarely study based on domestic multicenter data to construct prediction model and through external validation. Therefore, we utilized multicenter data to construct a simple but robust prediction model in development cohort and carried out external validation. Ultimately, we identified that CKD stage1-3, D-Dimer, BUN, CK-MB and PCT(>25ng/ml) were independent predictors of SA-AKI and constructed a reliable SA-AKI predictive model. Both SOFA score and APACHE II score are commonly used and effective rating index in critical medicine whose reliability have been recognized widely by the medical community especially in critical medicine [Citation25,Citation26]. Our nomogram model showed favorable performance compared with SOFA score and APACHE II score (), which might be an applicable tool for SA-AKI prediction in the future.
Patients with CKD have poor renal function reservation and more likely to suffer from AKI once they are attacked by acute status such as trauma, burning, inflammation and so on. Interestingly, previous researches mostly not categorized CKD stage according to the severity of renal dysfunction or excluded CKD patients [Citation8,Citation9]. In our study, we taken prior CKD stage1-3 into consideration to explore the relation between baseline renal function and AKI. We found that septic patients with CKD stage1-3 are more susceptible to developing AKI (OR:3.046, 95% CI:1.291-7.187, p = 0.011). Previous study found that survivors of AKI especially those with severe AKI have a higher risk of CKD [Citation27]. These two clinical syndromes are interrelated which AKI and CKD predispose to each other in a malicious circle and may result in negative impacts independently or synergistically. It reminded that clinicians should pay more attention to those who have abnormal baseline renal function before admission.
BUN is a protein metabolic waste from the liver and excreted by the kidneys, which is a typical biomarker to evaluate renal function on a regular basis. Previous study had shown that even slightly increased level of BUN was significantly related to an increasing risk of AKI in ICU setting [Citation8,Citation9,Citation28]. We also found that septic patients had a higher level of BUN when accompanied by AKI. However, BUN has limited specificity because changes in BUN levels are influenced by a variety of factors including blood volume, nutritional status and protein intake.
Inflammation plays an increasingly crucial role in the physiopathology of AKI. PCT acted as a monocyte chemoattractant at the site of inflammation, causing mesangial cell apoptosis and boosting the synthesis of inflammatory cytokines, which could predict the occurrence of SA-AKI in a retrospective study [Citation29]. Our study had similar finding to previous studies that PCT was more accurate at predicting SA-AKI than other inflammatory biomarkers such as WBC, NEUT and CRP [Citation30]. Especially, when the PCT level markedly elevated(>25ng/ml), the incidence of SA-AKI significantly increased. Notably, we found that PCT less than 25 ng/ml was not an independent predictor of SA-AKI, which may indicate that only the severe inflammatory response could cause SA-AKI.
Sepsis inflammation response can damage the vascular endothelium and arouse a chain reaction. Excessive platelets and coagulation factors consumption aggravated hyperfibrinolysis, thus causing coagulation disorders subsequently [Citation31,Citation32]. Microvascular dysfunction is considered to be one of the mechanisms of SA-AKI [Citation33] and it seems that some coagulative parameters could predict AKI. A retrospective study [Citation34] identified that more than 50% of patients with septic AKI had at least one abnormal coagulation index, which may prompt poor prognosis. Xin et al. [Citation35] observed that PLT and PTA were independent predictors of SA-AKI and then constructed a prediction model (AUC = 0.887) combined with PCT. Moreover, Xu et al. [Citation36] conducted an observational retrospective study that showed that increased D-dimer (OR = 1.098, 95% CI 1.002–1.202, p = 0.045) is a significant predictive factor for intra-abdominal patients with septic shock. In our study, we found that D-dimer as an indicator to judge coagulation function was also an independent predictor for SA-AKI, which was similar to previous studies.
CK-MB mainly exists in myocardial cells and has been widely used in cardiac ischemic disease owing to its favorable cost-performance ratio and simplicity. Early sepsis is characterized by circulatory dysfunction which referred to intravascular volume reduction and vasodilation, showing myocardial ischemia with the increase of CK-MB [Citation4]. However, the association between CK-MB and renal insufficiency in septic patients has not been clearly reported. Wei et al. [Citation37] performed a prospective single center observational research with 3369 patients, suggesting that CK-MB was an independent risk factor for predicting contrast-induced AKI among myocardial infarction patients. Moreover, the correlation analysis of CK-MB and some laboratory indictors suggested that with the increase of CK-MB, Scr and HCT also increased [Citation38]. In our study, we also found that myocardial injury due to sepsis may cause increase of CK-MB, which might lead damage to renal tubular cells after glomerular filtration, thereby causing renal dysfunction. More prospective studies are still needed to explore the relationship between CK-MB and AKI in septic patients in the future.
