Abstract
Objectives
Currently, there is no consensus on the optimal timing for the initiation of peritoneal dialysis (PD) after catheter placement.
Design
Systematic review and meta-analysis.
Exact date of data collection
From inception till July 31, 2023.
Main outcome measures
To assess the outcomes and safety of unplanned PD initiation (<14/7 days after catheter insertion) in cohort studies.
Results
Fifteen studies involving 3054 participants were included. (1) The risk of unplanned initiation of leakage and Obstruction was no difference in both the break-in period (BI) <14 and BI < 7 groups. (2) Catheter displacement was more likely to occur in the emergency initiation group with BI < 7. (3) No significant differences were observed between the two groups regarding infectious complications. (4) There was no difference in transition to HD between patients with BI < 7 and BI < 14 d.
Conclusion
Infectious complications of unplanned initiation of peritoneal dialysis did not differ from planned initiation. Emergency initiation in the BI < 7 group had higher catheter displacement, but heterogeneity was higher. There were no differences in leakage or obstruction in either group. Catheter survival was the same for emergency initiation of peritoneal dialysis compared with planned initiation of peritoneal dialysis and did not increase the risk of conversion to hemodialysis.
Registration
This meta-analysis was registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, number: CRD42023431369)
Introduction
The prevalence of chronic kidney disease is a major global health concern. According to recent reports, the global incidence of chronic kidney disease is approximately 11–13%, with the majority of patients presenting at stage 3 or above [Citation1]. The number of patients with end-stage kidney disease is growing at a rate of 1.9–2.3% per year, based on statistics from the United States Renal Data System (USRDS) [Citation2]. Worldwide, it is projected that the use of renal replacement therapy (RRT) will more than double to 5.439 million (3.899–7.640 million) people by 2030, with the maximum growth in Asia (0.968 million to a projected 2.162 million [1.571–3.014 million]) [Citation3]. In China, the prevalence of chronic kidney disease is 10.8% [Citation4]. Due to its insidious onset, patients often seek medical attention at a late stage, resulting in unplanned dialysis initiation, accounting for 30–50% of all dialysis initiations [Citation5].
Peritoneal dialysis (PD), the first-line RRT, is currently used in approximately 11% of the global dialysis population [Citation6]. Survival in PD was superior to HD in the first two years of treatment, with similar results after five years [Citation7]. Typically, patients who are suitable for PD but are unable to undergo timely placement of a PD catheter often undergo temporary central venous catheter placement to initiate HD [Citation8]. However, temporary central venous catheters are associated with an increased risk of complications including, but not limited to, sepsis and bacteremia requiring hospitalization, as well as catheter malfunctions necessitating replacement [Citation9].
There is significant interest in urgent-start PD, defined as the initiation of PD within 48–72 h of catheter placement. This strategy circumvents the need for temporary vascular access. It also facilitates PD uptake and is cost-effective in the short and long term [Citation10,Citation11]. One of the main obstacles in the use of PD is the waiting period after catheter insertion, which leads to clinical reluctance to choose PD as the preferred dialysis modality. Currently, there is no consensus regarding the optimal number of days between peritoneal catheter placement and dialysis initiation. Some studies suggest a range of 7–14 d [Citation12], and most international guidelines [Citation13–15] support a delay of two weeks for PD after catheter insertion to minimize the risk of complications.
Previous meta-analyses [Citation16] compared the Clinical Prognostic Outcome of unplanned peritoneal dialysis, and we updated the literature based on this, as well as adding and focusing on the comparison of outcomes with BI < 7. Therefore, our study evaluated the outcomes and safety of different urgent initiation strategies compared to conventional PD concerning the BI period, including mechanical and infectious complications, technique survival rates, and transition to HD.
Methods
The study was conducted and reported according to the PRISMA [Citation17] (Preferred Reporting Item for Systematic Reviews and Meta-Analyses) guidelines. All analyses were based on previously published studies; therefore, no ethical approval or patient consent was required.
