Abstract
Background
The association between proteinuria levels and cardiovascular disease (CVD) development and all-cause mortality in chronic kidney disease (CKD) patients remains controversial.
Methods
In this investigation, we conducted a retrospective analysis involving 1138 patients who were registered in the CKD-Research of Outcomes in Treatment and Epidemiology (ROUTE) study. The primary outcome of this study was the composite of cardiovascular events or all-cause death. Cox proportional hazards regression, smooth curve fitting, piecewise linear regression, and subgroup analyses were used.
Results
The mean age of the included individuals was 67.3 ± 13.6 years old. Adjusted hazard ratios (HRs) for UPCR in middle and high groups, compared to the low group, were 1.93 (95% CI: 1.28–2.91) and 4.12 (95% CI: 2.87–5.92), respectively, after multivariable adjustment. Further adjustments maintained significant associations; HRs for middle and high groups were 1.71 (95% CI: 1.12–2.61) and 3.07 (95% CI: 2.08–4.54). A nonlinear UPCR-primary outcome relationship was observed, with an inflection point at 3.93 g/gCr.
Conclusion
Among non-dialyzed patients with stage G2-G5 CKD, a nonlinear association between UPCR and the primary outcome was observed. A higher UPCR (when UPCR < 3.93 g/gCr) was an independent predictor of the primary outcome. Importantly, our study predates SGLT2 inhibitor use, showcasing outcomes achievable without these medications. Future research considerations will involve factors like SGLT-2 inhibitor utilization.
Introduction
Chronic kidney disease (CKD) is an important contributor to morbidity and mortality from noncommunicable diseases [Citation1]. By 2040, CKD is estimated to become the fifth leading cause of death globally – one of the largest projected increases in any major cause of death [Citation2]. Cardiovascular disease (CVD) is a leading cause of death in patients with CKD and is therefore a major focus of preservative care in this population [Citation3]. Proteinuria is associated with graded cardiovascular and all-cause mortality and acts as a risk multiplier across all levels of renal function [Citation4,Citation5]. Many previous studies have shown that higher albuminuria is independently associated with a higher risk of cardiovascular events and clinical outcomes [Citation6–9]. In a large Canadian study, Nagata et al. also found that heavy proteinuria independently increased the risk of death, myocardial infarction (MI), and progression of CKD [Citation10]. However, some studies have reported conflicting results. A study conducted by Li et al. showed that proteinuria was not an independent determinant of CVD events [Citation11]. Another study also reported an independent association between descending thoracic aortic calcium and eGFR but not with the urinary albumin-to-creatinine ratio (UACR) [Citation12]. Thus, the status of proteinuria as a risk marker for CVD development and all-cause mortality remains controversial. In this study, we aimed to investigate the relationship between the urinary protein-to-creatinine ratio (UPCR) and cardiovascular events and all-cause death among patients with stage G2-G5 CKD who were not undergoing dialysis and to further evaluate the prognostic utility of the UPCR.
Methods
Study population and design
This retrospective analysis utilized data derived from the Chronic Kidney Disease Research of Outcomes in Treatment and Epidemiology (CKD-ROUTE) [Citation13]. A comprehensive description of the CKD-ROUTE study has been previously provided [Citation14–16]. In brief, the CKD-ROUTE study was a prospective, observational cohort investigation that included a representative Japanese population not undergoing dialysis but diagnosed with stage G2-G5 CKD based on the Kidney Disease Improving Global Outcomes (KDIGO) classification [Citation17]. A total of 1138 patients were recruited from Tokyo Medical and Dental University Hospital and its 15 affiliated hospitals within the Tokyo metropolitan area of Japan [Citation14]. In the CKD-ROUTE study, participants were eligible for inclusion if they met the following criteria: (1) they were newly visiting or were referred to the participating nephrology centers for the first time between October 2010 and December 2011, (2) they were over 20 years of age, and (3) they had stage G2-G5 CKD. Patients meeting any of the following criteria were excluded: (1) individuals with a malignancy diagnosed or treated within the previous 2 years, (2) transplant recipients, (3) patients with active gastrointestinal bleeding at the time of enrollment, and (4) patients who did not provide written informed consent [Citation14].
This study represents a secondary analysis of the CKD-ROUTE study data, specifically aimed at exploring the relationship between UPCR and the outcomes within this cohort. After excluding 88 subjects due to missing data related to outcomes or UPCR, the final analysis encompassed 1050 subjects.
