Abstract
Objective
This study aimed to explore the serum levels of gasdermin D (GSDMD) in uremic (end-stage kidney disease, ESKD) patients and their correlation with vascular calcification (VC) and clinical results.
Methods
This prospective observational cohort study enrolled 213 ESKD patients who were undergoing regular maintenance hemodialysis (MHD) for > 3 months in our hospital from August 2019 to July 2022. The abdominal aortic calcification score (AACS) was used to assess the VC condition of patients with ESKD. Serum GSDMD, caspase-1, interleukin (IL)-6, IL-1β, IL-18 and C-reactive protein (CRP) levels were measured using enzyme-linked immunosorbent assay (ELISA). Demographic and clinical data were obtained. All patients were followed up for 1 year, and patients with major adverse cardiovascular events (MACE) were defined as having a poor prognosis. All data used SPSS 26.0 to statistical analyses.
Results
The serum total cholesterol (TC) levels of patients in the AACS > 4 group were significantly elevated compared with those in the AACS ≤ 4 group. In addition, ESKD patients with an AACS > 4 had significantly higher serum levels of GSDMD, caspase-1, IL-6, IL-18 and IL-1β. Moreover, Pearson’s analysis supported a positive correlation between GSDMD and caspase-1, IL-6, and IL-1β. In addition, we found that GSDMD levels were positively correlated with the clinical data (AACS scores and serum TC levels) of patients with ERSD. Additionally, ROC curves showed that the serum levels of GSDMD could be a potential predictive biomarker of moderate/severe VC and prognosis in patients with ESKD. Finally, the results of logistic regression indicated that GSDMD and AACS scores were risk factors for poor prognosis in patients with ESKD.
Conclusion
Serum GSDMD levels were remarkably elevated in patients with ESKD with moderate/severe calcification. In addition, serum levels of GSDMD could be a potential predictive biomarker of moderate/severe VC and prognosis in patients with ESKD.
Introduction
Chronic kidney disease (CKD) is a progressive disease that poses a global health burden [Citation1]. It has been reported to affect over 10% of the general population worldwide [Citation2], and if left untreated, it eventually progresses to end-stage kidney disease (ESKD), also known as uremia. CKD is independently associated with an increased risk of cardiovascular disease (CVD) [Citation3,Citation4]. Therefore, early screening of CKD patients at risk of developing cardiovascular disease and providing specialized care and treatment are important in preventing adverse outcomes.
Vascular calcification (VC) is an independent risk factor of CVD [Citation5]. Various factors can influence VC, including advanced age, hypertension, diabetes, smoking, and malnutrition [Citation6,Citation7]. Pyroptosis is a newly discovered pathway of cell death [Citation8] and is closely associated with cardiovascular diseases (CVD), such as atherosclerosis, myocardial infarction, ischemia-reperfusion injury, heart failure, and coronary artery calcification [Citation9–14]. A recent study suggested that irisin protected against VC via inducing autophagy and inhibiting vascular smooth muscle cells pyroptosis in CKD [Citation15]. Collectively, these studies suggest that the pyroptosis pathway contributes to the occurrence of vascular calcification. However, relevant clinical research in this field is still lacking. Gasdermin D (GSDMD) is an effector of pyroptosis pathway [Citation16]. In a model of acute kidney injury induced by reperfusion injury, GSDMD protected kidney tubules from necrosis-mediated damage [Citation17]. Additionally, GSDMD influences vascular smooth muscle cell pyroptosis and vascular remodeling induced by Ang II [Citation18]. This indicates that GSDMD plays a role in acute kidney injury and VS. However, there are currently no clinical studies investigating the relationship between GSDMD and VC in ESKD patients, as well as its impact on patient prognosis.
In this prospective observational cohort study, we aimed to explore the serum levels of GSDMD in ESKD patients and their correlation with VC and clinical results. This study might reveal the clinical significance of GSDMD in ESKD patients and provide novel research targets for ESKD treatment.