Search strategy
We performed a search of the medical literature using the PubMed, Cochrane Library, Embase, Web of Science, CNKI (China National Knowledge Infrastructure), and WANFANG DATA databases for manuscripts published from database inception through July 31, 2023 to identify studies that evaluated the outcomes and safety of early-start PD using search terms such as ‘(“Peritoneal Dialysis” [Mesh]) OR (((Dialyses, Peritoneal [Title/Abstract]) OR (Dialysis, Peritoneal [Title/Abstract])) OR (Peritoneal Dialyses [Title/Abstract])).’ Additionally, ‘early-start OR urgent start’ was used to search the PubMed database. All database search records are shown in Table S1. We also searched for observational studies, case reports, reviews, guidelines, original reports, and meta-analyses of PD. Two investigators independently reviewed the titles and abstracts of all retrieved articles ().
Inclusion and exclusion criteria
For the inclusion criteria methodology, we referred to the previously published article by Yin et al. [Citation16]. Studies were evaluated using the following eligibility criteria: (i) cohort studies (prospective or retrospective) published as original research; (ii) studies involving patients with chronic kidney disease >18 years of age; (iii) studies comparing the outcomes of emergency-initiated PD (<14 d after catheter insertion) with those of routine PD (≥14 d after catheter insertion); and (iv) studies that provided data on patient/renal outcomes for >2 years or adverse event rate data. Studies with the following characteristics were excluded: (i) patients who had received any type of HD intervention, (ii) patients who had received any type of PD prior to enrollment, and (iii) studies that evaluated interventions only in the exposed or control groups.
Outcome assessment
The primary outcomes assessed were mechanical and infectious complications, including peritonitis, exit-site infection, leakage, obstruction, and catheter displacement. The secondary outcomes included technical survival and transition to HD.
Study selection and data extraction
Two researchers (HJ and WBQ) reviewed the literature. They applied the inclusion and exclusion criteria by screening the title followed by the abstract, then carefully read the full text and extracted the data that met the standard (κ = 0.851, p=.001). A third investigator (LYM) resolved any discrepancies.
Data collection forms were used to extract the following information from each study: first author, year of publication, sample size and geographic location of the study, study design, insertion technique, mean follow-up time, exposure versus control, and assessment of outcomes during the study period ().
Table 1. Characteristics of included trials.
Assessment of the risk of bias
Two authors (ZY and DLM) evaluated the quality of the study independently using the ROBINS-I (Risk of Bias in Nonrandomized Studies of Interventions) tool [Citation31].
Each of these items was assigned as Low risk of bias/Moderate risk of bias/Serious risk of bias/Critical risk of bias/No information (). Discrepancies were resolved by a third investigator (HQ).
Table 2. Quality Assessment sheet.
Statistical analysis
All statistical analyses were performed using Stata SE. Heterogeneity was assessed using the I2 test; values above 50% were considered to represent substantial heterogeneity. If the heterogeneity was small (p > .1, I2 <50%), a fixed-effect model was used for the meta-analysis. If the heterogeneity was large (p ≤ .1, I2 >50%), a random-effects model was used for the meta-analysis. Subgroup analysis, sensitivity analysis, and meta-regression were used to determine the sources of heterogeneity and BI. Funnel plots were used to detect publication bias in the literature, and Egger’s test was performed to quantify the publication bias of the funnel plots using Stata SE version 16.0 software, with p < .05 indicating publication bias. Single-zero events in events Stata SE has been automatically corrected at 0.5. Dichotomous data are summarized as risk ratios (RR). Statistical significance was set at p < .05.