Laboratory tests
Upon enrollment, a comprehensive data collection process was initiated, which involved documenting medical histories, assessing lifestyle behaviors (including self-feeding ability), and recording current medications. Furthermore, blood and urine samples were procured for an extensive array of measurements, including white blood cell count, hemoglobin (Hb) levels, platelet count, total protein, albumin, urea nitrogen, creatinine, sodium, potassium, chloride, calcium, phosphorus, alkaline phosphatase, intact parathyroid hormone (PTH), glucose, HbA1c, iron levels, unsaturated iron binding capacity, ferritin, C-reactive protein (CRP), urinary occult blood, urinary protein, and urinary creatinine [Citation15]. The estimated glomerular filtration rate (eGFR) was computed using a modified version of the three-variable Modification of Diet in Renal Disease (MDRD) equation, which was developed by the Japanese Society of Nephrology to account for Japanese physical characteristics [Citation18]: eGFR = 194 × serum creatinine −1.094 × age −0.287 (if female, × 0.739).
Proteinuria was evaluated using both the dipstick test and the UPCR. The specific details regarding the methodology for UPCR measurement can be referenced in the previous literature [Citation14–16]. Participants underwent assessments of their clinical condition during regular hospital visits every 6 months. All participants received treatment in accordance with the standard therapeutic protocols recommended by the Japanese guidelines for CKD [Citation14,Citation19].
Study endpoints
The primary outcome of this study was the composite of cardiovascular events (defined as ischemic heart disease, congestive heart failure, peripheral arterial disease, or stroke) or all-cause death, which included death due to any reason [Citation14]. Secondary outcomes included the individual components of the primary outcome: (1) cardiovascular events and (2) all-cause death.
Statistical analysis
We addressed missing data for certain variables through imputation techniques. When dealing with continuous variables with missing data, we opted to impute them by either their mean or median values, depending on the specific circumstances [Citation20]. Based on prior literatures [Citation21,Citation22] and clinical practices in CKD, urinary protein levels exceeding 3.5 g in a 24-h period indicate nephrotic-range proteinuria, while levels ranging from 1.0 to 3.5 g represent moderate proteinuria. These classifications are frequently utilized to assess partial relief in treatment efficacy and for stratifying urinary protein levels. Therefore, we adopted these two thresholds for stratifying urinary protein levels in our study. All study participants were categorized into three groups based on the UPCR: the low group (<1.0), the middle group (≥1.0-<3.5), and the high group (≥3.50 g/gCr). Continuous variables were presented using either the mean ± standard deviation (SD) (for normally distributed data) or the median (quartiles) (for skewed distribution), while categorical variables were expressed as frequencies or percentages. To assess potential differences in means and proportions among these groups, we employed a variety of statistical tests, including one-way ANOVA (for normally distributed data), the Kruskal–Wallis H test (for skewed data), and the chi-square test (for categorical variables).
To evaluate the impact of UPCR on the outcomes, we conducted Cox proportional hazards regression analyses, both with and without adjusting for potential confounding variables. Hazard ratios (HRs) were reported per 1 g/gCr increment of UPCR and per 1 SD increase in UPCR. To ensure the robustness of our analysis, we performed a sensitivity analysis where UPCR was transformed into a categorical variable, and the p value for trend was calculated. This sensitivity analysis aimed to validate the results obtained with UPCR as a continuous variable and explore the potential for nonlinear relationships. We further visualized our findings by generating Kaplan-Meier plots based on UPCR groupings. Additionally, we employed a smoothing spline curve to depict the adjusted relationships between UPCR and the primary outcome. As part of our exploratory analyses, we investigated the possibility of effect modifications in the association between UPCR levels and outcomes through stratified analyses and interaction testing.
All of the analyses were performed with the statistical software package R (http://www.R-project.org, The R Foundation) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). p Values less than 0.05 (two-sided) were considered statistically significant.
Results
Patient characteristics
The baseline characteristics of all patients are presented in . Of the 1050 participants, 30.0% were female and 70.0% were male; the mean age of the patients was 67.3 years (SD, 13.6 years), with a range from 22 to 94 years. The UPCR level was skewed, and the median value and interquartile range of the UPCR level were 0.74(0.13–2.85) g/gCr. The mean body mass index (BMI) was 23.8 ± 4.1 kg/m2. We categorized the UPCR into groups for analysis. The cutoff points for the low group, the middle group, and the high group were <1.0, ≥1.0-<3.5, and ≥3.50 g/gCr, respectively. The percentages of the low group, the middle group, and the high group were 56.2%, 22.3%, and 21.5%, respectively. Compared to patients with a low UPCR, patients with a higher UPCR had a younger age, higher SBP, lower level of Hb, lower serum albumin level, lower eGFR, higher rate of hypertension, diabetes, and history of CVD, higher prevalence of urinary occult blood, higher rate of use of renin-angiotensin-aldosterone system inhibitors (RAASis), higher use of diuretics, higher rate of diabetic nephropathy, and higher rate of CKD stage 5.