Methods
Subjects
This prospective observational cohort study enrolled 213 patients with ESKD who underwent regular maintenance hemodialysis (MHD) for over 3 months in our hospital from August 2019 to July 2022. The exclusion criteria were as follows:1) patients treated with combined peritoneal dialysis; 2) patients with combined acute coronary syndrome and active infection; 3) patients who had received immunosuppressive, anti-infection, and anti-inflammatory drug treatments in the past month; and 4) patients with severe liver disease, cancer, or significant cardiovascular problems. Serum samples from 100 healthy volunteers were collected as controls. A total of 289 participants were initially recruited for the study, and based on the inclusion and exclusion criteria, 213 patients with ESKD were ultimately included. In addition, all control participants were age-matched healthy volunteers without any underlying medical conditions who underwent medical checkups at our hospital. All study subjects were above 18 years of age.
For the treatment of ESKD patients, MHD was performed 2-3 times per week, with each session lasting 4 h. The dialyzer used was a high-flux membrane with a surface area of 1.4m2. The dialysis machine used was the DIALOG, Germany, with bicarbonate dialysate and a dialysate flow rate of 500 mL/min. Regular heparin or low-molecular-weight heparin was used for anticoagulation, and the blood flow rate ranged from 220 to 300 mL/min. All cases received individualized treatment for hypertension control and correction of anemia, among other interventions.
This study was approved by the ethics committee of our hospital (Z0568-02). All participants agreed to participate in this study and signed an informed consent form.
Blood sampling measurement
Serum GSDMD, caspase-1, interleukin (IL)-6, IL-1β, IL-18 and C-reactive protein (CRP) levels were measured using enzyme-linked immunosorbent assay (ELISA). Serum samples were collected on the first Monday of patient inclusion, prior to MHD. Fasting cubital venous blood samples (5 mL) were collected within 24 h after admission for all cases. Samples were centrifuged at 2000 × g for 15 min, and ELISA was performed using commercially available kits (GSDMD MBS2705515 MyBioSource, caspase-1 MBS264676 MyBioSource, IL-6 MBS175877 MyBioSource, CRP MBS177184 MyBioSource, IL-1β MBS175901 MyBioSource, IL-18 MBS2020065 MyBioSource).
The abdominal aortic calcification score (AACS) measurement
Patients were evaluated for AACS after serum sampling. An X-ray imaging system (TU-51, Hitachi, Japan) was used to capture lateral abdominal radiographs. Two experienced clinical radiologists independently analyzed the images using a blinded reading method. A semi-quantitative scoring method based on the Kauppila score [Citation19] was utilized to assess the images. The score was determined based on the degree of calcification in the anterior and posterior walls of each segment of the abdominal aorta. A score of 1 was assigned for involvement of less than 33%, a score of 2 for involvement between 33% and 66%, and a score of 3 for involvement greater than 66%. The scores for each segment were summed to obtain the abdominal aortic calcification score (AACS, range 0-24). A score of 0 on the AACS scale indicated no calcification, a score greater than 0 but less than or equal to 4 indicated mild calcification, and a score greater than 4 indicated moderate or severe calcification [Citation20,Citation21].
Data collection and scale scoring
Demographic and clinical statistics, including age, BMI, sex, diastolic blood pressure (DBP), and systolic blood pressure (SBP), were collected. Routine blood tests were performed using an automatic biochemical analyzer (Hitachi 7600; Hitachi Corporation, Japan), and the levels of fasting plasma glucose (FPG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), serum albumin (ALB), serum creatinine (Scr), blood urea nitrogen (BUN), hemoglobulin (HB), calcium (Ca), phosphate (P), parathyroid hormone (PTH), fasting plasma glucose (FPG), magnesium (Mg) were recorded. In addition, all patients were followed up for 1 year, and patients with one of the following conditions during follow-up were defined as major adverse cardiovascular events (MACE) [Citation22] (poor prognosis group): cardiac death, heart failure, cardiogenic shock, recurrent myocardial infarction, and arrhythmia with hemodynamic disturbances.