Results
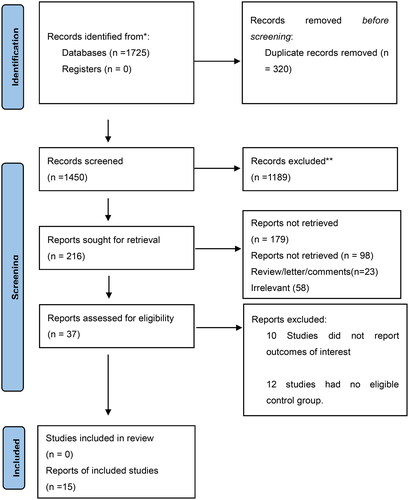
A total of 1725 articles were identified. After checking for duplicates, 320 articles were excluded. After reading the titles, 1189 articles were excluded. After reading the titles and abstracts of the remaining 216 articles, 121 were case reports or reviews and 58 were deemed irrelevant. The remaining 37 articles were subjected to a detailed full-text review. Twenty-two studies were deemed irrelevant. Finally, 15 studies involving 3054 participants met the inclusion criteria. presents the main characteristics (country, sample size, design, outcomes, and follow-up periods) of the included randomized controlled trials. A flowchart depicting the search strategy is shown in .
Mechanical complications
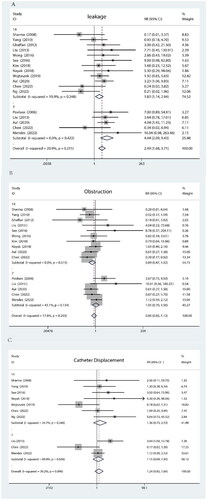
All mechanical complications are summarized in . Analyzed with BI as a subgroup.
Leakage
As depicted in Figure A, the heterogeneity between studies was minimal (I2 = 20.9%). No significant difference in leakage was observed for BI < 14 (p = .248). Similarly, there was no significant variation in the risk of leakage when BI < 7 d (p = .422).
Obstruction
Figure B illustrates that the heterogeneity between studies was insignificant (I2 = 17.8%). No significant difference in catheter obstruction was found for BI < 14 (p = .515). Likewise, no substantial distinction in catheter obstruction existed for BI < 7 (p = .134).
Catheter displacement
As displayed in Figure C, a moderate level of heterogeneity between studies was observed (I2 = 39.2%). For BI < 14, no notable disparity regarding catheter displacement was identified (p = .240). However, it is worth noting that catheter displacement is more likely to occur with a BI < 7 d, according to statistical analysis results indicating significance at p = .036. We performed meta-regression analysis with BI, Year, and Country and found that all p-values exceeded .1. We performed article-by-article culling for BI < 7 and found that the heterogeneity was over 50, so we did not cull any of the articles.
Infectious complications
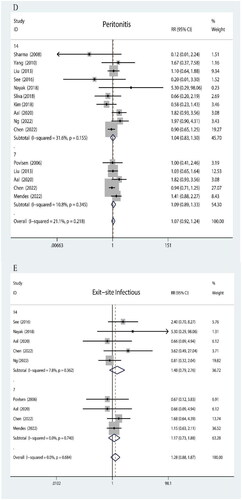
The infectious complications are shown in .
Peritonitis
As depicted in Figure D, the heterogeneity among studies was minimal (I2 = 21.1%). In patients with a BI score less than 14, there was no statistically significant difference observed in post-dialysis peritonitis incidence (p = .155). Similarly, comparable results were obtained for patients with a BI score less than 7 (p = .345).
Exit-Site infection
As illustrated in Figure E, no heterogeneity was found across the included studies. Among individuals with a BI score below 14, no significant variation was detected in catheter exit-site infection rates (p = .362). Likewise, for those with a BI score below 7, no notable distinction was observed in exit-site infection occurrence (p = .740).
Secondary outcome measures
The results of the secondary outcome measures are shown in Figure S1.