Table 1. Baseline characteristics of the study participants according to the urinary protein/creatinine ratio (UPCR).
The results of the univariate analysis are shown in . The results of the univariate analysis showed that age, SCr, UPCR, hypertension, prevalence of CVD, diabetes, and use of diuretics were positively correlated with the incidence of the primary outcome. We also found that the level of Hb and ALB and the eGFR were negatively correlated with the incidence of the primary outcome.
Table 2. Results of univariate analysis.
Association between the UPCR and the study endpoints
During a median follow-up of 22.7 months, 173 patients (16.5%) developed the primary outcome, 113 (10.8%) experienced cardiovascular events, and 73 (7.0%) died. Among the 173 patients (16.5%) who experienced the primary outcome, it is crucial to note that this number isn’t a direct sum of cardiovascular events and deceased patients. Some patients may have encountered multiple endpoint events, contributing to this figure. For instance, individuals who experienced a cardiovascular event might have subsequently passed away, leading to their inclusion in both the cardiovascular event and all-cause death categories. This overlap accounts for the discrepancy between the sum of cardiovascular events and deaths and the total count for the primary outcome. shows the prevalence rate of the study endpoints in the different UPCR groups. The HRs and 95% CIs for the three outcomes are displayed in , which presents the multivariable-adjusted HRs for the study endpoints per 1 g/gCr and 1 SD increases in the UPCR. When changes in the UPCR were assessed as continuous variables, an increase in the UPCR was associated with a higher risk of the primary outcome in the three models.
Figure 1. The prevalence rate of the study endpoints in the different UPCR groups. CVD: cardiovascular disease; UPCR: urinary protein/creatinine ratio.
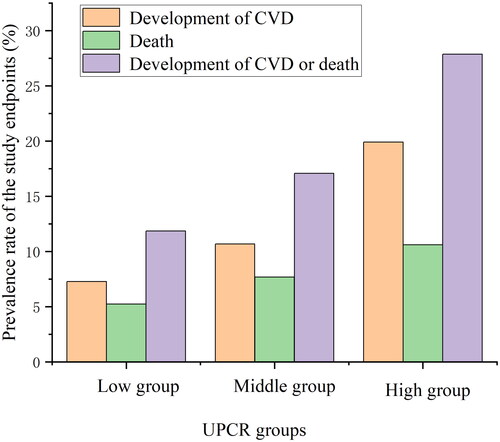
Table 3. Multivariable association of the urinary protein/creatinine ratio with the study endpoints.
For the purpose of sensitivity analysis, we also handled the UPCR as a categorical variable. After adjusting for sex, age, and BMI, the adjusted HRs for the primary outcome in the middle group and the high group compared to those in the low group were 1.93 (95% CI: 1.28–2.91) and 4.12 (95% CI: 2.87–5.92), respectively. After further adjustment (for the eGFR, hypertension, diabetes, prevalence of CVD, and use of RAASi), these associations remained significant; compared with that for the low group, the HR for the middle group was 1.71 (95% CI: 1.12–2.61), and the HR for the high group was 3.07 (95% CI: 2.08–4.54). We found that the trend across the groups was significant (p for trend <0.00001) (). There was also a statistically significant trend for cardiovascular events alone and all-cause mortality when considered alone ().
We also found that the relationship between the UPCR and the incidence of the primary outcome was nonlinear (after adjusting for sex, age, BMI, eGFR, hypertension, diabetes, prevalence of CVD, and use of RAASi) (). By using a two-piecewise linear regression model, we calculated that the inflection point for the UPCR was 3.93 (loglikelihood ratio test p = 0.002). On the left of the inflection point, we observed a positive relationship between the UPCR and the incidence of the primary outcome (HR: 1.334, 95% CI: 1.184 − 1.504, p < 0.0001). On the right side of the inflection point, however, the relationship tended to be saturated (HR: 1.026, 95% CI: 0.957–1.099, p = 0.4766). Details are shown in . Kaplan–Meier curves showed that patients within the higher UPCR categories (the high group) had a higher cumulative incidence of the primary outcome ().