Statistical analysis
The normal distribution of the data was confirmed by Kolmogorov-Smirnov analysis. Normally distributed data were expressed as mean ± SD, while non-normally distributed data were expressed as median (range). The Mann-Whitney test or Student’s t test was used for comparisons between the two groups. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for comparisons among three or more groups. The chi-square test was used to determine the rates. Pearson’s rank correlation and Spearman’s rank correlation were used for correlation analysis. The role of serum GSDMD in the diagnosis of VC and prediction of patient prognosis was analyzed using ROC curve analysis. Logistic regression analysis was performed for risk factors of poor prognosis. Differences were considered statistically significant at p < 0.05. All data used SPSS 26.0 to statistical analyses.
Results
Clinical characteristics of all participants
This prospective observational cohort study included two groups of ESKD patients undergoing maintenance hemodialysis in our hospital: those with AACS scores ≤ 4 (AACS ≤ 4 group, n = 121) and those with AACS scores greater than 4 (AACS > 4 group, n = 92). The basic information is shown in . Comparing the demographic, clinical, and kidney function indicators of the two groups, the serum TC and P levels of patients in the AACS > 4 group were significantly elevated, while the serum Mg levels were significantly decreased compared with those patients in the AACS ≤ 4 group (p < 0.05). No other significant differences were found in age, sex, BMI, SBP, DBP, TG, HDLC, LDLC, ALB, Scr, or BUN between the two groups.
Table 1. Demographic and clinical data of all subjects.
Serum levels of GSDMD and inflammatory factors in ESKD patients
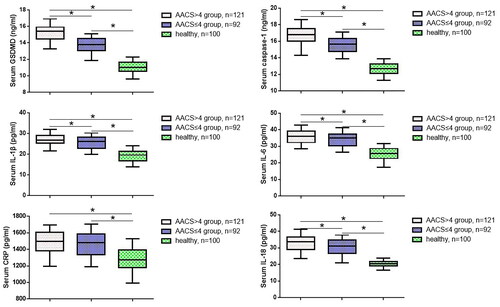
To further investigate the relationship between GSDMD, vascular calcification, cell apoptosis factors, and inflammatory cytokines in ESKD patients, we measured the levels of GSDMD, caspase-1, IL-6, IL-1β, IL-18 and CRP in the serum using ELISA. As shown in , compared to the other two groups, ESKD patients with AACS > 4 had significantly higher serum levels of GSDMD, caspase-1, IL-6, IL-18 and IL-1β, as did those with AACS ≤ 4 compared to the healthy control group. Additionally, there was no significant difference in the CRP levels between the AACS ≤ 4 and AACS > 4 groups. Pearson’s analysis supported a positive correlation between GSDMD and the levels of caspase-1 and IL-1β ().
Table 2. Correlation analysis among GSDMD, caspase-1, IL-6, IL-1β and CRP.
Correlation between serum GSDMD levels and ESKD patients’ clinical outcome
We subsequently used Spearman’s rank correlation analysis to examine the relationship between GSDMD levels and clinical factors in patients with ESKD. As shown in , no significant correlation was found between GSDMD levels and age, BMI, SBP, DBP, FPG, TC, TG, HDLC, or LDLC. However, GSDMD levels positively correlated with SBP (p = 0.036), serum TC levels (p < 0.001), serum P levels (p < 0.001), serum FPG levels (p = 0.015), serum Mg levels (p < 0.001) and AACS scores (p < 0.001). These results indicate that GSDMD is associated with the clinical outcomes of patients with ESKD.
Table 3. Correlation between serum GSDMD levels and the clinical data of ESKD patients.