Technical survival rate
The heterogeneity between studies was medium (I2 = 45.3%). We performed meta-regression analysis with BI, Year, and Country and found that all p-values exceeded 0.1. Therefore, we performed another sensitivity analysis, used a case-by-case elimination method, and found that Chen et al. [Citation20] was the source of heterogeneity. Chen et al.’s study excluded patients who were switched to hemodialysis within 3 months of peritoneal dialysis being performed without stating a reason, possibly compromising their outcome. Therefore, we excluded this article from the final analysis, showing a lower heterogeneity. In the BI < 14 group, there was no significant difference in the Technical Survival Rate (p = .842). Additionally, the results for BI < 7 were also similar (p = .248).
Transition to HD
The heterogeneity between studies was large (I2 = 64.2%). We performed meta-regression analysis with BI, Year, and Country and found that all p-values exceeded .1. Therefore, we performed another sensitivity analysis, used a case-by-case elimination method and found that Silva et al. [Citation24] was the source of heterogeneity. When we excluded this article, the results showed no heterogeneity. As demonstrated in G, there was no significant difference in Transition to HD in the BI < 14 group (p = .606), and similar results were observed in the BI < 7 group (p = .942).
Publication bias and sensitivity analysis
The publication bias funnel plot showed no publication bias in the study results (Figure S2) and Egger’s test (p = .349). In addition, sensitivity analysis showed no destructive changes in the combined results, indicating that the combined results of the study were robust (Figure S3).
Discussion
Based on this meta-analysis, the following conclusions were drawn: (1) The risk of unplanned initiation of leakage and obstruction was no different in the BI < 14 and BI < 7 groups. (2) Catheter displacement was more likely to occur in the emergency initiation group with BI < 7. (3) No significant differences were observed between the two groups regarding infectious complications. (4) There was no difference in transition to HD between patients with BI < 7 and BI < 14 d.
Mechanical complications are the most common cause of peritoneal dialysis failure, and weak abdominal walls, obesity, steroids, intraperitoneal pressure, and large amounts of dialysate increase the risk of leakage due to physical strain [Citation32]. Pericatheter leakage is a major concern with early PD initiation. The rate of mechanical complications was higher in patients who started due to an emergency than in patients who underwent planned initiation, with a higher frequency of immediate postoperative leakage ranging from 7% to 24% [Citation33]. The absolute occurrence rate of catheter leakage still fell within the range reported in the existing literature on urgent-start PD, ranging from 2.2% at six months to 33% at three months [Citation6,Citation28]. Banli et al. [Citation34] also reported a leakage rate of 4.9% in patients who started PD early (after six days), whereas Mendes et al. [Citation18] reported a difference in mechanical complications (leakage) between the two groups, with a leakage rate of 13.27% in the unplanned group. However, Young-Il et al. [Citation35] studied 51 patients who immediately underwent PD after catheter placement and reported very low leakage rates of 1.9% at one month and no leakage after one month. Our meta-analysis showed no significant leakage when the BI was <7/14 d (p > .1). We believe that the early use of the catheter limits the wound healing time and the growth of peri catheter tissue granulation [Citation25]. Several strategies have described ways to minimize the risk of dialysate leakage associated with urgent PD catheter placement. These measures include using only supine dwell PD with lower dwell volumes, avoiding dwelling while the patient is upright, insertion of the catheter via laparoscopy, and use of purse-string sutures [Citation21,Citation25].
Obstruction is another major problem during the urgent initiation of PD. The early initiation of PD may stimulate omental wrapping, leading to catheter obstruction [Citation27,Citation30,Citation36]. See et al. [Citation25] reported a significantly higher incidence of catheter displacement after urgent initiation of PD compared to routine dialysis (12% vs. 1%, p = .047). Ng et al. [Citation19] also reported a migration rate of 21.7% in the early-start PD group, which was significantly higher than that in the planned-start PD group, with a rate of 1.8% (Bonferroni-corrected p = 0.011). Nayak et al. [Citation8] found that although the rate of catheter obstruction in the routine group was 16.7% (four cases) and 25% in the urgent group, the difference between the two groups was not statistically significant (p = .452). In this meta-analysis, BI < 14 d showed no significant differences from the routine group in terms of catheter displacement and obstruction. BI < 7 had higher catheter displacement. However, the heterogeneity of the studies was high; therefore, more prospective studies are needed to further investigate this.