Figure 2. A nonlinear relationship was observed between UPCR levels and the incidence of the primary outcome. The red line denotes fitted curves, and the blue line represents 95% confidence intervals for the association between UPCR and the primary outcome. Adjusted for sex, age, BMI, eGFR, hypertension, diabetes, the prevalence of CVD, and the use of RAASi.
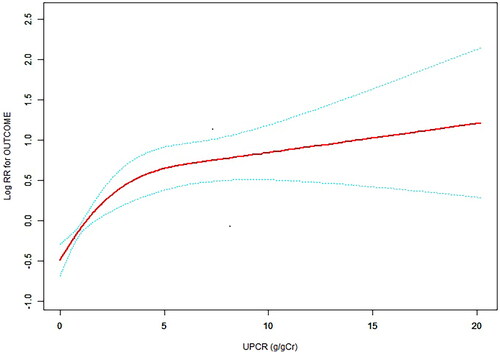
Figure 3. Multivariable-adjusted Kaplan–Meier plot for the association of the UPCR groups with the incidence of the primary outcome; Kaplan–Meier plot adjusted for sex, age, BMI, eGFR, hypertension, diabetes, the prevalence of CVD, and the use of RAASi.
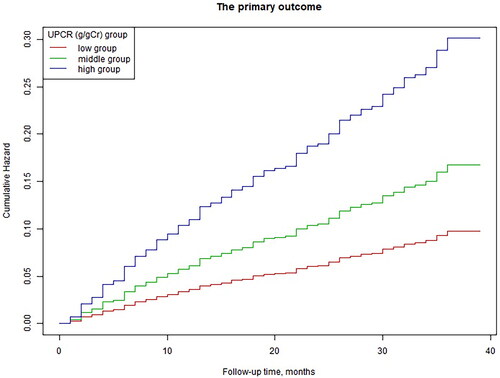
Table 4. The results of the two-piecewise linear regression model.
Stratified analysis of the study endpoint incidence
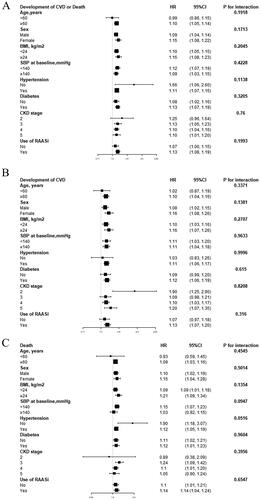
We performed further exploratory subgroup analyses to assess the associations between the UPCR and the study endpoints. We performed analyses stratified by age (<60, ≥60 years), sex, BMI (<24, ≥24 kg/m2), baseline SBP (<140, ≥140 mmHg), hypertension (no, yes), diabetes (no, yes), CKD stage (2, 3, 4, 5), and use of RAASis (no, yes). In our cohort, the impact of the UPCR on the study endpoints was not affected by age, sex, BMI, baseline SBP, hypertension, diabetes, CKD stage, or use of RAASis during the follow-up period (p for all interactions > 0.05) ().
Discussion
The incidence of CKD continues to surge on a global scale, as indicated by multiple studies [Citation1,Citation23,Citation24]. In Japan, the prevalence of CKD is approximately 13% [Citation25], with rates of 63.1 and 71.8 per 1000 individuals under 75 years old in 2017 (crude and age-adjusted, respectively) [Citation26]. Moreover, the number of CKD patients undergoing renal replacement therapy is increasing annually, with the annual medical cost of managing dialysis patients being approximately 4 million Japanese yen [Citation27]. However, only about one-third of the population has prescriptions targeting complications such as diabetes and hypertension [Citation26]. These statistics emphasize the ongoing need for improvements in CKD recognition and management. CKD serves as a precursor to end-stage kidney disease and poses a risk for CVD and premature death [Citation28,Citation29]. Proteinuria is an independent risk factor for CKD progression. Therefore, analyzing the relationship between proteinuria and cardiovascular events and all-cause death in the Japanese CKD population is essential.
This study investigated the relationships between the UPCR and the study endpoints in patients with stage G2-G5 CKD who were not undergoing dialysis. We have demonstrated that the UPCR was positively associated with the incidence of the study endpoints after adjusting for other covariates. In the Cox regression model, a high UPCR was associated with an increased risk of the primary outcome and secondary outcomes after adjusting for age, sex, BMI, eGFR, hypertension, diabetes, prevalence of CVD, and use of RAASis (compared with the low group, in the high group: HR for the primary outcome 3.07, p < 0.00001; HR for CVD development 3.05, p < 0.00001; HR for all-cause mortality 3.52, p = 0.00006). By stratifying the patients into subgroups, the results showed that the impacts of the UPCR on the study endpoints were not affected by age, sex, BMI, baseline SBP, hypertension, diabetes, CKD stage, or use of RAASis (p for all interactions > 0.05).