Predictive value of GSDMD for moderate/severe VC and prognosis in ESKD patients
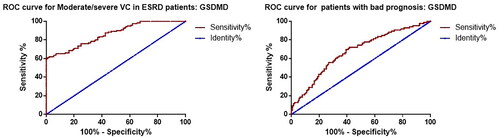
We constructed ROC curves to assess the predictive value of GSDMD for moderate/severe VC (AACS > 4) and prognosis in patients with ESKD. The results showed that GSDMD could be a potential predictive biomarker of moderate/severe VC in ESKD patients, with an AUC of 0.861, cutoff value of 14.43 ng/ml, sensitivity of 76.0%, and specificity of 75.0% (). In addition, we investigated the role of GSDMD in predicting the prognosis of ESKD patients. A total of 86 (40.4) patients developed MACE during the follow-up period. We divided all patients into two groups, good prognosis (n = 127) and poor prognosis (n = 86), based on the occurrence of MACE during follow-up and performed ROC curve analysis. The results showed that the AUC of GSDMD in predicting poor prognosis in ESKD patients was 0.677, with a cutoff value of 14.59 ng/ml, a sensitivity of 69.8%, and a specificity of 60.6%.
Risk factors of ESKD patients with poor prognosis by logistic regression analysis
Finally, we used the entry method for logistic regression to analyze the risk factors of ESKD patients with moderate/severe VC. For the logistic regression analysis, we used the entry method, and used serum biomarkers with significant differences as covariates. The results showed that GSDMD, Caspase-1, P, Mg, IL-1β and IL-18 were risk factors for moderate/severe VC in ESKD patients (). Furthermore, we analyzed the risk factors of ESKD patients with poor prognosis. As shown in , the results of logistic regression suggested that GSDMD was a risk factor for poor prognosis in patients with ESKD.
Table 4. Logistic regression analysis for risk factors of moderate/severe VC in ESKD patients compared to mild VC patients.
Table 5. Logistic regression for risk factors of ESKD patients with poor prognosis.
Discussion
Evidence suggests that over 50% of patients with ESKD die from CVD [Citation23]. Therefore, for ESKD patients, the earlier the attention to vascular function, the better is the prognosis. Thus, there is an urgent need to develop new biomarkers and comprehensive approaches to promptly diagnose and treat VC in ESKD patients. In this study, we found that serum levels of GSDMD could be a potential predictive biomarker of moderate/severe VC and prognosis in ESKD patients.
The AACS score is a semiquantitative score used to measure abdominal aortic calcification (AAC) [Citation24]. We divided all ESKD patients into two groups using the AACS score and compared their clinical data. The results showed that the serum TC levels of patients in the AACS > 4 group were significantly elevated compared to those in the AACS ≤ 4 group, suggesting a potential link between lipid metabolism and vascular calcification in patients with ESKD. Other studies have attempted to identify differential expression of serum biomarkers in patients with CKD or ESKD. Lee et al. confirmed that serum pro-brain natriuretic peptide levels were independently associated with abdominal aortic calcification (AAC) scores in CKD dialysis patients [Citation25]. Ito et al. found that higher serum magnesium levels were significantly associated with lower abdominal aortic calcification volume in ESKD patients [Citation26]. Observational studies on dialysis patients have shown that higher blood magnesium concentration is associated with a lower risk of vascular calcification [Citation27,Citation28]. Both in vitro and in vivo models have demonstrated that magnesium can prevent the transdifferentiation of osteoblastic vascular smooth muscle cells [Citation29]. Furthermore, recent research indicates that magnesium can inhibit the maturation of calcium phosphate particles, which may be a fundamental mechanism behind its anti-calcification properties [Citation30]. Consistently, in our study, we also observed a significant decrease in serum magnesium levels in patients with Aortic Arch Calcification Score (AACS) >4 compared to those with AACS ≤4. In addition, a clinical study by Zhou et al. [Citation31] which included 102 CKD patients undergoing peritoneal dialysis (PD), it was observed that serum indoxyl sulfate, diabetes, and high-sensitivity C-reactive protein were independent factors influencing the occurrence of vascular calcification in PD patients. Interestingly, in our study, there were no significant differences in diabetes or serum CRP levels between groups with AACS ≤ 4 and AACS > 4. This difference may be attributed to the different populations studied, as we included ESKD patients in whom primary etiological factors and molecular mechanisms evolve over time, which could contribute to the variation in these study findings, highlighting the need for a better understanding of the VC development process.