In malpositioning cases, using laxatives and mobility may aid catheter repositioning [Citation37]. However, early PD does not provide sufficient time for formulating routine prescriptions for intestinal cleansing and spontaneous resetting. Aal et al. [Citation21] suggested that although 20% of patients experience catheter displacement, leading to functional impairment, repositioning for treatment without catheter replacement or altering the dialysis modality is a safe and reasonable alternative for urgent PD initiation in patients without HD access.
Severe infectious complications result in two-thirds of laparoscopic catheter losses, with peritonitis being the most common infection. Studies have shown that the incidence of peritonitis ranges from 0% to 15.4% in patients undergoing urgent PD initiation [Citation6,Citation30,Citation38]. In the report by Abdel et al. [Citation21], the incidence of peritonitis in the select start and urgent-start groups were 15.1% and 27.6%, respectively. Ng et al. [Citation19] reported no statistically significant differences in the rates of peritonitis, exit-site infection, or catheter-related leaks among subgroups within the first year of PD. In this meta-analysis, there was no significant difference in infectious complications (peritonitis, exit-site infections) between the BI < 14/7 groups (p > .1). Similar results were observed in a study by Wojtaszek et al. [Citation22] in which there were no differences in the rates of peritonitis or exit-site or tunnel infections between the urgent- and routine-start groups during the first year of PD or throughout the observation period. A Danish study [Citation30] reported that 15.4% of patients who underwent urgent-start PD developed peritonitis within the first three months, while 15.4% of patients in the planned-start PD group also experienced peritonitis, with no difference between the two groups. Strict disinfection protocols, preoperative antibiotic prophylaxis, experienced nephrologists performing catheter insertions, and training of patients and healthcare personnel by a specialized nursing team may help control the occurrence of early peritonitis.
This meta-analysis revealed that PD patients with a BI < 14/7 d had similar catheter technique survival rates, and no difference in transfer to HD. However, it is important to note that the sample size for BI < 7 d was limited, and the results should be interpreted cautiously. A favorable technique survival rate is crucial for successful PD implementation and avoiding transfer to HD. Despite the recognized benefits of PD, its utilization rate among dialysis patients is only 6.9%, with many patients who initially choose PD eventually switching to HD [Citation39,Citation40]. In our analysis, four articles examined catheter technique survival rates and transitions to HD. The catheter technique survival rates reported in these studies ranged from 75% to 99%, with catheter malfunction and infectious complications being the main reasons for this phenomenon. Nayak et al. [Citation8] reported three patients who chose to discontinue PD because of catheter obstruction caused by catheter displacement without undergoing catheter repositioning. Liu et al. [Citation27] noted that within the first six months after catheter insertion, 21 patients transitioned to HD, with catheter malfunction (n = 12) being the primary cause, followed by peritonitis (n = 8). Only catheter malfunction (hazard ratio [HR]: 20.087; 95% CI: 7.326–55.074; p < .001) and peritonitis (HR: 4.533; 95% CI: 1.748–11.751; p = .002) were identified as independent risk factors for technique failure. The duration of the catheter adaptation period was not associated with technique failure (p > .05). However, this risk did not exist for PD patients who received assistance from nurses or family members.
Authors contributions
Ji H and Baoqiao W drafted and revised the manuscript and collected data. Yue Z and Limiao D help to screen the literature and collect data. Yueming L drafted and revised the manuscript and designed the tables/figures, Juan J drafted and revised the manuscript. Qiang H initiated the collaboration, as well as directed, drafted, and revised the manuscript.
Ethics statement
An ethics statement is not applicable because this study is based exclusively on published literature.
Acknowledgements
We would like to thank all those involved in the work.
Disclosure statement
No potential conflict of interest was reported by the author(s)
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Additional information
Funding
References
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – A systematic review and meta-analysis. PLoS One. 2016;11(7):1. doi: 10.1371/journal.pone.0158765.