This is consistent with previous studies that reported an association between proteinuria and adverse outcomes [Citation30–32]. Wei-Yu Su et al. enrolled 482 predialysis patients with CKD stage 3–5 and found that a high UPCR was associated with a higher risk of overall and cardiovascular mortality [Citation33]. A recent cohort study of 1581 individuals, with a median observation period of 8.2 years, showed that an albumin-to-creatinine ratio (ACR) between 30 and 300 mg/g was associated with an increased risk for MI and all-cause mortality [Citation34]. Wu et al. reported that proteinuria was an independent predictor of all-cause mortality in a general population of 95,391 northern Chinese individuals [Citation35]. A retrospective cohort study of 66,311 primary-care diabetic patients also demonstrated a positive association between the UACR and risks of CVD events and mortality, and the risks started even in patients with high-normal albuminuria [Citation36].
In this study, we also used a two-piecewise linear regression model to show a nonlinear relationship between the UPCR and the primary outcome. In our study, the inflection point obtained from GAM after adjusting for potential confounders (sex, age, BMI, eGFR, hypertension, diabetes, prevalence of CVD, and use of RAASi) was 3.93 g/gCr. A recent study reported an association between elevated albuminuria and CVD, and the threshold of albuminuria was 30 mg/g [Citation37]. Another cohort study also showed that multivariable relative risks of CVD mortality for proteinuria range from 1.2 to 2.9 g/g [Citation38]. Kühn et al. found that ACR between 30 and 300 mg/g was associated with an increased risk for MI and all-cause mortality [Citation34]. The threshold of the UPCR in our study was different from those studies. We speculate that the reasons may include the different research population and our adjustment for different confounders. Our study showed that when the UPCR was below 3.93 g/gCr, the risk of the primary outcome increased with increasing UPCR. However, when above 3.93 g/gCr, this relationship tended to be saturated. Therefore, more attention should be given to patients whose UPCR is below 3.93 g/gCr due to the greater risk of the primary outcome. These data may help identify those at high risk of adverse outcomes and suggest a benefit of integrating the UPCR into future cardiovascular prediction models for CKD patients.
Endothelial dysfunction and inflammation have been implicated as potential mechanisms underlying the relationship between proteinuria and CVD. A study suggested that urinary protein excretion not only reflects localized subclinical renal disease but also a more generalized vascular endothelial dysfunction [Citation39–41]. A marker of vascular micro-necrosis, high-sensitivity troponin T (hs-TnT), has been found to independently predict transitions in albuminuria grade [Citation42–44]. Levels of circulating von Willebrand Factor (vWF) antigen, which is released in greater concentrations in response to endothelial cell damage, have been shown to be higher in patients with microalbuminuria than in controls [Citation45–47]. Some studies found that the use of vascular endothelial growth factor (VEGF) antagonists as angiogenesis inhibitors for the treatment of patients with cancer can increase the incidence of proteinuria and hypertension. The effect was reversed upon withdrawal of therapy [Citation48,Citation49]. It further showed the relationship between proteinuria and endothelial dysfunction. A few studies [Citation50,Citation51] also demonstrated that there was a significant correlation between the degree of proteinuria and CRP level. Microalbuminuria is accompanied by elevated CRP. Another inflammatory biomarker, asymmetric dimethylarginine (ADMA), which causes endothelial dysfunction through inhibition of nitric oxide production, was shown to be associated with proteinuria [Citation52,Citation53]. Therefore, endothelial dysfunction and inflammation are potential mechanisms between proteinuria and CVD.
In our investigation, we observed a non-linear association between UPCR and the primary outcome, characterized by an inflection point around 3.93 g/gCr. We proposed several potential explanations for this saturation phenomenon. First, elevated levels of proteinuria often signify not only localized subclinical renal disease but also systemic vascular endothelial dysfunction. The pronounced systemic endothelial dysfunction associated with high proteinuria might limit further impacts on cardiovascular risk. Second, the coexistence of high proteinuria with established cardiovascular risk factors, such as hypertension, diabetes, and CKD progression, might already exert a substantial influence on cardiovascular risk, potentially diminishing the additional effect of increased proteinuria. Lastly, the identified inflection point around 3.93 g/gCr might reflect specific biological features in CKD patients, including declining glomerular filtration rates and limitations in renal tubular reabsorption, contributing to the observed non-linear relationship between UPCR and cardiovascular risk. We acknowledge that while these hypotheses are plausible, further in-depth mechanistic studies are necessary to elucidate why high proteinuria saturates the relationship with cardiovascular risk. We plan to undertake additional basic research to delve deeper into this intriguing phenomenon.