Compared with previous researches on SA-AKI prediction in critically ill patients, our research has several novel contributions. Firstly, our study based on multicenter data from eight Grade-A tertiary hospitals in Shanghai, which is the first multicenter clinical research about SA-AKI prediction and enhance the generalizability of model in clinical use. Secondly, lack of external validation has been pointed out as a shortcoming of previous AKI prediction models [Citation39]. In order to demonstrate the clinical utility of our proposed model in different populations, our study performed external validation and internal validation. Thirdly, compared with traditional score systems such as SOFA and APACHE II score, our model has a more favorable predictive performance. Last but not the least, our model can easily promote and applicate to clinical use owing to only basic and commonly tested clinical data were enrolled.
5. Limitations
Nevertheless, we should acknowledge our study has some limitations. Firstly, given its retrospective design, the causal relationship of the study factors and AKI is difficult to clarify, and only its correlation can be confirmed. Prospective experiments are still needed further confirmation in future research. Secondly, we only enrolled indicators within the first 24 h of admission and did not dynamically analyze the changes of indicators during hospitalization. Owing to a lack of corresponding follow-up data, it is difficult for us to confirm the impact of risk indicators on subsequent AKI progression. Thirdly, due to the limited sensitivity and tracking difficulties of urine output, we solely considered changes in Scr for AKI diagnosis in our study, which may led to overlooking some acute renal damage. Lastly, as a multicenter cohort, the insufficient sample size makes it impossible to use machine learning for further study. In the future, we will collect more larger sample sizes and utilize machine learning for enhanced and continuous prediction of SA-AKI by a combination of multiorgan and dynamic clinical data. However, we still suppose that the constructed model is useful for clinicians to timely identify critical patients with sepsis at high risk of developing AKI.
6. Conclusion
The prediction of SA-AKI is intricate due to its high heterogeneity. In contrast to previous models, our predictive model is a valuable supplement. We developed a simple SA-AKI prediction model on basis of five easily obtained indicators and validated it both internally and externally, which might be a useful tool for clinical applications.
Ethics approval
Our study protocol was conducted in accordance with the Declaration of Helsinki. The study was reviewed and approved by ethics committee of Ruijin Hospital ([2021] Research Approval No. 59) and the requirement for informed consent was waived owing to retrospective study.
Supplemental Material
Download PDF (167 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):1–12. doi: 10.1001/jama.2016.0287.
- Alobaidi R, Basu RK, Goldstein SL, et al. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35(1):2–11. doi: 10.1016/j.semnephrol.2015.01.002.
- Anderko RR, Gómez H, Canna SW, et al. Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation. Intensive Care Med Exp. 2022;10(1):6. doi: 10.1186/s40635-022-00433-y.
- Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4(1):22. doi: 10.1186/s40560-016-0148-1.
- Coelho S, Cabral G, Lopes JA, et al. Renal regeneration after acute kidney injury. Nephrology . 2018;23(9):805–814. doi: 10.1111/nep.13256.
- Xie T, Xin Q, Chen R, et al. Clinical value of prognostic nutritional index and neutrophil-to-lymphocyte ratio in prediction of the development of sepsis-induced kidney injury. Dis Markers. 2022;2022:1449758–1449759. doi: 10.1155/2022/1449758.
- Fan C, Ding X, Song Y. A new prediction model for acute kidney injury in patients with sepsis. Ann Palliat Med. 2021;10(2):1772–1778. doi: 10.21037/apm-20-1117.
- Deng F, Peng M, Li J, et al. Nomogram to predict the risk of septic acute kidney injury in the first 24 h of admission: an analysis of intensive care unit data. Ren Fail. 2020;42(1):428–436. doi: 10.1080/0886022X.2020.1761832.
- Yue S, Li S, Huang X, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. 2022;2022:9367873–9367812. doi: 10.1155/2022/9367873.
- Yang S, Su T, Huang L, et al. A novel risk-predicted nomogram for sepsis associated-acute kidney injury among critically ill patients. BMC Nephrol. 2021;22(1):173. doi: 10.1186/s12882-021-02379-x.
- Kellum JA, Lameire N, Group K a G W. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454.
- Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011.