Pyroptosis is characterized by GSDMD-mediated pore formation, cell swelling, rapid rupture, and the subsequent release of pro-inflammatory mediators [Citation10]. In recent years, the relationship between cell pyroptosis and vascular calcification has garnered widespread attention, and many studies have investigated this association. Evidence suggests that the expression of many factors associated with pyroptosis (caspase-1 and NLRP3) changes during the occurrence and progression of vascular calcification [Citation32,Citation33]. Additionally, activation of the pyroptosis signaling pathway in vascular smooth muscle cells can promote the progression of vascular calcification [Citation34,Citation35]. Inflammation plays a crucial role in the relationship between pyroptosis and vascular calcification, as elevated levels of inflammatory factors such as IL-1β and IL-6 can induce pyroptosis [Citation36] and promote vascular calcification [Citation37]. Activated NLRP3 and caspase-1 can cleave pro-forms of IL-1β and IL-18 to produce mature cytokines or recognize and cleave GSDMD to mediate cell pyroptosis [Citation18]. A complex interplay appears to exist between pyroptosis, inflammation, and VC. Therefore, we measured the serum levels of pyroptosis key pathway proteins (GSDMD, caspase-1) and inflammatory factors (CRP, IL-6, and IL-1β) in all ESKD patients. The results supported that ESKD patients with AACS > 4 had significantly higher serum levels of GSDMD, caspase-1, IL-6, and IL-1β as well as a positive correlation between GSDMD and the levels of caspase-1, IL-6, and IL-1β. Other studies have also found significant elevation of IL-1β and IL-6 levels in patients undergoing dialysis [Citation38,Citation39]. Additionally, Gou et al. [Citation40] and Ding et al. [Citation41] identified that interactions and associations between GSDMD and these cytokines. These findings were consistent with those of the present study. However, we have, for the first time, identified the association between serum GSDMD levels, inflammatory factor levels, and VC in ESKD patients. Furthermore, our results demonstrated that serum GSDMD levels can predict the prognosis of patients with ESKD.
This study has several limitations that merit consideration. First, the sample size was relatively small, which could have affected the generalizability of the findings. Second, our analysis assessed only a limited number of inflammatory factors, which may have excluded other potentially relevant variables. Thirdly, all patients had a shorter follow-up period. Lastly, further in-depth research is needed to elucidate the molecular mechanisms by which GSDMD is involved in ESKD development. In the future, more experiments should be conducted to investigate this.
Conclusion
This study showed that serum GSDMD levels were remarkably elevated in patients with ESKD with moderate/severe VC. In addition, serum levels of GSDMD could be a potential predictive biomarker of moderate/severe VC and prognosis in patients with ESKD. This study may provide new targets and a comprehensive approach for cardiovascular protection in patients with ESKD.
Consent for publication
All authors agreed the submission and the policy of the journal and copyright.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The Second Xiangya Hospital of Central South University of Science and Technology.
Availability of data and material
All data in this study can be obtained by proper request from the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:1–8. doi:10.1007/978-981-13-8871-2_1.
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. doi:10.1016/j.kisu.2021.11.003.
- Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686.
- Hu EA, Steffen LM, Grams ME, et al. Dietary patterns and risk of incident chronic kidney disease: the atherosclerosis risk in communities study. Am J Clin Nutr. 2019;110(3):713–721. doi:10.1093/ajcn/nqz146.
- Jun JE, Lee Y-B, Lee S-E, et al. Elevated serum uric acid predicts the development of moderate coronary artery calcification independent of conventional cardiovascular risk factors. Atherosclerosis. 2018;272:233–239. doi:10.1016/j.atherosclerosis.2018.02.014.
- Ejaz AA, Nakagawa T, Kanbay M, et al. Hyperuricemia in kidney disease: a major risk factor for cardiovascular events, vascular calcification, and renal damage. Semin Nephrol. 2020;40(6):574–585. doi:10.1016/j.semnephrol.2020.12.004.
- Choi SR, Lee Y-K, Cho AJ, et al. Malnutrition, inflammation, progression of vascular calcification and survival: inter-relationships in hemodialysis patients. PLoS One. 2019;14(5):e0216415. doi:10.1371/journal.pone.0216415.
- Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. doi:10.1038/s41392-021-00507-5.
- Zeng C, Wang R, Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int J Biol Sci. 2019;15(7):1345–1357. doi:10.7150/ijbs.33568.
- Wang Q, Wu J, Zeng Y, et al. Pyroptosis: a pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta. 2020;510:62–72. doi:10.1016/j.cca.2020.06.044.
- Xu Y-J, Zheng L, Hu Y-W, et al. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi:10.1016/j.cca.2017.11.005.
- Liu W, Shen J, Li Y, et al. Pyroptosis inhibition improves the symptom of acute myocardial infarction. Cell Death Dis. 2021;12(10):852. doi:10.1038/s41419-021-04143-3.
- Toldo S, Mauro AG, Cutter Z, et al. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–H1568. doi:10.1152/ajpheart.00158.2018.
- Habimana O, Salami OM, Peng J, et al. Therapeutic implications of targeting pyroptosis in cardiac-related etiology of heart failure. Biochem Pharmacol. 2022;204:115235. doi:10.1016/j.bcp.2022.115235.
- Pang Q, Wang P, Pan Y, et al. Irisin protects against vascular calcification by activating autophagy and inhibiting NLRP3-mediated vascular smooth muscle cell pyroptosis in chronic kidney disease. Cell Death Dis. 2022;13(3):283. doi:10.1038/s41419-022-04735-7.
- Burdette BE, Esparza AN, Zhu H, et al. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11(9):2768–2782. doi:10.1016/j.apsb.2021.02.006.
- Tonnus W, Maremonti F, Belavgeni A, et al. Gasdermin D-deficient mice are hypersensitive to acute kidney injury. Cell Death Dis. 2022;13(9):792. doi:10.1038/s41419-022-05230-9.
- Fang Z, Wu G, Sheng J, et al. Gasdermin D affects aortic vascular smooth muscle cell pyroptosis and ang II-induced vascular remodeling. Heliyon. 2023;9(6):e16619. doi:10.1016/j.heliyon.2023.e16619.
- Cui R-R, Mao D-A, Yi L, et al. Apelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/akt signaling pathways. Amino Acids. 2010;39(5):1193–1200. doi:10.1007/s00726-010-0555-x.
- Peeters MJ, van den Brand JAJG, van Zuilen AD, et al. Abdominal aortic calcification in patients with CKD. J Nephrol. 2017;30(1):109–118. doi:10.1007/s40620-015-0260-7.
- Elmasri K, Hicks Y, Yang X, et al. Automatic detection and quantification of abdominal aortic calcification in dual energy X-ray absorptiometry. Proc Comput Sci. 2016;96:1011–1021. doi:10.1016/j.procs.2016.08.116.
- Bosco E, Hsueh L, McConeghy KW, et al. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21(1):241. doi:10.1186/s12874-021-01440-5.
- Modi ZJ, Lu Y, Ji N, et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: an analysis of the US renal data system. JAMA Cardiol. 2019;4(4):353–362. doi:10.1001/jamacardio.2019.0375.
- Chen H-C, Wang W-T, Hsi C-N, et al. Abdominal aortic calcification score can predict future coronary artery disease in hemodialysis patients: a 5-year prospective cohort study. BMC Nephrol. 2018;19(1):313. doi:10.1186/s12882-018-1124-x.
- Lee SM, Kim SE, Lee JY, et al. Serum myostatin levels are associated with abdominal aortic calcification in dialysis patients. Kidney Res Clin Pract. 2019;38(4):481–489. doi:10.23876/j.krcp.19.019.
- Ito M, Yamaguchi M, Katsuno T, et al. Association between serum magnesium levels and abdominal aorta calcification in patients with pre-dialysis chronic kidney disease stage 5. PLoS One. 2021;16(6):e0253592. doi:10.1371/journal.pone.0253592.
- Eelderink C, Te Velde-Keyzer CA, Frenay AS, et al. Serum calcification propensity and the risk of cardiovascular and all-Cause mortality in the general population: the PREVEND study. Arterioscler Thromb Vasc Biol. 2020;40(8):1942–1951. doi:10.1161/atvbaha.120.314187.