Our study has several strengths and limitations. The strengths of this study include that we addressed and explored nonlinearity and that we adjusted for potential confounding factors to minimize residual confounders. We acknowledge that there are several limitations to this study. First, this was a post hoc analysis of the CKD-ROUTE study. Despite our best efforts to adjust for clinically relevant characteristics, because of the nature of the observational study design, the possibility of residual confounding remains. Second, in this study, we lacked centralized measurements and standardization of laboratory parameters. Third, this study evaluated the UPCR only once, and the association between the effect of the UPCR and the study outcomes over time could not be estimated. Fourth, we recognize that our study, being observational in nature, did not directly investigate mechanistic data or extensively explore the relationship between proteinuria and endothelial cells. Further investigations are needed to uncover the specific mechanisms through which proteinuria affects vascular endothelial function. Fifth, unfortunately, our study encountered a limitation due to the unavailability of relevant data concerning the usage of sodium-dependent glucose transporters 2 (SGLT-2) inhibitors among CKD patients during our research period. As this study was a secondary analysis of the CKD-ROUTE study data, the absence of information on SGLT-2 inhibitor usage restricted our ability to explore and incorporate the impact or influence of these inhibitors within our analysis. This constraint limited our comprehensive evaluation of SGLT-2 inhibitors in the context of proteinuric kidney disease during the period under study. Finally, this study was performed with CKD patients who were not undergoing dialysis, and the generalizability of our findings to the general population remains to be determined.
Conclusions
In summary, our findings indicate a positive association between UPCR and the risks of the primary outcome, CVD development, and all-cause mortality, in patients with stage G2-G5 CKD who were not undergoing dialysis. Importantly, this association remained consistent regardless of renal function and traditional cardiovascular risk factors, such as hypertension and diabetes. Notably, the relationship between UPCR and the incidence of the primary outcome exhibited a nonlinear pattern, with UPCR being positively correlated with the incidence when UPCR levels were below 3.93 g/gCr. Overall, UPCR serves as a convenient and practical tool for identifying patients at a heightened risk of adverse outcomes and those with a less favorable prognosis. It is crucial to emphasize that this study predates the use of SGLT2 inhibitors, reflecting outcomes achievable without these medications. Furthermore, considerations for future research will encompass factors such as the use of SGLT-2 inhibitors.
Author contributions
Haiying Song and Qijun Wan designed the study and wrote the manuscript. Haiying Song, Yuheng Liao, and Haofei Hu performed the statistical analysis. All authors participated in writing and revising the manuscript and read and approved the final manuscript.
Ethics statement
The CKD-ROUTE study was approved by the ethical committees of Tokyo Medical and Dental University, School of Medicine (No. 883), and all other institutions participating in the study and was conducted in accordance with the ethical principles of the Declaration of Helsinki. The protocol was registered with the UMIN Clinical Trials Registry (UMIN000004461).
Informed consent statement
Patient consent was waived due to the retrospective and non-interventional nature of the study.
Acknowledgments
We gratefully acknowledge Iimori Soichiro, Naito Shotaro, Noda Yumi, Sato Hidehiko, Nomura Naohiro, Sohara Eisei, Okado Tomokazu, Sasaki Sei, Uchida Shinichi, Rai, Tatemitsu for the data of this study.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):1–11. doi: 10.1016/S0140-6736(20)30045-3.
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5.
- Thomas B, Matsushita K, Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. doi: 10.1681/ASN.2016050562.
- Di Angelantonio E, Chowdhury R, Sarwar N, et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341(sep30 1):c4986–c4986. doi: 10.1136/bmj.c4986.
- Li J, Liu FH, Guo J, et al. Retrospective analysis of renal prognosis in elderly coronary artery disease patients complicated with renal insufficiency. Aging (Albany NY). 2021;13(19):22856–22866. doi: 10.18632/aging.203579.
- Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6.
- Provenzano M, Chiodini P, Minutolo R, et al. Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: multicentre prospective study in nephrology clinics. Nephrol Dial Transplant. 2020;35(1):138–147.
- Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234.
- Mok Y, Ballew SH, Sang Y, et al. Albuminuria as a predictor of cardiovascular outcomes in patients with acute myocardial infarction. J Am Heart Assoc. 2019;8(8):e010546.