- Collins AJ, Foley RN, Chavers B, et al. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 59(1 Suppl 1):A7. A7
- Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–8. doi: 10.1016/S0140-6736(14)61601-9.
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6.
- Vanga A. Offering patients therapy options in unplanned start: development and implementation of an education program for unplanned-start patients. Adv Perit Dial. 2015;31:69–73.
- Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. Am J Kidney Dis. 2012;59(3):400–408. doi: 10.1053/j.ajkd.2011.08.034.
- Vonesh EF, Snyder JJ, Foley RN, et al. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 2004;66(6):2389–2401. doi: 10.1111/j.1523-1755.2004.66028.x.
- Nayak KS, Subhramanyam SV, Pavankumar N, et al. Emergent start peritoneal dialysis for End-Stage renal disease: outcomes and advantages. Blood Purif. 2018;45(4):313–319. doi: 10.1159/000486543.
- Vats HS. Complications of catheters: tunneled and nontunneled. Adv Chronic Kidney Dis. 2012;19(3):188–194. doi: 10.1053/j.ackd.2012.04.004.
- Alkatheeri AM, Blake PG, Gray D, et al. Success of urgent-start peritoneal dialysis in a large Canadian renal program. Perit Dial Int. 2016;36(2):171–176. doi: 10.3747/pdi.2014.00148.
- Blake PG, Jain AK. Urgent start peritoneal dialysis: defining what it is and why it matters. Clin J Am Soc Nephrol. 2018;13(8):1278–1279. doi: 10.2215/CJN.02820318.
- Ivarsen P, Povlsen JV. Can peritoneal dialysis be applied for unplanned initiation of chronic dialysis? Nephrol Dial Transplant. 2014;29(12):2201–2206. doi: 10.1093/ndt/gft487.
- Ranganathan D, John GT, Yeoh E, et al. A randomized controlled trial to determine the appropriate time to initiate peritoneal dialysis after insertion of catheter (timely PD study). Perit Dial Int. 2017;37(4):420–428. doi: 10.3747/pdi.2016.00066.
- Dombros N, Dratwa M, Feriani M, et al. European best practice guidelines for peritoneal dialysis. 3 peritoneal access. Nephrol Dial Transplant. 2005;20(Suppl 9):ix8–ix12.
- Piraino B, Bernardini J, Brown E, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31(6):614–630. doi: 10.3747/pdi.2011.00057.
- Yin Y, Cao Y, Yuan L. Outcome and safety of unplanned-start peritoneal dialysis according to Break-In periods: a systematic review and meta-analysis. Blood Purif. 2021;50(2):161–173. doi: 10.1159/000510550.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647.
- Mendes ML, Alves CA, Marinho LCR, et al. Unplanned vs. planned peritoneal dialysis as initial therapy for dialysis patients in chronic kidney replacement therapy. Int Urol Nephrol. 2022;54(6):1417–1425. doi: 10.1007/s11255-021-03029-9.
- Ng AKH, Tan SN, Tay ME, et al. Comparison of planned-start, early-start and deferred-start strategies for peritoneal dialysis initiation in end-stage kidney disease. Ann Acad Med Singap. 2022;51(4):213–220. doi: 10.47102/annals-acadmedsg.2021495.
- Yifang CHEN, Xiuling CHEN, Hui GAO, et al. Patients initiated peritoneal dialysis within 7 days after peritoneal catheterization. Chin J Nephrol Dial Transplant. 2022;31(1):33–38.
- Abdel Aal AK, Mahmoud K, Moustafa AS, et al. Comparative study on the outcomes of Elective-Start versus urgent-start peritoneal dialysis catheter placement. Radiol Res Pract. 2020;2020:3751827. doi: 10.1155/2020/3751827.