- Závada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25(12):3911–3918. doi: 10.1093/ndt/gfp766.
- De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care. 2016;20(1):69. doi: 10.1186/s13054-016-1218-4.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022 Nov;102(5S):S1–S127. doi: 10.1016/j.kint.2022.06.008. PMID: 36272764.
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Surg. 2015;102(3):148–158. doi: 10.1002/bjs.9736.
- Mcneish DM. Using lasso for predictor selection and to assuage overfitting: a method long overlooked in behavioral sciences. Multivariate Behav Res. 2015;50(5):471–484. doi: 10.1080/00273171.2015.1036965.
- Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi: 10.1016/j.eururo.2018.08.038.
- Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863.
- Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock–a significant independent risk factor for mortality: results from the German prevalence study. Nephrol Dial Transplant. 2008;23(3):904–909. doi: 10.1093/ndt/gfm610.
- Yue S, Li S, Huang X, et al. Machine learning for the prediction of acute kidney injury in patients with sepsis. J Transl Med. 2022;20(1):215. doi: 10.1186/s12967-022-03364-0.
- Kwak SH, Ahn S, Shin MH, et al. Identification of biomarkers for the diagnosis of sepsis-associated acute kidney injury and prediction of renal recovery in the intensive care unit. Yonsei Med J. 2023;64(3):181–190. doi: 10.3349/ymj.2022.0324.
- Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–417. doi: 10.1038/s41581-023-00683-3.
- Zhang L, Wang Z, Zhou Z, et al. Developing an ensemble machine learning model for early prediction of sepsis-associated acute kidney injury. iScience. 2022;25(9):104932. doi: 10.1016/j.isci.2022.104932.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751.
- Fernando SM, Rochwerg B, Seely AJE. Clinical implications of the third international consensus definitions for sepsis and septic shock (sepsis-3). CMAJ. 2018;190(36):E1058–E1059. doi: 10.1503/cmaj.170149.
- Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019.
- Samimagham HR, Kheirkhah S, Haghighi A, et al. Acute kidney injury in intensive care unit: incidence, risk factors and mortality rate. Saudi J Kidney Dis Transpl. 2011;22(3):464–470.
- Jeeha R, Skinner DL, De Vasconcellos K, et al. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology. 2018;23(12):1090–1095. doi: 10.1111/nep.13174.
- Fu G, Zhan HC, Li HL, et al. Association between procalcitonin and acute kidney injury in patients with bacterial septic shock. Blood Purif. 2021;50(6):790–799. doi: 10.1159/000512351.
- Fani F, Regolisti G, Delsante M, et al. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol. 2018;31(3):351–359. doi: 10.1007/s40620-017-0452-4.
- Jansen MPB, Florquin S, Roelofs J. The role of platelets in acute kidney injury. Nat Rev Nephrol. 2018;14(7):457–471. doi: 10.1038/s41581-018-0015-5.
- Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35(1):96–107. doi: 10.1016/j.semnephrol.2015.01.010.
- Pan L, Mo M, Huang A, et al. Coagulation parameters may predict clinical outcomes in patients with septic acute kidney injury. Clin Nephrol. 2021;96(5):253–262. doi: 10.5414/CN110459.
- Xin Q, Xie T, Chen R, et al. A predictive model based on inflammatory and coagulation indicators for sepsis-induced acute kidney injury. J Inflamm Res. 2022;15:4561–4571. doi: 10.2147/JIR.S372246.
- Xu Z, Cheng B, Fu S, et al. Coagulative biomarkers on admission to the ICU predict acute kidney injury and mortality in patients with septic shock caused by intra-abdominal infection. Infect Drug Resist. 2019;12:2755–2764. doi: 10.2147/IDR.S218592.
- Wei W, Zhang L, Zhang Y, et al. Predictive value of creatine kinase MB for contrast-induced acute kidney injury among myocardial infarction patients. BMC Cardiovasc Disord. 2021;21(1):337. doi: 10.1186/s12872-021-02155-7.
- Jiang XT, Ding L, Huang X, et al. Elevated CK-MB levels are associated with adverse clinical outcomes in acute pancreatitis: a propensity score-matched study. Front Med . 2023;10:1256804. doi: 10.3389/fmed.2023.1256804.
- Guan C, Li C, Xu L, et al. Risk factors of cardiac surgery-associated acute kidney injury: development and validation of a perioperative predictive nomogram. J Nephrol. 2019;32(6):937–945. doi: 10.1007/s40620-019-00624-z.