- Nagata M, Ninomiya T, Kiyohara Y, et al. Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am J Epidemiol. 2013;178(1):1–11. doi: 10.1093/aje/kws447.
- Li LC, Lee YT, Lee YW, et al. Aortic arch calcification predicts the renal function progression in patients with stage 3 to 5 chronic kidney disease. Biomed Res Int. 2015;2015:131263.
- Roos CJ, Delgado V, de Koning EJ, et al. Associations of atherosclerosis in the descending thoracic aorta on CTA with arterial stiffness and chronic kidney disease in asymptomatic patients with diabetes mellitus. Int J Cardiovasc Imaging. 2014;30(6):1151–1159. doi: 10.1007/s10554-014-0441-9.
- Soichiro I, Shotaro N, Yumi N, et al. Data from: prognosis of chronic kidney disease with normal-range proteinuria: the CKD-ROUTE study. Dryad Dataset. 2018;13:e0190493.
- Iimori S, Naito S, Noda Y, et al. Prognosis of chronic kidney disease with normal-range proteinuria: the CKD-ROUTE study. PLoS One. 2018;13(1):e0190493. doi: 10.1371/journal.pone.0190493.
- Iimori S, Noda Y, Okado T, et al. Baseline characteristics and prevalence of cardiovascular disease in newly visiting or referred chronic kidney disease patients to nephrology centers in Japan: a prospective cohort study. BMC Nephrol. 2013;14(1):152. doi: 10.1186/1471-2369-14-152.
- Iimori S, Naito S, Noda Y, et al. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology (Carlton). 2015;20(9):601–608. doi: 10.1111/nep.12493.
- Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444.
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034.
- Tamura K, Yutoh J, Matsushita K, et al. Hypertension with chronic kidney disease: anti-hypertensive therapy recommended for the management of hypertension with CKD in JSN-CKD GL 2013 and JSH2014. Nihon Rinsho. 2015;73(11):1876–1884.
- Erviti J, Alonso A, Oliva B, et al. Oral bisphosphonates are associated with increased risk of subtrochanteric and diaphyseal fractures in elderly women: a nested case-control study. BMJ Open. 2013;3(1):e002091. doi: 10.1136/bmjopen-2012-002091.
- Ozeki T, Nagata M, Katsuno T, et al. Nephrotic syndrome with focal segmental glomerular lesions unclassified by columbia classification; pathology and clinical implication. PLoS One. 2021;16(1):e0244677. doi: 10.1371/journal.pone.0244677.
- Sethi S, Zand L, Nasr SH, et al. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J. 2014;7(6):531–537. doi: 10.1093/ckj/sfu100.
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–1270. doi: 10.1038/ki.2011.368.
- Nagai K, Asahi K, Iseki K, et al. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2021;25(8):885–892. doi: 10.1007/s10157-021-02049-0.
- Japan nephrology society. Special issue: clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54(8):1034–191.
- Takeuchi M, Shinkawa K, Yanagita M, et al. Prevalence, recognition and management of chronic kidney disease in Japan: population-based estimate using a healthcare database with routine health checkup data. Clin Kidney J. 2021;14(10):2197–2202. doi: 10.1093/ckj/sfab016.
- Nitta K, Masakane I, Hanafusa N, et al. Annual dialysis data report 2017, JSDT renal data registry. Ren Replace Ther. 2019;5(1):53. doi: 10.1186/s41100-019-0248-1.
- Weaver DJ, Mitsnefes M. Cardiovascular disease in children and adolescents with chronic kidney disease. Semin Nephrol. 2018;38(6):559–569. doi: 10.1016/j.semnephrol.2018.08.002.
- de Jager DJ, Vervloet MG, Dekker FW. Noncardiovascular mortality in CKD: an epidemiological perspective. Nat Rev Nephrol. 2014;10(4):208–214. doi: 10.1038/nrneph.2014.8.
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5.
- Lin CC, Chen TY, Li CI, et al. Renal markers and risks of all cause and cardiovascular mortality from the taichung community based cohort study. Sci Rep. 2021;11(1):14143. doi: 10.1038/s41598-021-93627-5.
- Kim HY, Kweon SS, Lee YH, et al. Cystatin C-based estimated GFR and albuminuria are independently associated with all-cause and CVD mortality in korean population: the dong-gu study. Maturitas. 2021;143:178–183. doi: 10.1016/j.maturitas.2020.10.016.