- Wojtaszek E, Grzejszczak A, Grygiel K, et al. Urgent-start peritoneal dialysis as a bridge to definitive chronic renal replacement therapy: short- and long-term outcomes. Front Physiol. 2018;9:1830. doi: 10.3389/fphys.2018.01830.
- Kim K, Son YK, Lee SM, et al. Early technical complications and long-term survival of urgent peritoneal dialysis according to break-in periods. PLOS One. 2018;13(10):e0206426. Published 2018 Oct 26. doi: 10.1371/journal.pone.0206426.
- Silva BC, Adelina E, Pereira BJ, et al. Early start peritoneal dialysis: technique survival in long-term follow-up. Kidney Blood Press Res. 2018;43(6):1699–1705. doi: 10.1159/000495386.
- See EJ, Cho Y, Hawley CM, et al. Early and late patient outcomes in urgent-start peritoneal dialysis. Perit Dial Int. 2017;37(4):414–419. doi: 10.3747/pdi.2016.00158.
- Wong LP, Li NC, Kansal S, et al. Urgent peritoneal dialysis starts for ESRD: initial multicenter experiences in the United States. Am J Kidney Dis. 2016;68(3):500–502. doi: 10.1053/j.ajkd.2016.03.426.
- Liu Y, Zhang L, Lin A, et al. Impact of break-in period on the short-term outcomes of patients started on peritoneal dialysis. Perit Dial Int. 2014;34(1):49–56. doi: 10.3747/pdi.2012.00293.
- Yang YF, Wang HJ, Yeh CC, et al. Early initiation of continuous ambulatory peritoneal dialysis in patients undergoing surgical implantation of tenckhoff catheters. Perit Dial Int. 2011;31(5):551–557. doi: 10.3747/pdi.2009.00171.
- Sharma AP, Mandhani A, Daniel SP, et al. Shorter break-in period is a viable option with tighter PD catheter securing during the insertion. Nephrology. 2008;13(8):672–676. doi: 10.1111/j.1440-1797.2008.01000.x.
- Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant. 2006;21(Suppl 2):ii56–59. doi: 10.1093/ndt/gfl192.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- Crabtree JH, Shrestha BM, Chow KM, et al. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39(5):414–436. doi: 10.3747/pdi.2018.00232.
- Li PK, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508. doi: 10.3747/pdi.2016.00078.
- Banli O, Altun H, Oztemel A, et al. Early start of CAPD with the seldinger technique. Perit Dial Int. 2005;25(6):556–559. doi: 10.1177/089686080502500610.
- Jo Y-I, Song J-O, Park J-H, et al. Idiopathic eosinophilic peritonitis in continuous ambulatory peritoneal dialysis: experience with percutaneous catheter placement. Nephrology. 2007;12(5):437–440. doi: 10.1111/j.1440-1797.2007.00794.x.
- Figueiredo A, Goh BL, Jenkins S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int. 2010;30(4):424–429. doi: 10.3747/pdi.2010.00087.
- Peppelenbosch A, van Kuijk WH, Bouvy ND, et al. Peritoneal dialysis catheter placement technique and complications. NDT Plus. 2008;1(Suppl 4):iv23–iv28. doi: 10.1093/ndtplus/sfn120.
- Bitencourt Dias D, Mendes ML, Burgugi Banin V, et al. Urgent-Start peritoneal dialysis: the first year of Brazilian experience. Blood Purif. 2017;44(4):283–287. doi: 10.1159/000478970.
- Al-Jaishi AA, Jain AK, Garg AX, et al. Hemodialysis vascular access creation in patients switching from peritoneal dialysis to hemodialysis: a population-based retrospective cohort. Am J Kidney Dis. 2016;67(5):813–816. doi: 10.1053/j.ajkd.2015.11.025.
- Pulliam J, Li NC, Maddux F, et al. First-year outcomes of incident peritoneal dialysis patients in the United States. Am J Kidney Dis. 2014;64(5):761–769. doi: 10.1053/j.ajkd.2014.04.025.