- Ter Braake AD, Govers LP, Peeters MJ, et al. Low plasma magnesium concentration and future abdominal aortic calcifications in moderate chronic kidney disease. BMC Nephrol. 2021;22(1):71. doi:10.1186/s12882-021-02267-4.
- Ter Braake AD, Vervloet MG, de Baaij JHF, et al. Magnesium to prevent kidney disease-associated vascular calcification: crystal clear? Nephrol Dial Transplant. 2022;37(3):421–429. Feb 25 doi:10.1093/ndt/gfaa222.
- Ter Braake AD, Eelderink C, Zeper LW, et al. Calciprotein particle inhibition explains magnesium-mediated protection against vascular calcification. Nephrol Dial Transplant. 2020;35(5):765–773. doi:10.1093/ndt/gfz190.
- Zhou S-J, Wang X-X, Tang W, et al. Lower serum irisin levels are associated with increased abdominal aortic calcification in peritoneal dialysis patients. Kidney Dis (Basel). 2021;7(3):219–226. doi:10.1159/000512514.
- Zhang X, Li Y, Yang P, et al. Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler Thromb Vasc Biol. 2020;40(3):751–765. doi:10.1161/ATVBAHA.119.313414.
- Chen T-C, Yen C-K, Lu Y-C, et al. The antagonism of 6-shogaol in high-glucose-activated NLRP3 inflammasome and consequent calcification of human artery smooth muscle cells. Cell Biosci. 2020;10(1):5. doi:10.1186/s13578-019-0372-1.
- Jiang Y, Liu H, Yu H, et al. Circular RNA Calm4 regulates hypoxia-induced pulmonary arterial smooth muscle cells pyroptosis via the Circ-Calm4/miR-124-3p/PDCD6 axis. Arterioscler Thromb Vasc Biol. 2021;41(5):1675–1693. doi:10.1161/ATVBAHA.120.315525.
- Ma G, Yu Z, Nan F, et al. HCMV-IE2 promotes atherosclerosis by inhibiting vascular smooth muscle cells’ pyroptosis. Front Microbiol. 2023;14:1177391. doi:10.3389/fmicb.2023.1177391.
- Wang H, Wang Z, Wang L, et al. IL-6 promotes collagen-induced arthritis by activating the NLRP3 inflammasome through the cathepsin B/S100A9-mediated pathway. Int Immunopharmacol. 2020;88:106985. doi:10.1016/j.intimp.2020.106985.
- Zhao X-K, Zhu M-M, Wang S-N, et al. Transcription factor 21 accelerates vascular calcification in mice by activating the IL-6/STAT3 signaling pathway and the interplay between VSMCs and ECs. Acta Pharmacol Sin. 2023;44(8):1625–1636. doi:10.1038/s41401-023-01077-8.
- Batra G, Lakic TG, Lindbäck J, et al. Interleukin 6 and cardiovascular outcomes in patients with chronic kidney disease and chronic coronary syndrome. JAMA Cardiol. 2021;6(12):1440–1445. doi:10.1001/jamacardio.2021.3079.
- Desjardins M-P, Sidibé A, Fortier C, et al. Association of interleukin-6 with aortic stiffness in end-stage renal disease. J Am Soc Hypertens. 2018;12(1):5–13. doi:10.1016/j.jash.2017.09.013.
- Gou X, Xu W, Liu Y, et al. IL-6 prevents lung macrophage death and lung inflammation injury by inhibiting GSDME-and GSDMD-mediated pyroptosis during pneumococcal pneumosepsis. Microbiol Spectr. 2022;10(2):e02049-21. doi:10.1128/spectrum.02049-21.
- Ding B, Geng S, Hou X, et al. Berberine reduces renal cell pyroptosis in golden hamsters with diabetic nephropathy through the Nrf2-NLRP3-caspase-1-GSDMD pathway. Evidence-Based Complementary Altern Med. 2021;2021:1–13. doi:10.1155/2021/5545193.