- Su WY, Wu PY, Huang JC, et al. Increased proteinuria is associated with increased aortic arch calcification, Cardio-Thoracic ratio, rapid renal progression and increased overall and cardiovascular mortality in chronic kidney disease. Int J Med Sci. 2020;17(8):1102–1111. doi: 10.7150/ijms.45470.
- Kühn A, van der Giet M, Kuhlmann MK, et al. Kidney function as risk factor and predictor of cardiovascular outcomes and mortality among older adults. Am J Kidney Dis. 2021;77(3):386–396.e1. doi: 10.1053/j.ajkd.2020.09.015.
- Wu J, Jia J, Li Z, et al. Association of estimated glomerular filtration rate and proteinuria with all-cause mortality in community-based population in China: a result from kailuan study. Sci Rep. 2018;8(1):2157. doi: 10.1038/s41598-018-20554-3.
- Fung CS, Wan EY, Chan AK, et al. Association of estimated glomerular filtration rate and urine albumin-to-creatinine ratio with incidence of cardiovascular diseases and mortality in chinese patients with type 2 diabetes mellitus - a population-based retrospective cohort study. BMC Nephrol. 2017;18(1):47. doi: 10.1186/s12882-017-0468-y.
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. Jama. 2010;303(5):423–429. doi: 10.1001/jama.2010.39.
- Yilmaz MI, Sonmez A, Saglam M, et al. ADMA levels correlate with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol. 2008;19(2):388–395. doi: 10.1681/ASN.2007040461.
- de Chickera SN, Bota SE, Kuwornu JP, et al. Albuminuria, reduced kidney function, and the risk of ST - and non-ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2018;7(20):e009995.
- Yilmaz MI, Romano M, Basarali MK, et al. The effect of corrected inflammation, oxidative stress and endothelial dysfunction on fmd levels in patients with selected chronic diseases: a Quasi-Experimental study. Sci Rep. 2020;10(1):9018. doi: 10.1038/s41598-020-65528-6.
- Pedrinelli R, Giampietro O, Carmassi F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344(8914):14–18. doi: 10.1016/s0140-6736(94)91047-2.
- Askin L, Tanriverdi O, Turkmen S. Clinical importance of high- sensitivity troponin T in patients without coronary artery disease. North Clin Istanb. 2020;7(3):305–310. doi: 10.14744/nci.2019.71135.
- Swoboda PP, McDiarmid AK, Erhayiem B, et al. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6(0 ):e005539.
- Mackinnon B, Deighan CJ, Norrie J, et al. The link between circulating markers of endothelial function and proteinuria in patients with primary glomerulonephritis. Clin Nephrol. 2005;63(3):173–180. doi: 10.5414/cnp63173.
- Bitzan M, Hammad RM, Bonnefoy A, et al. Acquired thrombotic thrombocytopenic purpura with isolated CFHR3/1 deletion-rapid remission following complement blockade. Pediatr Nephrol. 2018;33(8):1437–1442. doi: 10.1007/s00467-018-3957-8.
- Rensma SP, Stehouwer CDA, Van Boxtel MPJ, et al. Associations of arterial stiffness with cognitive performance, and the role of microvascular dysfunction: the Maastricht study. Hypertension. 2020;75(6):1607–1614. doi: 10.1161/HYPERTENSIONAHA.119.14307.
- Shye M, Hanna RM, Patel SS, et al. Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin Kidney J. 2020;13(6):969–980. doi: 10.1093/ckj/sfaa049.
- Zhang W, Feng LJ, Teng F, et al. Incidence and risk of proteinuria associated with newly approved vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: an up-to-date meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. 2020;13(3):311–320. doi: 10.1080/17512433.2020.1734450.
- Sinha SK, Shaheen M, Singh VR, et al. How clinically relevant is C-reactive protein for blacks with metabolic syndrome to predict microalbuminuria? Metab Syndr Relat Disord. 2021;19(1):39–47. doi: 10.1089/met.2019.0121.
- Liu L, Gao B, Wang J, et al. Clinical significance of single and persistent elevation of serum high-sensitivity C-reactive protein levels for prediction of kidney outcomes in patients with impaired fasting glucose or diabetes mellitus. J Nephrol. 2021;34(4):1179–1188. doi: 10.1007/s40620-020-00848-4.
- Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157.
- Maas R, Mieth M, Titze SI, et al. Drugs linked to plasma homoarginine in chronic kidney disease patients-a cross-sectional analysis of the German chronic kidney disease cohort. Nephrol Dial Trans-Plant. 2020;35(7):1187–1195. doi: 10.1093/ndt/gfy342.